Abstract
The present study explores the ability of activated carbon prepared from sulphuric acid-treated cottonseed cake (SCSC) by chemical activation with sulphuric acid for the removal of Pb(II) from an aqueous solution. Batch experiments were carried out by varying several conditions such as contact time, solution pH, adsorbent dose and temperature along with commercial activated carbon (CAC). According to the experimental results, the equilibrium time and optimal pH range were found to be 3 h and 4.0–6.0, respectively. The equilibrium data were analysed based on the Freundlich, Langmuir, Redlich–Peterson and Dubinin–Radushkevich isotherms using nonlinear regression analysis and fit well with the Langmuir model. Based on the Langmuir isotherm, the adsorption capacity was found to be 115.86 mg/g for SCSC, which was substantially (5.3 times) greater than that of CAC (21.69 mg/g) at 300 K. The thermodynamic investigations indicated that the adsorption reactions were spontaneous and exothermic in nature. The results of the kinetic study showed that the adsorption of Pb(II) could be described by the pseudo-second-order equation, suggesting that the adsorption process is presumably chemisorptions with film diffusion. A single-stage batch adsorber was designed for different adsorbent dose using the Langmuir equation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution due to toxic heavy metals has been a major cause of concern. Industrial and domestic wastewater is responsible for causing several damages to the environment and adversely affecting the health of the people. Metals can be distinguished from other toxic pollutants, since they are non-biodegradable and can accumulate in living tissues, thus becoming concentrated throughout the food chain [1]. Lead is found to be one of the toxic heavy metals in liquid effluents generated by various industries such as metal finishing, metal plating and metal processing industries [2]. According to the Indian Standard Institution, the allowable limit for discharge of lead into drinking water is 0.05 mg/L, and in land surface water, it is 0.1 mg/L [3]. If the lead(II) concentration exceeds the allowable limits then it may cause harmful effects to human and aquatic organisms. Therefore, it is necessary to treat lead-contaminated wastewater prior to its discharge to the environment. The most commonly employed techniques for the treatment of lead(II)-contaminated wastewaters include chemical precipitation, adsorption, ion-exchange, electrolysis, evaporation, and membrane separation [4]. Among the mentioned methods, adsorption is proved to be one of the simplest and most effective techniques. As of now, adsorption by activated carbon is accepted to be the best available technology for the reduction of heavy metals, except that its manufacturing cost is quite high. Hence, low-cost activated carbons based on agricultural solid wastes have been investigated for a long time. Agricultural by-products and waste materials used for the production of activated carbons include watermelon shell and walnut shell [5], chestnut shell [6], orange peel [7], pomelo peel [8], grapefruit peel [9], cassava peel [10], peanut husk [11], palm shell [12], gingelly oil cake [13], olive cake [14], graphitic carbon nitride [15], and graphene oxide [16, 17]. These materials have shown attractive experimental results for lead(II) removal.
The main objective of this paper was to evaluate the potential for developing an effective, new, low-cost adsorbent from sulphuric acid-treated cottonseed cake (SCSC) and compare it with the commercial activated carbon (CAC) for the removal of Pb(II) from aqueous solutions. The influence of several operating parameters for the adsorption of Pb(II) ions such as contact time, solution pH, adsorption dose on various initial concentrations and temperature were investigated in batch mode. The kinetic data were fitted to different adsorption kinetic models such as the pseudo-first order, pseudo-second order and intraparticle diffusion equation, and the isotherm data were fitted to the Freundlich, Langmuir, Redlich–Peterson and Dubinin–Radushkevich isotherm equations. Thermodynamic parameters were also carried out to estimate the standard free energy (∆G°), enthalpy change (∆H°) and entropy change (∆S°).
Materials and methods
Preparation of carbon adsorbent
The cottonseed cake sample, which was produced at a cottonseed oil factory, was used in this study. The oilcake was washed with water, dried in a hot-air oven at 100–110 °C for 24 h. Then it was treated with concentrated sulphuric acid in 1:2 weight ratios and kept in an air oven at 160 ± 5 °C for 24 h. The carbonized material was washed with distilled water and soaked in 1 % sodium bicarbonate for 24 h to remove any free acid. The material was then washed with distilled water, dried and sieved to a 20–50 ASTM mesh size for use in the experiments (SCSC). The CAC (SD fine chemicals, Ltd.) was procured from the market and sieved to a 20–50 ASTM mesh size and used for evaluation purposes. The characteristics of both carbons were tested as per ISI-877 [18] and given in Table 1.
Preparation of the Pb(II) solutions
Stock aqueous solution of Pb(II) having a concentration of 1000 mg/L was prepared by dissolving 1.59 g of Pb(NO3)2 in 1000 mL of distilled water. Lead(II) solutions of desired concentrations were prepared by dilution of the stock solution. The pH of the solution was adjusted to the required value by using 0.1-N HCl or 0.1-N NaOH solutions.
Batch adsorption studies
The batch experiments were conducted in polythene bottles of 300 mL capacity. 100 mL of the solution containing 10 mg/L of Pb(II) ions adjusted to the desired pH were taken in bottles and equilibrated for specific periods of time in a temperature-controlled shaker. At the end of the equilibration period, the solutions were centrifuged and the Pb(II) concentrations were determined with an atomic absorption spectrophotometer. Adsorption isotherm and kinetic studies were carried out with different initial concentrations of Pb(II) by modifying the carbon dosage at a constant level. Pb(II) removal (%) was calculated using the following equation [16]:
The adsorption capacity of Pb(II) ions adsorbed per gram of adsorbent (mg/g) was calculated by:
where C 0 and C e are the initial and equilibrium Pb(II) concentrations (mg/L); V is the volume of the Pb(II) solution (L); M is the mass of the adsorbent used (g).
Results and discussion
Examination of the carbon characteristics (Table 1) shows that SCSC has considerable bulk density and high ion-exchange capacity. The moisture content of SCSC suggests that the acid treatment made the carbon porous in nature. Even though SCSC shows lower surface area when compared with CAC, it has shown considerable ion-exchange capacity, which is responsible for Pb(II) removal.
Characterisation of the SCSC and CAC
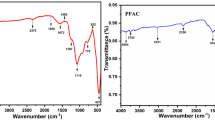
The FTIR spectrum and SEM images of the SCSC and CAC were used to characterise the adsorbent material. The FTIR spectrum of the SCSC and CAC are shown in Fig. 1a, b. It could be seen from spectra for SCSC that the peak at 3370–3470 cm−1 corresponds to the OH stretching [19]. The peak at 2869 cm−1 indicated the presence of aliphatic C-H groups in SCSC [19]. The bands appearing at 1629–1548 cm−1 in SCSC and 1543 cm−1 in CAC are ascribed to the formation of oxygen functional groups, like a highly conjugated C=O stretching in carboxylic acid groups [20]. The peak at 1369 cm−1 in SCSC due to asymmetric S=O stretching of the sulphonic acid group [21]. The presence of hydroxyl, carboxylic and sulphonic acids groups were confirmed in SCSC by the FTIR spectra and, in the case of CAC, only the carboxylic acid group was confirmed. These SCSC carbon containing polar functional groups (–OH, C=O, S=O) could be involved in chemical bonding and may be responsible for the adsorption of metals [22]. Further, according to Pearson’s theory, hard acids prefer to interact with hard bases and soft acids with soft bases. Pb is a soft Lewis acid, binds with the functional groups containing ‘S’, which are soft Lewis bases. Hence, S=O functional groups, which are present on the adsorbent, show very high coordination with the Pb2+ ions.
Scanning electron microscopy (SEM) was employed to observe the physical surface morphology of the adsorbents. SEM images of the SCSC and CAC are depicted in Fig. 2a, b, respectively. The surface of the adsorbent appears to be irregular and porous. On the basis of this observation, it can be concluded that the adsorbent has an adequate morphology for Pb(II) ion adsorption. Similar results were reported in Refs. [23, 24].
Effect of agitation time
The effect of contact time on the adsorption of Pb(II) onto SCSC and CAC are shown in Fig. 3a. The experimental data showed a rapid increase in the initial lead adsorption up to 1 h. After this, the adsorption rate became slower, and finally, no further significant adsorption was noted beyond 3 h. This could be attributed to a larger number of vacant binding sites available for adsorption during the initial stage, which was carried out via physical adsorption or ion exchange on the surface of the adsorbent. After prolonging the contact time, the occupation of the remaining vacant sites was difficult as a result of the repulsive forces between Pb2+ ions in solid and liquid phases. Hence the optimum equilibrium time was taken as 3 h for subsequent experiments.
Effect of pH
The pH of the solution has a significant impact on the uptake of heavy metals, since it determines the surface charge of the adsorbent, the degree of ionization and speciation of the adsorbate. Thus, the effect of H+ ion concentration in the aqueous solutions on the percentage removal of lead(II) ions was studied at different pH values ranging from 2.0 to 8.0. These studies were not extended to a higher pH because of the precipitation of the lead ions forming hydroxides. It is clear from Fig. 3b, that the adsorption of Pb(II) is increased with the increase in initial pH of the solution and maximum removal occurred at a pH within the range 4.0–6.0 for SCSC and at pH 6.0 for CAC. The pH dependence of adsorption efficiency could be explained by the functional groups involved in metal uptake and metal chemistry. The FTIR spectroscopic analysis shows that the SCSC has a variety of functional groups such as carboxyl, hydroxyl and sulphonic acid groups which are involved in almost all the potential binding mechanisms. Moreover, depending on the solution pH, the functional groups participate in metal ion bindings. At low pH values, H+ ions engage on the whole adsorption sites on the SCSC surface and a lesser amount of lead could be sorbed because of electrostatic repulsion with H+ ions on the adsorbent surface [25]. As the pH is increased, more negatively charged surface became available which facilitated greater Pb(II) uptake on the surface of adsorbents. At pH > 6.0, the decrease in adsorption efficiency was due to formation of soluble hydroxylated complexes of lead ions and their competition with active sites; as a result, the retention would decrease again. High Pb(II) sorption efficiency was observed from pH 4.0–6.0 for SCSC and at 6.0 for CAC, as judged from Fig. 3b. This area is favorable because no precipitation occurred in the Pb(II) sorption system. Based on the high sorption efficiency of the adsorbents, pH 5.0 for SCSC and pH 6.0 for CAC was chosen for determining the other experimental parameters for subsequent study.
The mechanism of Pb(II) removal may also be explained based on an ion exchange model. A pure carbon surface is considered to be non-polar, but in actual practice some carbon–oxygen complexes (CXO, COX and CXO2) are usually present, which render the surface slightly polar [26]. The surface oxygen complexes hydrolyse water molecules as shown below;
Since the SCSC is prepared upon treatment with H2SO4 followed by NaHCO3 treatment, groups such as CXO Na+, CXO Na2 2+, CXSO3H and CXSO3Na are also assumed to be present. Hence, it is expected that Na+ may be exchanged with Pb(II) and soluble complexes of Pb(II) as follows:
The above mechanism was verified quantitatively by the release of sodium ions in the treated water due to the adsorption of lead ions.
Effect of adsorbent dose
The adsorbent dose is an important parameter because it determines the capacity of the adsorbent for a given initial lead(II) ion concentration. The effect of adsorbent dose on the adsorption of Pb(II) is shown in Fig. 3c. It was observed that the percentage of Pb(II) removal increases with increase in carbon dose from 0.05 to 0.1 g for SCSC and 0.05–0.3 g for CAC. At the low carbon dose, it is known that the adsorbent surface becomes saturated with the metal ions, while the residual metal ion concentration in the solution is high. With an increase in carbon dose, the lead ion removal is intensified. For the carbon dose of 0.1 g for SCSC and 0.3 g for CAC, the incremental lead ion removal become very low as the surface metal ion concentration and the aqueous metal ion concentration reached the equilibrium state. For the higher adsorbent dose, the removal efficiency of lead ions onto SCSC and CAC was leveled off. It is evident that with 100 mL of Pb(II) solution containing 10 mg/L of Pb(II) ions, a minimum adsorbent dose of 0.1 g SCSC is required for the maximum removal of 99.3 %, and, in the case of CAC, 0.3 g is required for the removal of 55 % only. The data clearly indicate that SCSC is nearly three times more effective than CAC and this may be due to a moderate ion-exchange capacity exhibited by SCSC as compared to CAC.
Adsorption isotherms
In general, an adsorption isotherm is an invaluable curve describing the phenomenon governing the retention (or release) or mobility of a substance from the aqueous porous media or aquatic environments to a solid-phase at a constant temperature and pH. Different isotherm models have been used to determine the adsorption efficiency for adsorption of heavy metals from aqueous media. In this study, four adsorption isotherm models (Freundlich [27], Langmuir [28], Redlich–Peterson [29] and Dubinin–Radushkevich [30] isotherms) were used for Pb(II) adsorption on SCSC and CAC.
where, C e is the concentration of nickel in the aqueous solution at equilibrium, K F and n are the Freundlich constants, denoting the adsorption capacity and intensity; q m is the monolayer adsorption capacity, and K L is the Langmuir constant related to binding energy; K R, a R and g are the Redlich–Peterson constant related to the adsorption capacity, affinity of the binding sites and adsorption intensity; q mD is the D–R monolayer adsorption capacity and β is the adsorption energy. The β value gives the mean free energy (E) of adsorption per molecule of adsorbate, when it is transferred to the surface of the solid infinity in the solution, and can be computed using the following relationship:
The isotherm parameters, correlation coefficients (R 2), sum of squares error (SSE), and root mean squared error (RMSE) values were estimated from the plot of q e versus C e and are listed in Table 2. Based on the R 2, SSE and RMSE values from Table 2, the Langmuir isotherm model exhibited a better fit to the adsorption equilibrium data of Pb(II) ions onto SCSC and CAC. The value of the Redlich–Peterson exponent (g) is closer to 1, which means that the Langmuir isotherm is preferable. Hence, the good fit of equilibrium data in Langmuir isotherm expressions confirms the monolayer coverage process of Pb(II) ions onto SCSC and CAC. According to the Langmuir isotherm, the monolayer saturation capacity (q m) of Pb(II) ions was found to be 115.86 mg/g for SCSC, which was about 5.3 times greater than that of CAC (21.69 mg/g). The low E values obtained in the Dubinin–Radushkevich study (Table 2) indicate that a weak interaction exists between the sorbate and sorbent. Based on the characteristics of the carbon, an ion-exchange mechanism may also be operating in SCSC during the Pb(II) removal process.
The comparison of the maximum monolayer adsorption capacity of Pb(II) onto various adsorbents was presented in Table 3. These results indicate that SCSC is a better adsorbent for removal of Pb(II) compared to other sorbents. Therefore, it could be concluded that the SCSC has good potential for the removal of Pb(II) from aqueous solutions.
Thermodynamics studies
Studies with Pb(II) ions onto SCSC and CAC were carried out at different temperatures (300, 310, and 320 K) and are shown in Fig. 4a. The effect of temperature on the adsorption of Pb(II) ions were carried out using 100 mL of 10 mg/L of Pb(II) solutions, with an adsorbent dose of 0.1 g and equilibrated for 3 h at pH 5.0 for SCSC and 6.0 for CAC. The equilibrium constant at different temperatures can be calculated with the following equation:
where, K c is the equilibrium constant (L/g), C a is the equilibrium concentration of metal ion adsorbed per unit mass on the adsorbent (mg/L) and C e is the equilibrium metal ion concentration (mg/L). The values of free energy change (∆G°) at different temperatures were calculated from the following relation:
where R is the gas constant (8.314 kJ/mol K) and T is the absolute temperature. The Van’t Hoff equation was applied to calculate the enthalpy change (∆H°) and entropy change (∆S°):
∆S° and ∆H° can be calculated from the intercept and slope of the linear plot of ln K c versus 1/T (Fig. 4b) and are given in Table 4. The negative value of ∆G° implies that the adsorption of Pb(II) ions onto SCSC was spontaneous and feasible. The negative ∆H° value indicates the exothermic nature of adsorption and the negative ∆S° value can be used to describe the randomness at the SCSC and CAC solution interface during the adsorption of Pb(II) ions.
Kinetic models
In order to investigate the adsorption mechanism and rate controlling steps, the adsorption kinetic data were modelled using pseudo-first order, pseudo-second order and intraparticle diffusion equations.
The linear form of the pseudo-first order rate equation by Lagergren [36] is given as:
where q e (mg/g) and q t (mg/g) are the adsorption amount at equilibrium and at time t (min), respectively. k 1 (min−1) is the rate constant of the pseudo-first order adsorption process. The constants were determined experimentally by plotting of log (q e − q t) versus t.
A pseudo-second order model was also generally applied to fit the experimental data. The linear form of the pseudo-second order model [37] can be expressed as:
where k 2 (g/mg/min) is the rate constant of adsorption. By plotting a curve of t/q t against t, q e and k 2 can be evaluated. The initial adsorption rate, h 0 (mg/g/min) is defined as:
In order to compare quantitatively the applicability of kinetic models in fitting to data, the percentage relative deviation (P%) was calculated by the following equation:
where q e(exp) and q e(cal) are experimental and calculated values of Pb(II) adsorbed on the adsorbents; N is the number of measurements made. It is found that the lower value of percentage deviation (P%), the better is the fit. It is generally accepted that when P% value is <5, the fit is considered to be excellent [38].
The kinetic data were analysed by an intra-particle diffusion model to elucidate the diffusion mechanism [39]:
where q t is the amount of sorbate on the surface of the sorbent at time t (mg/g), k d (mg/g/min1/2) is the intra-particle diffusion rate constant and I (mg/g) is a constant that gives an idea about the thickness of the boundary layer. According to this model, the plot of q t versus (t 1/2) is linear and passes through the origin, and then intraparticle diffusion is the sole rate-limiting step.
The kinetic parameters and correlation coefficients are listed in Table 5. From Table 5, the correlation coefficients for the pseudo-second order equation are much closer to unity. The calculated q e values are very close to the experimental q e values. Furthermore, the P% is also found to be <5 % in the case of the pseudo-second order equation. These results confirm that the adsorption kinetics of Pb(II) ions onto the SCSC and CAC is mainly governed by a pseudo-second order equation. This suggests that the rate-limiting step of this sorption system may be controlled by chemical sorption involving valence forces through sharing or exchange of electrons between sorbent and sorbate. Figure 5a, b show plots of the Weber and Morris intra-particle diffusion model for the sorption of Pb(II) onto SCSC and CAC. The deviation in the plots from the origin for all concentrations indicated that pore diffusion was not the only rate-controlling factor, but some other processes like film diffusion were also involved in the sorption process. The low correlation coefficient values obtained for the intra-particle diffusion model also indicate that adsorption is not occurring in the pores of the sorbent in accordance with surface adsorption. Moreover, distinct ionic species might be present simultaneously in the solution and, hence, each of them are being adsorbed at variable rates as a function of their affinity to the individual functional groups; this also depends on the binding mechanism.
In order to further assess the nature of the diffusion process responsible for the adsorption of Pb(II) on SCSC and CAC, attempts were made to calculate the pore and film diffusion coefficients for various concentrations of Pb(II) using the following the equation [40]:
where D p is the pore diffusion coefficient (cm2/s), r is the radius of the sorbent (cm), and t 1/2 is the half-life period (s).
where D f is the film diffusion coefficient (cm2/s), d is the film thickness (cm), and C*/C is equilibrium loading of the sorbent. According to Michelsen et al. [26], if film diffusion is to be the rate determining process in the adsorption of heavy metals on a sorbent, the values of film diffusion coefficient (D f) should be between 10−6 and 10−8 cm2/s. If pore diffusion D p were to be the rate determining process, its value should be in the range of 10−11–10−13cm2/s. The pseudo-second order rate constant (k 2) was applied to calculate the film and pore diffusion coefficients for the present study. It is evident from Table 6 that the removal of Pb(II) follows the film diffusion process, since the coefficient values are closer to the range of 10−6–10−8 cm2/s.
Single-stage batch adsorber
As previously mentioned, adsorption equilibrium data are essential for designing an adsorption system. The schematic diagram for a single batch adsorber was found to be similar to that used in the removal of nickel ions by cashew nut shell [41]. The Pb(II) solution to be treated contains V (L) of solvent and the Pb(II) concentration is reduced from C 0 to C e in the adsorption process. The amount of adsorbent used is M (g) of Pb(II)-free SCSC adsorbent and the Pb(II) concentration changes from q o = 0 to q e. The mass balance equates the Pb(II) removed from the solution to that picked up by the SCSC adsorbent and is
The Langmuir adsorption isotherm data may be applied to Eq. 19 since the Langmuir adsorption isotherm gave the best fit to experimental data.
Figure 6 shows the plot between the calculated amounts of SCSC required to remove lead(II) ions from a solution of initial concentration 50 mg/L for 80, 85, 90 and 95 % lead(II) removal at different solution volumes (1–10 L) for a single-stage batch adsorption system, for which the design procedure is outlined.
Desorption and regeneration studies
In order to investigate desorption of metal ion from metal-loaded adsorbents, the metal-loaded adsorbent was treated with HCl, which has been reported as an efficient metal desorbent. Desorption studies were performed with different concentrations of hydrochloric acid. From the results of this study (data not shown), with the increasing of hydrochloric acid concentration, the desorption rate also increased initially and then become almost stable. The maximum percentage recovery of Pb(II) was 99 % for SCSC and 90 % for CAC with a 0.3-M HCl solution. In the adsorption process, to keep the processing cost down and to open the possibility of recovering the metal(s) extracted from the liquid phase, it is desirable to regenerate the adsorbent material. In this study, 0.3-M HCl was used to regenerate the carbon over five cycles of operation. A slight increase in the sorption of Pb(II) could be observed after each and every cycle with SCSC, improving the recovery of Pb(II) ions. This increase in the sorption (99–99.9 %) may occur because additional surface active sites present on the sorbent surface open after repeated regeneration cycles. However, the recovery of Pb(II) ions was slightly decreased at the end of the fifth cycle (Fig. 7a). For CAC, both the adsorption and desorption values decreased rapidly (Fig. 7b). Therefore, SCSC has a greater potential for repeated uses and the recovery of Pb(II) ions.
Conclusion
The present investigation shows that the carbon prepared from an agricultural waste by-product such as cottonseed is able to remove 99.3 % of Pb(II) ions from aqueous solution whereas CAC was able to remove only 55 %. Compared with CAC, the SCSC has a wider applicable pH range, from 4.0 to 6.0. Equilibrium data agreed well with the Langmuir isotherm and the adsorption capacity (q m) of SCSC is 5.3 times greater than CAC. The adsorption is followed by pseudo-second order kinetics, which indicates chemisorptions with the film diffusion process being an essential rate controlling step. The thermodynamic study showed that the adsorption was a spontaneous and exothermic process. A single-stage batch adsorber was designed using the Langmuir isotherm equation at room temperature. The regeneration study shows that SCSC could be effectively utilized for the removal of Pb(II) ions for five cycles of operation under study when compared with CAC. The above results showed that SCSC is an effective, economical and environmentally friendly adsorbent for the removal of Pb(II) from aqueous solutions.
References
U. Kumar, M. Bandyopadhyay, Biores. Technol. 97, 104 (2006)
L. Wang, J. Zhang, R. Zhao, Y. Li, C. Li, C. Zhang, Biores. Technol. 101, 5808 (2010)
ISI, Tolerance limits for industrial effluents prescribed by Indian Standards Institution. IS: 2490 (Part II), New Delhi, India
P. Senthil Kumar, Environ. Prog. Sustain. 33(1), 55 (2014)
J.J.M. Barbosa, C.L. Velandia, A.D.P. Maldonado, L. Giraldo, J.C.M. Pirajan, Adsorption 19, 675 (2013)
H. Demiral, E. Baykul, M. Deniz Gezer, S. Erkoc, A. Engin, M. Celalettin Baykul, Sep. Sci. Technol. 49, 2711 (2014)
S. Liang, X. Guo, Q. Tian, Desalination 275, 212 (2011)
Q.S. Pan, W. Liu, M.G. Mao, H.P. Yan, D.S. Huang, B. Zhou, B.S. Wang, N. Wu, S.J. Xu, L. Shi, Adv. Mater. Res. 726–731, 2320 (2013)
Y.Y. Pei, J.Y. Liu, Adv. Mater. Res. 391–392, 968 (2011)
H.I. Owamah, J. Mater. Cycles Waste Manag. 16, 347 (2014)
Q. Li, J. Zhai, W. Zhang, M. Wang, J. Zhou, J. Hazard. Mater. 141, 163 (2006)
Y.B. Onundi, A.A. Mamun, M.F. Al Khatib, Y.M. Ahmed, Int. J. Environ. Sci. Technol. 7(4), 751 (2010)
K.B. Nagashanmugam, K. Srinivasan, J. Environ. Sci. Eng. 52, 349 (2010)
S. Doyurum, A. Celik, J. Hazard. Mater. 138, 22 (2006)
R. Hu, X. Wang, S. Dai, D. Shao, T. Hayat, A. Alsaedi, Chem. Eng. J. 260, 469 (2015)
S. Yang, C. Chen, Y. Chen, J. Li, D. Wang, X. Wang, W. Hu, ChemplusChem 80, 480 (2015)
G. Zhao, X. Ren, X. Gao, X. Tan, J. Li, C. Chen, Y. Huang, X. Wang, Dalton Trans. 40, 10945 (2011)
ISI 877, Methods of sampling and tests for activated carbon used for decolourising vegetable oils and sugar solutions (1977)
J. Hu, D. Shao, C. Chen, G. Sheng, J. Li, X. Wang, M. Nagatsu, J. Phys. Chem. B 114, 6779 (2010)
Y.F. Jia, K.M. Thomas, Langmuir 16, 1114 (2000)
R. Sudha, K. Srinivasan, P. Premkumar, Ecotoxicol. Environ. Saf. 117, 115 (2015)
S. Ricordel, S. Taha, I. Cisse, G. Dorange, Sep. Purif. Technol. 24, 389 (2001)
M.R. Awual, Chem. Eng. J. 266, 368 (2015)
M.R. Awual, T. Yaita, S. Suzuki, H. Shiwaku, J. Hazard. Mater. 291, 111 (2015)
S. Yang, J. Hu, C. Chen, D. Shao, X. Wang, Environ. Sci. Technol. 45, 3621 (2011)
L.D. Michelsen, P.G. Gideon, E.G. Pace, L.H. Kutal, Office Water Res. Technol. Bull. 74, 153 (1975)
H.M.F. Freundlich, J. Phys. Chem. 57, 385 (1906)
I. Langmuir, J. Am. Chem. Soc. 40, 1361 (1918)
O. Redlich, D.L. Peterson, J. Phys. Chem. 63(6), 1024 (1959)
M.M. Dubinin, L.V. Radushkevich, Chem. Zent. 1, 875 (1947)
K. Huang, H. Zhu, Environ. Sci. Pollut. Res. 20, 4424 (2013)
A. Shahat, M.R. Awual, M.A. Khaleque, M.Z. Alam, M. Naushad, A.M.S. Chowdhury, Chem. Eng. J. 273, 286 (2015)
M.R. Awual, M.M. Hasan, A. Shahat, Sens. Actuators B 203, 854 (2014)
M.R. Awual, M.M. Hasan, Sens. Actuators B 202, 395 (2014)
M.R. Awual, M.M. Hasan, Microporous Mesoporous Mater. 196, 261 (2014)
S. Lagergren, Kungliga Svenska Vetenskapsakad Handl. 24, 1 (1898)
Y.S. Ho, G. Mckay, Can. J. Chem. Eng. 76, 822 (1998)
E. Ayranci, O. Duman, J. Hazard. Mater. 124, 125 (2005)
W.J. Weber, J.S. Morris, J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 89, 31 (1963)
A.K. Bhattacharya, C.J. Venkobachar, J. Environ. Eng. Div. ASCE 110, 110 (1984)
P. SenthilKumar, S. Ramalingam, R.V. Abhinaya, K.V. Thiruvengadaravi, P. Baskaralingam, S. Sivanesan, Sep. Sci. Technol. 46, 2436 (2011)
Acknowledgments
The authors would like to thank the Chairman, Department of Chemistry, Erode Sengunthar Engineering College, Anna University, Chennai (India), for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Malathi, S., Krishnaveni, N. & Sudha, R. Adsorptive removal of lead(II) from an aqueous solution by chemically modified cottonseed cake. Res Chem Intermed 42, 2285–2302 (2016). https://doi.org/10.1007/s11164-015-2149-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2149-4