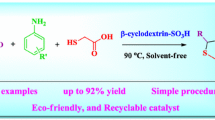
Abstract
An environmentally benign and efficient method has been developed for the synthesis of a series of 2H-indazolo[2,1-b]phthalazine-triones derivatives with β-cyclodextrine-SO3H as a recyclable catalyst by simply combining of various aldehydes with cyclic 1,3-diketones and phthalhydrazide under solvent free condition. The advantageous features of this methodology are high atom-economy, operational simplicity, shorter reaction time, convergence, and facile automation.
Graphical Abstract
A greener, efficient, and expeditious method has been developed for the synthesis of 2H-indazolo [2,1-b] phthalazine-triones derivatives with β-cyclodextrine-SO3H as a recyclable catalyst for the first time.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heterocyclic compounds acquired significance, as these are associated with a wide range of potential biological and pharmaceutical activities [1]. These N-containing heterocyclic compounds play a crucial role in the context of drug scaffolds, synthetic organic chemistry, and medicinal chemistry as well as material sciences [2]. 3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H, 13H)-triones derivatives constitute a key structural motif in a number of natural and synthetic bioactive molecules [3]. Heterocycles containing phthalazine moiety constitute as an important structural motif attributing to their pharmacological and biological activities [4, 5]. Besides these they are endowed with anticonvulsant [6], cardio tonic [7] and vasorelaxant activities [8] and also comply as anti-inflammatory, analgesic, antihypoxic and antipyretic agents [9]. Numerous protocols have been developed for the synthesis of 3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-triones using p-TSA [10], ionic liquids [11], (S)-CSA [12], dodecylphosphonic acid (DPA) [13], heteropoly acids [14], Ce(SO4)2·4H2O [15], solid acids [16], montmorillonite K-10 [17], N-halosulfonamides [11], silica-sulfuric acid [18], phosphomolybdic acid–silica (PMA-SiO2) [19] as catalysts. However, some of the synthetic strategies suffer from certain limitations such as expensive catalysts, low yields of products, long reaction times, use of toxic solvents, tedious procedures for preparation of catalysts and tedious work-up conditions. Hence, the development of an efficient, simple, easy work-up and environmentally benign protocol using a recyclable catalyst and a green solvent for the synthesis of 3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H, 13H)-triones derivatives is still desirable and in demand.
In recent decades, organic reactions under solvent‐free conditions have also attracted much interest from chemists particularly from the viewpoint of green chemistry [20]. Green chemistry approaches are significant due to the reduction in waste production, reduction in byproducts, reduction of energy and cost [21]. The possibility of performing multicomponent reactions under solvent‐free conditions using an environmentally friendly catalyst could enhance the efficiency from an economic as well as ecological point of view [22]. β-cyclodextrin-SO3H is well known supramolecular catalysts, which by reversible formation of host–guest complexes, activate the organic molecules and catalyze the reactions [23]. Our literature survey at this stage revealed that there is no single report on the synthesis of 3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H, 13H)-triones derivatives in solvent free mediated by β-cyclodextrin-SO3H, as a recyclable supramolecular catalyst. As part of our ongoing program toward the development of greener chemical approaches for the synthesis of novel reaction intermediates and heterocyclic moieties [24–28], we report herein the synthesis of 3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-triones by the reaction of phthalhydrazide, 1,3-diketones, and aldehydes using β-cyclodextrin-SO3H, as a recyclable supramolecular catalyst, in solvent free medium (Scheme 1).
Results and discussion
The studies were initiated to optimize the reaction conditions for a model reaction of phthalhydrazide, benzaldehyde and dimedone in presence of different catalyst and solvent (Table 1). In order to establish the real effectiveness of the catalyst for the synthesis of 2H-indazolo[2,1-b]phthalazine-trione derivatives, a comparative reaction was performed without catalyst using phthalhydrazide, benzaldehyde and dimidone in ethanol at reflux. It was found that only 35 % of product was obtained in the absence of catalyst even after 1 h (Table 1, entry 1). In order to develop a viable approach, the model reaction was investigated by employing different catalyst such as CaCl2, SiO2, FeCl3, Zn(OTf)2, ZnCl2, β-cyclodextrin, CuCl2, β-cyclodextrine-SO3H, Li(OTf), SnCl2·2H2O and CuF2. Among all screened catalyst β-cyclodextrine-SO3H gave the best result in view of yield and reaction time (Table 1, entry 8). In contrast CaCl2, SiO2, Zn(OTf)2, ZnCl2, Li(OTf) and SnCl2.2H2O did not afford the desired product in good yields (Table 1, entries 2, 3, 5, 6, 10 and 11). The β-cyclodextrine-SO3H is appeared to be more efficient than CuF2 in terms of yield and time for completion of the reaction (Table 1, entries 8 and 13). Careful analysis of screened Lewis acid catalyst, such as β-cyclodextrin, CuCl2, FeCl3 and CuF2 were effective, but promising results were obtained with β-cyclodextrine-SO3H catalyst in less time with better yield (Table 1, entries 4, 8, 9, 13 and 14).
To see the effect of solvent, we screened different solvents such as toluene, ethanol, acetonitrile, dimethylformamide, ethylene glycol, methanol, water, and tetrahydrofuran at room temperature. It was observed that under solvent condition required longer times to afford comparable yields (Table 1, entries 15–21). When the reaction was performed under solvent free conditions, high yield of target product was obtained (Table 1, entry 8). Moreover, we found that the yields were obviously affected by the amount of β-cyclodextrine-SO3H loaded. When 5 mol%, 10 mol%, and 20 mol% of β-cyclodextrine-SO3H were used, the yields were 91, 98, and 95 %, respectively (Table 1, entries 8, 22 and 23). Therefore, 10 mol% of β-cyclodextrine-SO3H was sufficient and optimal quantity for the completion of the reaction.
Thus, we selected the optimized reaction condition to examine the universality of this catalyst application with different electron rich and deficient substrates. It was gratifying to observe that most of the tested substrates exhibited satisfactory reactivity profiles, in all cases leading to a heterocyclization sequence that readily afforded the target structures (Table 2). Various substituted aldehydes undergo the reaction in the presence of catalytic amount of β-cyclodextrine-SO3H (10 mol%) in solvent free condition at 80 °C. As compared to aromatic aldehydes, the heteroaromatic aldehyde gave slightly lower yield (Table 2, entries 1–18, 26). Compared with heteroaromatic aldehydes, aliphatic aldehydes afforded relatively higher yields of the corresponding 2H-indazolo[2,1-b]phthalazine-trione (Table 2, entries 19–21, 26). In general, all the reactions were clean, and the 2H-indazolo[2,1-b]phthalazine-trione were obtained in good to excellent yields (85–98 %).
A possible mechanism of this one-pot reaction is expected on the basis of the reported literature [13]. A plausible mechanism for the formation of substituted 2H-indazolo[2,1-b]phthalazine-trione derivatives by using β-cyclodextrine-SO3H as a recyclable catalyst (Scheme 2).
The reusability of the β-cyclodextrine-SO3H catalyst is one of the most important benefits and makes it useful for commercial applications as well. Thus, the recovery and reusability of the catalyst were investigated. The recyclability of the catalyst was checked with model reaction (Table 3, entries 1–4). The catalyst was recovered after completion of the first fresh run, the reaction mixture cooled to room temperature and diluted with water. The catalyst was dissolved in water and product was precipitated out. The precipitated crude product was separated by simple filtration and β-cyclodextrine-SO3H was recovered by evaporating the aqueous layer under reduced pressure. The recovered β-cyclodextrine-SO3H (10 mol %) was dried at 90–100 °C for 12 h and tested in up to three more reaction cycles. The same catalyst (10 mol%) was reused for subsequent reactions (three runs) with fresh substrates under the same conditions. The catalyst showed excellent recyclability in all these reactions (Table 3), as the reaction times and yield remained almost the same with a slight reduction in catalytic activity.
The reported as well as synthesized novel compounds were further characterized by their spectral properties (1H, 13C NMR, and HRMS).
Conclusion
In summary, an environmentally benign and efficient method has been developed for the synthesis of a series of 2H-indazolo[2,1-b]phthalazine-triones derivatives in solvent free media with β-cyclodextrine-SO3H as a recyclable catalyst by simply combining of various aldehydes with cyclic 1,3-diketones and phthalhydrazide. In generally, the 2H-indazolo[2,1-b]phthalazine-triones is carried out in solvent-free conditions without any use of anhydrous condition. To the best of our knowledge this is the first report for using β-cyclodextrine-SO3H as a green catalyst for synthesis of entitled compounds. Shorter reaction times, good to excellent yields and more importantly, the recyclability of catalyst with a slight reduction in catalytic activity, make this protocol good and attractive.
Experimental
Materials and methods
Chemicals were purchased from Aldrich and Alfa Aesar chemical companies and used as it is. The NMR spectra were recorded in CDCl3 on a Jeol JNMECP 400 NMR instrument using TMS as an internal standard. The HRMS was recorded on a Jeol JMS-700 mass spectrometer.
General procedure for the synthesis of β-cyclodextrin-SO3H catalyst
A β-cyclodextrin-SO3H catalyst was prepared by adopting the literature procedure [29]. To a well stirred mixture of β-cyclodextrin (10.0 g, 4.5 mmol) in CH2Cl2 (50 mL), chlorosulfonic acid (2.00 g, 10 mmol) was added slowly at 0 °C during 3 h. The resulting mixture was stirred for another 2 h to remove HCl from the reaction vessel. Then, the mixture was filtered and washed with methanol (50 mL) and dried at room temperature to obtain sulfonated β-cyclodextrin as a white powder (10.56 g).
General procedure for the synthesis of 2H-indazolo [2,1-b] phthalazine-triones derivatives (1–25)
A mixture of phthalhydrazide (1.0 mmol), aldehyde (1 mmol), 5,5-dimethyl-1,3-cyclohexanedione or 1,3-cyclohexanedione (1.0 mmol), β-cyclodextrin-SO3H (10 mol %) was heated at 80 °C under solvent free condition for an appropriate time as mentioned in Table 1. After completion of the reaction as monitored by TLC the reaction mixture was allowed to cool to room temperature and the residue was diluted with water. The precipitate formed was collected by filtration at pump, washed with water, and dried. The residue recrystallized from ethanol to afford the pure product of 2H-indazolo[2,1-b]phthalazine-trione derivatives.
3,3-Dimethyl-13-phenyl-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (1)
Yellow powder, mp: 200–202 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.23 (s, 6H, –(CH3)2), 2.35 (s, 2H, –CH2–C=), 3.26 and 3.41 (2H, AB system, J = 16 Hz, CHaHbCO), 6.47 (s,1H, –CH–N), 7.61 (d, J = 8 Hz, 2H, Ar–H), 7.90–7.85 (m, 2H, Ar–H), 8.37–8.16 (m, 5H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 28.5 (–CH3), 28.7 (–CH3), 34.7 (–C(CH3)2), 38.1 (–CH2–C=), 50.9 (–CH2CO), 64.9 (–CH–N), 118.6 (–HC–C–CO–), 127.1 (Ar–C), 127.7 (Ar–C), 127.9 (Ar–C), 128.7 (Ar–C), 128.9 (Ar–C), 129.1 (Ar–C), 133.6 (Ar–C), 134.5 (Ar–C), 136.4(–N–C–CH2–), 150.9 (Ar–C), 154.3 (–N–CO–), 156.1 (–N–CO–), 192.2 (–CO–); HRMS m/z calcd for C23H20N2O3 [M+] 372.4165, found 372.4167.
3,3-Dimethyl-13-(4-bromophenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6, 11(2H, 13H)-trione (2)
Yellow powder, mp: 261–263 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.20 (6H, –(CH3)2), 2.32 (s, 2H, CH2–C=), 3.22 and 3.39 (2H, AB system, J = 16 Hz, CHaHbCO), 6.38 (s, 1H, –CH–N), 7.29 (d, J = 8 Hz, 2H, Ar–H), 7.44 (d, J = 8 Hz, 2H, Ar–H), 7.85–7.82 (m, 2H, Ar–H), 8.25–8.22 (m, 1H, Ar–H), 8.34–8.32 (m, 1H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 28.49 (–CH3), 28.78 (–CH3), 34.72 (–C(CH3)2), 38.10 (–CH2–C=), 50.96 (–CH2CO), 64.45 (–CH–N), 118.04 (–HC–C–CO–), 122.79 (Ar–C), 127.76 (Ar–C), 128.11 (Ar–C), 128.93 (Ar–C), 129.02 (Ar–C), 131.95 (Ar–C), 133.74 (Ar–C), 134.68 (Ar–C), 135.61 (–N–C–CH2–), 151.20 (Ar–C), 154.43 (–N–CO–), 156.03 (–N–CO–), 192.12 (–CO–); HRMS m/z calcd for C23H19BrN2O3 [M+] 450.0579, found 450.0577.
3,3-Dimethyl-13-(4-ethoxyphenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6, 11(2H, 13H)-trione (3)
Yellow powder, mp: 220–221 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.21 (6H, –(CH3)2), 1.35 (t, J = 12 Hz, 3H, –OCH2–CH 3), 2.33 (s, 2H, CH2–C=), 3.22 and 3.40 (2H, AB system, J = 16 Hz, CHaHbCO), 3.99–3.96 (m, 2H, –OCH 2–CH3), 6.39 (s, 1H, –CH–N), 6.81 (d, J = 8 Hz, 2H, Ar–H), 7.32 (d, J = 8 Hz, 2H, Ar–H), 7.80 (d, J = 4 Hz, 2H, Ar–H), 8.32–8.23 (m, 2H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 14.86 (–OCH2–CH3), 28.53 (–CH3), 28.76 (–CH3), 34.65 (–C(CH3)2), 38.09 (–CH2–C =), 51.02 (–CH2CO), 63.39 (–CH–N), 64.61 –OCH2–CH3), 114.64 (Ar–C), 118.61 (–HC–C–CO–), 127.68 (Ar–C), 127.92 (Ar–C), 128.23 (Ar–C), 128.54 (Ar–C), 129.22 (Ar–C), 133.47 (Ar–C), 134.48 (–N–C–CH2–), 150.75 (Ar–C), 154.26 (Ar–C), 156.05 (–N–CO–), 159.18 (–N–CO–), 192.19 (–CO–); HRMS m/z calcd for C25H24N2O4 [M+] 416.1736, found 416.1736.
3,3-Dimethyl-13-(4-fluorophenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H, 13H)-trione (4)
Yellow powder, mp: 218–220 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.20 (s, 6H, –(CH3)2), 2.33 (s, 2H, CH2–C=), 3.23 and 3.40 (2H, AB system, J = 16 Hz, CHaHbCO), 6.42 (s, 1H, –CH–N), 7.02–6.98 (m, 2H, Ar–H), 7.41–7.38 (m, 2H, Ar–H), 7.85–7.83 (m, 2H, Ar–H), 8.24–8.23 (m, 1H, Ar–H), 8.34–8.32 (m, 1H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 28.47 (–CH3), 28.72 (–CH3), 34.68 (–C(CH3)2), 38.03 (–CH2–C=), 50.92 (–CH2CO), 64.30 (–CH–N), 115.62 (Ar–C), 115.84 (Ar–C), 118.19 (–HC–C–CO–), 127.69 (Ar–C), 128.04 (Ar–C), 129.00 (Ar–C), 129.08 (Ar–C), 132.30 (Ar–C), 133.67 (Ar–C), 134.62 (–N–C–CH2–), 151.08 (Ar–C), 154.39 (–N–CO–), 156.01 (–N–CO–), 161.46 (Ar–C), 163.92 (Ar–C), 192.23 (–CO–); HRMS m/z calcd for C23H19FN2O3 [M+] 390.1380, found 390.1380.
3,3-Dimethyl-13-(4-methylphenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H, 13H)-trione (5)
Yellow powder, mp: 227–229 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.20 (s, 6H, –(CH3)2), 2.28 (s, 3H, –CH3), 2.32 (s, 2H, CH2–C=), 3.22 and 3.40 (2H, AB system, J = 16 Hz, CHaHbCO), 6.40 (s, 1H, –CH–N), 7.12 (d, J = 8 Hz, 2H, Ar–H), 7.30 (d, J = 8 Hz, 2H, Ar–H), 7.83–7.78 (m, 2H, Ar–H), 8.25–8.21 (m, 1H, Ar–H), 8.34–8.30 (m, 1H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 21.27 (–CH3), 28.48 (–C(CH3)2), 28.78 (–C(CH3)2), 34.67 (–C(CH3)2), 38.10 (–CH2–C=), 51.00 (–CH2CO), 64.88 (–CH–N), 118.70 (–HC–C–CO–), 127.13 (Ar–C), 127.72 (Ar–C), 127.94 (Ar–C), 129.02 (Ar–C), 129.19 (Ar–C), 129.48 (Ar–C), 133.49 (Ar–C), 134.48 (–N–C–CH2–), 138.48 (Ar–C), 150.79 (Ar–C), 154.24 (–N–CO–), 156.04 (–N–CO–), 192.16 (–CO–); HRMS m/z calcd for C22H22N2O3 [M+] 386.1630, found 386.1632.
3,3-Dimethyl-13-(4-isopropylphenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H, 13H)-trione (6)
Yellow powder, mp: 204–206 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.20–1.17 (m, 12H, –C(CH 3)2, CH(CH 3)2), 2.34 (s, 2H, CH2–C=), 2.87–2.80 (m, 1H, CH(CH3)2), 3.23 and 3.42 (2H, AB system, J = 16 Hz, CHaHbCO), 6.43 (s, 1H, –CH–N), 7.17 (d, J = 8 Hz, 2H, Ar–H), 7.32 (d, J = 8 Hz, 2H, Ar–H), 7.84–7.80 (m, 2H, Ar–H), 8.27–8.24 (m, 1H, Ar–H), 8.34–8.31 (m, 1H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 23.86 (–CH(CH3)2), 28.61 (–C(CH3)2), 28.72 (–C(CH3)2), 33.80 (–C(CH3)2), 34.69 (–CH(CH3)2), 38.07 (–CH2–C=), 51.01 (–CH2CO), 64.77 (–CH–N), 118.71 (–HC–C–CO–), 126.87 (Ar–C), 127.14 (Ar–C), 127.72 (Ar–C), 127.96 (Ar–C), 128.99 (Ar–C), 129.17 (Ar–C), 133.51 (Ar–C), 133.59 (Ar–C), 134.51 (–N–C–CH2–), 149.14 (Ar–C), 150.88 (Ar–C), 154.30 (–N–CO–), 156.08 (–N–CO–), 192.31 (–CO–); HRMS m/z calcd for C26H26N2O3 [M+] 414.1943, found 414.1945.
3,3-Dimethyl-13-(4-nitrophenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H, 13H)-trione (7)
Yellow powder, mp: 223–225 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.20 (6H, –C(CH 3)2), 2.33 (2H, –CH2–C=), 3.26 and 3.41 (2H, AB system, J = 16 Hz, CHaHbCO), 6.50 (s, 1H, –CH–N), 7.61 (d, J = 8 Hz, 2H, Ar–H), 7.90–7.85 (m, 2H, Ar–H), 8.17 (d, J = 8 Hz, 2H, Ar–H), 8.24–8.22 (m, 1H, Ar–H), 8.37–8.35 (m, 1H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 28.38 (–C(CH3)2), 28.70 (–C(CH3)2), 34.74 (–C(CH3)2), 38.03 (–CH2–C=), 50.80 (–CH2CO), 64.16 (–CH–N), 117.26 (–HC–C–CO–), 124.02 (Ar–C), 127.72 (Ar–C), 128.11 (Ar–C), 128.23 (Ar–C), 128.60 (Ar–C), 128.95 (Ar–C), 133.97 (Ar–C), 134.81 (–N–C–CH2–), 143.53 (Ar–C), 147.84 (Ar–C), 151.74 (Ar–C), 154.56 (–N–CO–), 155.93 (–N–CO–), 192.10 (–CO–); HRMS m/z calcd for C23H19N3O5 [M+] 417.1325, found 417.1327.
3,3-Dimethyl-13-(3,4,5 trimethoxyphenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H, 13H)-trione (8)
Yellow powder, mp: 232–234 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.24 (6H, –C(CH 3)2), 2.35 (s, 2H, –CH2–C=), 3.20 and 3.45 (2H, AB system, J = 16 Hz, CHaHbCO), 3.80 (d, J = 8 Hz, 9H, –OCH3), 6.38 (s, 1H, –CH–N), 6.63 (s, 2H, Ar–H), 7.86 (d, J = 8 Hz, 2H, Ar–H), 8.35–8.27 (m, 2H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 28.11 (–C(CH3)2), 28.97 (–C(CH3)2), 34.61 (–C(CH3)2), 38.04 (–CH2–C=), 50.92 (–CH2CO), 56.16 (–OCH3), 60.68 (–OCH3), 64.97 (–CH–N), 104.55 (Ar–C), 118.26 (–HC–C–CO–), 127.69 (Ar–C), 127.98 (Ar–C), 128.90 (Ar–C), 129.00 (Ar–C), 131.81 (Ar–C), 133.62 (Ar–C), 134.61 (–N–C–CH2–), 138.20 (Ar–C), 150.89 (Ar–C), 153.32 (Ar–C), 154.50 (–N–CO–), 156.09 (–N–CO–), 192.18 (–CO–); HRMS m/z calcd for C26H26N2O6 [M+] 462.1791, found 462.1793.
3,3-Dimethyl-13-(4-chlorophenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H, 13H)-trione (9)
Yellow powder, mp: 260–262 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.20 (6H, –C(CH 3)2), 2.32 (s, 2H, –CH 2–C=), 3.22 and 3.40 (2H, AB system, J = 16 Hz, CHaHbCO), 6.40 (s, 1H, –CH–N), 7.29 (d, J = 8 Hz, 2H, Ar–H), 7.36 (d, J = 8 Hz, 2H, Ar–H), 7.86–7.82 (m, 2H, Ar–H), 8.26–8.22 (m, 1H, Ar–H), 8.35–8.31 (m, 1H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 28.38 (–C(CH3)2), 28.66 (–C(CH3)2), 34.62 (–C(CH3)2), 37.98 (–CH2–C=), 50.83 (–CH2–CO–), 64.27 (–CH–N), 117.96 (–HC–C–CO–), 127.64 (Ar–C), 127.99 (Ar–C), 128.52 (Ar–C), 128.91 (Ar–C), 133.63(Ar–C), 134.43 (Ar–C), 134.57 (Ar–C), 134.95 (–N–C–CH2–), 151.09 (Ar–C), 154.31 (–N–CO–), 155.92 (–N–CO–), 192.06 (–CO–); HRMS m/z calcd for C23H19ClN2O3 [M+] 406.1084, found 406.1087.
13-(4-Methylphenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (10)
Yellow powder, mp: 244–246 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.24 (m, 2H, –CH2–CH 2–CH2–CO–), 2.42 (t, J = 8 Hz, 2H, –CH 2–CH2–CH2–CO–), 2.72 (s, 3H, –CH 3 ), 3.37–3.53 (m, 2H, –CH2–CH2–CH 2–CO–), 6.59 (s, 1H, –CH–N), 7.15–7.02 (m, 4H, Ar–H), 7.84–7.83 (m, 2H, Ar–H), 8.21–8.19 (m, 1H, Ar–H), 8.35–8.34 (m, 1H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 19.28 (–CH3), 22.31 (–CH2–CH2–CH2–CO–), 24.43 (–CH2–CH2–CH2–CO–), 36.85 (–CH2–CH2–CH2–CO–), 61.48 (–CH–N), 120.81 (–HC–C–CO–), 125.27 (Ar–C), 126.42 (Ar–C), 127.53 (Ar–C), 127.97 (Ar–C), 128.37 (Ar–C), 128.96 (Ar–C), 130.62 (Ar–C), 133.47 (Ar–C), 134.47 (Ar–C), 135.08 (–N–C–CH2–), 137.03 (Ar–C), 152.04 (Ar–C), 153.90 (–N–CO–), 155.98 (–N–CO–), 192.40 (–CO–); HRMS m/z calcd for C22H18N2O3 [M+] 358.1317, found 358.1319.
13-(4-Chlorophenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (11)
Yellow powder, mp: 272–273 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.26–2.23 (m, 2H, –CH2–CH 2–CH2–CO–), 2.49–2.45 (m, 2H, –CH 2–CH2–CH2–CO–), 3.36–3.53 (m, 2H, –CH2–CH2–CH 2–CO–), 6.40 (s, 1H, –CH–N–), 7.32 (d, J = 8 Hz, 4H, Ar–H), 7.85 (s, 2H, Ar–H), 8.30 (d, J = 8 Hz, 2H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 22.42 (–CH2–CH2–CH2–CO–), 24.64 (–CH2–CH2–CH2–CO–), 37.02 (–CH2–CH2–CH2–CO–), 64.49 (–CH–N), 119.29 (–HC–C–CO–), 127.88 (Ar–C), 128.21 (Ar–C), 128.73 (Ar–C), 129.06 (Ar–C), 133.81 (Ar–C), 134.65 (Ar–C), 134.77 (Ar–C), 135.04 (–N–C–CH2–), 152.68 (Ar–C), 154.49 (–N–CO–), 156.15 (–N–CO–), 192.59 (–CO–); HRMS m/z calcd for C21H15ClN2O3 [M+] 378.0771, found 378.0773.
13-(4-Ethoxyphenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (12)
Yellow powder, mp: 219–220 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.35 (t, J = 12 Hz, 3H, –O–CH2–CH 3), 2.24 (m, 2H, –CH2–CH 2–CH2–CO–), 2.46 (t, J = 8 Hz, 2H, –CH 2–CH2–CH2–CO–), 3.33–3.53 (m, 2H, –CH2–CH2–CH 2–CO–), 3.99–3.96 (m, 2H, –O–CH 2–CH3), 6.40 (s, 1H, –CH–N), 6.82 (d, J = 8 Hz, 2H, Ar–H), 7.32 (d, J = 8 Hz, 2H, Ar–H), 7.82 (d, J = 4 Hz, 2H, Ar–H), 8.33–8.23 (m, 2H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 14.79 (–O–CH2–CH3), 22.31 (–CH2–CH2–CH2–CO–), 24.48 (–CH2–CH2–CH2–CO–), 36.94 (–CH2–CH2–CH2–CO–), 63.37 (–O–CH2–CH3), 64.57 (–CH–N), 114.57 (Ar–C), 119.67 (–HC–C–CO–), 127.67 (Ar–C), 127.90 (Ar–C), 128.06 (Ar–C), 128.52 (Ar–C), 128.92 (Ar–C), 129.15 (Ar–C), 133.44 (Ar–C), 134.47 (–N–C–CH2–), 152.19 (Ar–C), 154.21 (–N–CO–), 156.04 (–N–CO–), 159.14 (Ar–C), 192.63 (–CO–); HRMS m/z calcd for C23H20N2O4 [M+] 388.1423, found 388.1425.
13-(4-Bromophenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (13)
Yellow powder, mp: 280–281 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.25–2.22 (m, 2H, –CH2–CH 2–CH2–CO–), 2.46–2.44 (t, J = 8 Hz, 2H, –CH 2–CH2–CH2–CO–), 3.31–3.54 (m, 2H, –CH2–CH2–CH 2–CO–), 6.38 (s, 1H, –CH–N), 7.30 (d, J = 8 Hz, 2H, Ar–H), 7.44 (d, J = 4 Hz, 2H, Ar–H), 7.84 (s, 2H, Ar–H), 8.30 (d, J = 8 Hz, 2H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 22.40 (–CH2–CH2–CH2–CO–), 24.62 (–CH2–CH2–CH2–CO–), 36.99 (–CH2–CH2–CH2–CO–), 64.54 (–CH–N), 119.21 (–HC–C–CO–), 122.87 (Ar–C), 127.86 (Ar–C), 128.19 (Ar–C), 129.02 (Ar–C), 131.98 (Ar–C), 133.80 (Ar–C), 134.76 (Ar–C), 135.55 (–N–C–CH2–), 152.68 (Ar–C), 154.47 (–N–CO–), 156.13 (–N–CO–), 192.56 (–CO–); HRMS m/z calcd for C21H15BrN2O3 [M+] 422.0266, found 422.0268.
13-(3-Methylphenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (14)
Yellow powder, mp: 222–225 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.25–2.21 (m, 2H, –CH2–CH 2–CH2–CO–), 2.30 (s, 3H, –CH3), 2.46–2.41 (t, J = 8 Hz, 2H, –CH 2–CH2–CH2–CO–), 3.32–3.51 (m, 2H, –CH2–CH2–CH 2–CO–), 6.38 (s, 1H, –CH–N), 7.06 (s, 1H, Ar–H), 7.20 (t, J = 12 Hz, 3H, Ar–H), 7.80 (t, J = 12 Hz, 2H, Ar–H), 8.23 (t, J = 8 Hz, 1H, Ar–H), 8.31 (d, J = 8 Hz, 1H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 21.52 (–CH3), 22.30 (–CH2–CH2–CH2–CO–), 24.50 (–CH2–CH2–CH2–CO–), 36.95 (–CH2–CH2–CH2–CO–), 65.00 (–CH–N), 119.82 (–HC–C–CO–), 124.18 (Ar–C), 127.70 (Ar–C), 127.88 (Ar–C), 127.92 (Ar–C), 128.54 (Ar–C), 128.98 (Ar–C), 129.13 (Ar–C), 129.55 (Ar–C), 133.48 (Ar–C), 134.49 (Ar–C), 136.34 (-N–C-CH2-), 138.26 (Ar–C), 152.18 (Ar–C), 154.18 (–N–CO–), 156.02 (–N–CO–), 192.47 (–CO–); HRMS m/z calcd for C22H18N2O3 [M+] 358.1317, found 358.1319.
13-(3,4,5-Methoxyphenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (15)
Yellow powder, mp: 249–250 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.27–2.23 (m, 2H, –CH2–CH 2–CH2–CO–), 2.53–2.45 (m, 2H, –CH 2–CH2–CH2–CO–), 3.32–3.55 (m, 2H, –CH2–CH2–CH 2–CO–) 3.81 (d, J = 12 Hz, 9H, –OCH3), 6.39 (s, 1H, –CH–N), 6.63 (s, 2H, Ar–H), 7.86 (t, J = 8 Hz, 2H, Ar–H), 8.36–8.28 (m, 2H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 22.32 (–CH2–CH2–CH2–CO–), 24.52 (–CH2–CH2–CH2–CO–), 36.96 (–CH2–CH2–CH2–CO–), 56.26 (–OCH3), 60.68 (–OCH3), 64.97 (–CH–N), 104.61 (Ar–C), 119.42 (–HC–C–CO–), 127.73 (Ar–C), 128.03 (Ar–C), 128.93 (Ar–C), 129.03 (Ar–C), 131.79 (Ar–C), 133.62 (Ar–C), 134.62 (–N–C–CH2–), 152.23 (Ar–C), 153.34 (Ar–C), 154.48 (–N–CO–), 156.11 (-N-CO-), 192.54 (-CO-); HRMS m/z calcd for C24H22N2O6 [M+] 434.1478, found 434.1480.
13-(2-Methoxyphenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (16)
Yellow powder, mp: 276–277 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.24–2.20 (m, 2H, –CH2–CH 2–CH2–CO–), 2.43–2.39 (m, 2H, –CH 2–CH2–CH2–CO–), 3.37–3.49 (m, 2H, –CH2–CH2–CH 2–CO–), 3.72 (s, 3H, –OCH3), 6.59 (s, 1H, –CH–N), 6.97–6.83 (m, 2H, Ar–H), 7.45–7.24 (m, 2H, Ar–H), 7.82 (d, J = 4 Hz, 2H, Ar–H), 8.50 (d, J = 8 Hz, 2H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 22.54 (–CH2–CH2–CH2–CO–), 24.64 (–CH2–CH2–CH2–CO–), 37.12 (–CH2–CH2–CH2–CO–), 56.01 (–OCH3), 62.83 (–CH–N), 111.67 (Ar–C), 118.89 (–HC–C–CO–), 121.10 (Ar–C), 123.90 (Ar–C), 127.76 (Ar–C), 127.96 (Ar–C), 129.25 (Ar–C), 130.05 (Ar–C), 130.21 (Ar–C), 133.41 (Ar–C), 134.42 (–N–C–CH2–), 152.72 (Ar–C), 154.11 (–N–CO–), 156.32 (Ar–C), 157.48 (–N–CO–), 192.66 (–CO–); HRMS m/z calcd for C22H18N2O4 [M+] 374.1267, found 374.1269.
13-(2-Methylphenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (17)
Yellow powder, mp: 267–268 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.26–2.20 (m, 2H, –CH2–CH 2–CH2–CO–), 2.44–2.41 (m, 2H, –CH 2–CH2–CH2–CO–), 2.72 (s, 3H, –CH3), 3.36–3.52 (m, 2H, –CH2–CH2–CH 2–CO–), 6.60 (s, 1H, –CH–N), 7.16–7.01 (m, 4H, Ar–H), 7.83–7.81 (m, 2H, Ar–H), 8.23–8.20 (m, 1H, Ar–H), 8.36–8.33 (m, 1H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 19.30 (–CH3), 22.34 (–CH2–CH2–CH2–CO–), 24.46 (–CH2–CH2–CH2–CO–), 36.88 (–CH2–CH2–CH2–CO–), 61.53 (–CH–N), 120.91 (–HC–C–CO–), 125.24 (Ar–C), 126.41 (Ar–C), 127.61 (Ar–C), 127.97 (Ar–C), 128.42 (Ar–C), 128.98 (Ar–C), 129.07 (Ar–C), 130.70 (Ar–C), 133.44 (Ar–C), 134.47 (Ar–C), 135.02 (–N–C–CH2–), 137.10 (Ar–C), 151.98 (Ar–C), 153.93 (–N–CO–), 156.04 (–N–CO–), 192.39 (–CO–); HRMS m/z calcd for C22H18N2O3 [M+] 358.1317, found 358.1315.
13-(2-Bromophenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (18)
Yellow powder, mp: 261–262 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.29–2.22 (m, 2H, –CH2–CH 2–CH2–CO–), 2.46–2.42 (m, 2H, –CH 2–CH2–CH2–CO–), 3.40–3.47 (m, 2H, –CH2–CH2–CH 2–CO–), 6.68 (s, 1H, –CH–N), 7.16–7.7.12 (m, 1H, Ar–H), 7.30 (t, J = 12 Hz, 1H, Ar–H), 7.43–7.39 (m, 1H, Ar–H), 7.51 (d, J = 8 Hz, 1H, Ar–H), 7.86–7.83 (m, 2H, Ar–H), 8.24–8.22 (m, 1H, Ar–H), 8.36–8.34 (m, 1H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm), 22.36 (–CH2–CH2–CH2–CO–), 24.61 (–CH2–CH2–CH2–CO–), 36.97 (–CH2–CH2–CH2–CO–), 65.95 (–CH–N), 115.12 (–HC–C–CO–), 127.01 (Ar–C), 127.78 (Ar–C), 127.88 (Ar–C), 128.13 (Ar–C), 128.86 (Ar–C), 129.14 (Ar–C), 130.16 (Ar–C), 133.69 (Ar–C), 133.93 (Ar–C), 134.63 (–N–C–CH2–), 154.34 (Ar–C), 156.28 (–N–CO–), 164.49 (–N–CO–), 192.42 (–CO–); HRMS m/z calcd for C21H15BrN2O3 [M+] 422.0266, found 422.0268.
13-Ethyl-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (19)
Yellow powder, mp: 196–198 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 0.59 (t, J = 12 Hz, 3H, –CH2–CH 3), 1.97–1.92 (m, 1H, –CH 2–CH3), 2.15–2.10 (m, 2H, –CH2–CH 2–CH2–CO–), 2.46–2.31 (m, 3H, –CH 2–CH3–CH 2–CH2–CH2–CO–), 3.15–3.29 (m, 2H, –CH2–CH2–CH 2–CO–), 5.52 (s, 1H, –CH–N), 7.78–7.70 (m, 2H, Ar–H), 8.17 (t, J = 12 Hz, 2H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 7.10 (–CH2–CH3), 22.05 (–CH2–CH2–CH2–CO–), 22.33 (–CH2–CH3), 24.46 (–CH2–CH2–CH2–CO–), 36.90 (–CH2–CH2–CH2–CO–), 63.40 (–CH–N), 117.63 (–HC–C–CO–), 127.38 (Ar–C), 127.76 (Ar–C), 128.83 (Ar–C), 128.95 (Ar–C), 133.32 (Ar–C), 134.36 (–N–C–CH2–), 153.20 (Ar–C), 154.49 (–N–CO–), 155.90 (–N–CO–), 193.22 (–CO–); HRMS m/z calcd for C17H16N2O3 [M+] 296.1161, found 296.1163.
13-Propyl-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (20)
Yellow powder, mp: 172–174 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 0.86 (t, J = 12 Hz, 3H, –CH2–CH2–CH 3), 1.21–1.10 (m, 2H, –CH2–CH 2–CH3), 2.07–2.01 (m, 1H, –CH 2–CH2–CH3), 2.26–20 (m, 2H, –CH2–CH 2–CH2–CO–), 2.59–2.34 (m, 3H, –CH 2–CH2–CH3, –CH 2–CH2–CH2–CO–), 3.28–3.42 (m, 2H, –CH2–CH2–CH 2–CO–), 5.68 (d, J = 4 Hz, 1H, –CH–N), 7.92–7.83 (m, 2H, Ar–H), 8.36–8.31 (m, 2H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 13.74 (–CH2–CH2–CH3), 16.58 (–CH2–CH2–CH3), 22.31 (–CH2–CH2–CH2–CO–), 24.46 (–CH2–CH2–CH2–CO–), 31.52 (–CH2–CH2–CH3), 36.92 (–CH2–CH2–CH2–CO–), 62.79 (–CH–N), 118.33 (–HC–C–CO–), 127.44 (Ar–C), 127.81 (Ar–C), 128.84 (Ar–C), 128.99 (Ar–C), 133.36 (Ar–C), 134.41 (–N–C–CH2–), 152.98 (Ar–C), 154.98 (–N–CO–), 155.97 (–N–CO–), 193.31 (–CO–); HRMS m/z calcd for C18H18N2O3 [M+] 310.1317, found 310.1319.
13-Isopropyl-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione (21)
Yellow powder, mp: 141–143 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 0.97–0.88 (m, 6H, –CH(CH 3)2), 2.19 (s, 2H, –CH2–CH 2–CH2–CO–), 2.63–2.37 (m, 3H, –CH(CH3)2, –CH 2–CH2–CH2–CO–), 3.14–3.48 (m, 2H, –CH2–CH2–CH 2–CO–), 5.58 (s, 1H, –CH–N), 7.88–7.81 (m, 2H, Ar–H), 8.32–8.28 (m, 2H, Ar–H); 13C NMR (CDCl3, 100 MHz) δ (ppm) 17.97 (–CH(CH3)2), 18.29 (–CH(CH3)2), 22.15 (–CH2–CH2–CH2–CO–), 24.60 (–CH2–CH2–CH2–CO–), 31.22 (–CH(CH3)2), 37.11 (–CH2–CH2–CH2–CO–), 67.00 (–CH–N), 118.10 (–HC–C–CO–), 127.62 (Ar–C), 127.88 (Ar–C), 128.81 (Ar–C), 129.10 (Ar–C), 133.40 (Ar–C), 134.52 (–N–C–CH2–), 153.79 (Ar–C), 155.20 (–N–CO–), 156.11 (–N–CO–), 193.24 (–CO–); HRMS m/z calcd for C18H18N2O3 [M+] 310.1317, found 310.1319.
References
V.P. Litvinov, Russ. Chem. Rev. 72, 69 (2003)
W.B. Chen, Z.-J. Wu, Q.-L. Pei, L.-F. Cun, X.-M. Zhang, W.-C. Yuan, Org. Lett. 12, 3132 (2010)
S. Grasso, G. DeSarro, N. Micale, M. Zappala, G. Puia, M. Baraldi, C. Demicheli, J. Med. Chem. 43, 2851 (2000)
J.S. Kim, H.K. Rhee, H.J. Park, S.K. Lee, C.O. Lee, H.Y. Park Choo, Bioorg. Med. Chem. 16, 4545 (2008)
S.S. El-Saka, A.H. Soliman, A.M. Imam, Afinidad J 66, 167 (2009)
L. Zhang, L.P. Guan, X.Y. Sun, C.X. Wei, K.Y. Chai, Z.S. Quan, Chem. Biol. Drug Des. 73, 313 (2009)
H. Wu, X.M. Chen, Y. Wan, H.Q. Xin, H.H. Xu, R. Ma, C.H. Yue, L.L. Pang, Lett. Org. Chem. 6, 219 (2009)
E.L. Piatnitski, M.A.J. Duncton, A.S. Kiselyov, R. Katoch-Rouse, D. Sherman, D.L. Milligan, C. Balagtas, W.C. Wong, J. Kawakami, J.F. Doody, Bioorg. Med. Chem. Lett. 15, 4696 (2005)
J. Sinkkonen, V. Ovcharenko, K.N. Zelenin, I.P. Bezhan, B.A. Chakchir, F. Al-Assar, K. Pihlaja, Eur. J. Org. Chem. 12, 2046 (2002)
M. Sayyafi, M. Sayyedhamzeh, H.R. Khavasi, A. Bazgir, Tetrahedron 64, 2375 (2008)
R. Ghorbani-Vaghei, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Amiri, M.M. Ghavidel, Tetrahedron 67, 1930 (2011)
G. Shukla, R.K. Verma, G.K. Verma, M.S. Singh, Tetrahedron Lett. 52, 7195 (2011)
M. Kidwai, R. Chauhan, A. Jahan, Chin. Sci. Bull. 57, 2273 (2012)
H.J. Wang, X.N. Zhang, Z.H. Zhang, Monatsh. Chem. 141, 425 (2010)
E. Mosaddegh, A. Hassankhani, Tetrahedron Lett. 52, 488 (2011)
A. Corma, H. Garcia, Catal. Today 38, 257 (1997)
M.V. Reddy, G.C.S. Reddy, Y.T. Jeong, Tetrahedron 68, 6820 (2012)
H.R. Shaterian, M. Ghashang, M. Feyzi, Appl. Catal. A 345, 128 (2008)
G. Sabitha, C. Srinivas, A. Raghavendar, J.S. Yadav, Helv. Chim. Acta 93, 1375 (2010)
T. Khan, Z.N. Siddiqui, New J. Chem. 38, 4847 (2014)
G.J. Hernandez, E. Juaristi, J. Org. Chem. 75, 7107 (2010)
M.S. Singh, S. Chowdhury, RSC Adv. 2, 4547 (2012)
J. Wu, X. Du, J. Ma, Y. Zhang, Q. Shi, L. Luo, B. Song, S. Yang, D. Hu, Green Chem. 16, 3210 (2014)
A.B. Atar, Y.S. Jeong, Y.T. Jeong, Tetrahedron 70, 5207 (2014)
A.B. Atar, Y.T. Jeong, Tetrahedron Lett. 54, 1302 (2013)
A.B. Atar, Y.T. Jeong, Tetrahedron Lett. 54, 5624 (2013)
A.B. Atar, J.S. Kim, K.T. Lim, Y.T. Jeong, New J. Chem. 39, 396 (2015)
A.B. Atar, J.T. Kim, K.T. Lim, Y.T. Jeong, Synth. Commun. 44, 2679 (2014)
S. Asghari, M. Tajbakhsh, B.J. Kenari, S. Khaksar, Chin. Chem. Lett. 22, 127 (2011)
Acknowledgments
This research work was supported by the BK 21 plus program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Atar, A.B., Lee, S.D., Cho, B.G. et al. β-Cyclodextrine-SO3H: the most efficient catalyst for one-pot synthesis of 2H-indazolo[2,1-b]phthalazine-triones under solvent-free conditions. Res Chem Intermed 42, 1707–1728 (2016). https://doi.org/10.1007/s11164-015-2113-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2113-3