Abstract
The CyanoP protein is a cyanobacterial homolog of the PsbP protein, which is an extrinsic subunit of photosystem II (PSII) in green plant species. The molecular function of CyanoP has been investigated in mutant strains of Synechocystis but inconsistent results have been reported by different laboratories. In this study, we generated and characterized a Synechocystis mutant in which entire region of the CyanoP gene was eliminated. After repeated subculture in CaCl2-depleted medium, growth retardation was clearly observed for a CyanoP knockout mutant of Synechocystis sp. PCC 6803 (∆P). The PSII-mediated oxygen-evolving activity of the ∆P cells was more susceptible to depletion of CaCl2 than that of wild-type cells. The 77 K fluorescence emission spectra indicated that energy coupling between phycobilisome and PSII was perturbed in both wild-type and ∆P cells under CaCl2-depleted conditions, and was more evident for the ∆P mutant. To examine the association of CyanoP with PSII complexes, we tested several detergents for solubilization of thylakoid membranes and showed that CyanoP was partly included in fractions containing large protein complexes in gel-filtration analysis. These results indicate that CyanoP constitutively stabilizes PSII functionality in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photosystem II (PSII) consists of both membrane-intrinsic and membrane-extrinsic subunits, and functions as a water/plastoquinone oxidoreductase [1]. On the thylakoid lumenal side of PSII, a metal cluster comprising four Mn ions, one Ca2+ ion, and five oxo ligands (the Mn cluster) catalyzes the oxygen-evolving reaction. In addition, two Cl− ions are bound near the Mn cluster [2]. The membrane-intrinsic subunits of PSII are involved in pigment and/or cofactor binding in photochemical reactions, and the membrane-extrinsic subunits surround the catalytic Mn cluster and are crucial in stabilizing the Mn cluster and retaining the PSII cofactor ions [3].
It is known that the membrane-extrinsic subunits of PSII are largely different among oxygenic photosynthetic organisms. In cyanobacteria, three PSII extrinsic proteins, PsbO, PsbU, and PsbV, bind to the thylakoid lumenal side of PSII, whereas PsbU and PsbV have been replaced by PsbP and PsbQ in green plant PSII [4, 5]. In addition, cyanobacteria have homologs of PsbP and PsbQ, CyanoP and CyanoQ, respectively [6, 7]; however, these are not included in the current structural model and their manner of interaction with the PSII complex is still unknown.
Although the molecular function of cyanobacterial PsbP (CyanoP) has been characterized by several groups, the results are rather inconsistent. Slower photoautotrophic growth, and lower PSII activity under Ca2+ and/or Cl−-depleted conditions, has been reported for the Synechocystis strain lacking CyanoP [8]. However, these phenotypes were not clearly observed in other studies [9, 10]. Later, comparative experiments using three mutant strains from different laboratories, insertional and partial-deletion knockout mutants carrying different antibiotic resistance cassettes, suggested CyanoP had a constrictive effect in stabilizing the donor side of PSII [11], although the precise location of CyanoP is still unknown. In this study, we created a new Synechocystis mutant in which the entire region of the CyanoP gene was eliminated by homologous recombination, and effects of Ca2+ and Cl− depletion on the growth and photosynthetic activity were reinvestigated. Furthermore, to examine the location of CyanoP in thylakoid membranes, several detergents were tested to solubilize thylakoid membranes without affecting the association of CyanoP with thylakoid protein complexes.
Experimental
Cell strains, growth conditions, and biochemical preparations
A CyanoP knockout mutant of Synechocystis sp. PCC 6803 (∆P) was newly created in this study. The HT3 strain, which contains a histidine-tagged version of the CP47 membrane protein, has frequently been used for isolation of PSII complexes from Synechocystis 6803 cells [12]. Cells were grown on a rotary shaker at 30 °C under 30–40 μmol photons m−2 s−1. In each subculture, cells grown for 5–6 days were diluted to OD730 = 0.05 with fresh BG11 or BG11 (− CaCl2) medium prepared as described elsewhere [13]. Thylakoid membranes were isolated as described elsewhere [6].
Construction of the CyanoP mutant (∆P)
The entire coding region of the sll1418 gene was removed by homologous recombination as described elsewhere [14]. The upstream and the downstream regions of the sll1418 were amplified by PCR with the following two sets of primers: 5′-GCTTCGCTCTGTATAGACTCTTGTC-3′ (up-F, the 5′ primers shown in Fig. 1) and 5′-ACATCAGAGATTTTGAGACACAACGTGGCTTACCTTTCCTTACCCATTAAACCTC-3′ (up-R-4 KLf), and 5′-CACCAACTGGTCCACCTACAACAAAGCTCTTCCATTGCCTAGTAACTAC-3′AGT (dnF-4KRf) and 5′-CAGAGCCTTTGCCTTTGAC-3′ (dnR, the 3′ primers shown in Fig. 1). The underlined parts are complementary to the ends (4KLf and 4KRr, see below) of the kanamycin-resistance cassette from pUC4K, which was also amplified by PCR in two overlapping parts, 4KL and 4KR, by using the following two sets of primers: AGCCACGTTGTGTCTCAAAATCTCTGATGT (4KLf) and GAGAAATCACCATGAGTGACGACTGAATCC (4KLr), and AAGCTTTTGCCATTCTCACCGGATTCAGTC (4KRf) and AGAGCTTTGTTGTAGGTGGACCAGTTGGTG (4KRr). Finally, we linked four fragments, up, 4KL, 4KR, and dn, by successive PCR to obtain a disruption cassette. The DNA was mixed with the wild-type cells, and mutants were then selected by screening with 30 μg ml−1 kanamycin. Complete gene segregation was confirmed by PCR. Accumulation of CyanoP and other thylakoid proteins was examined by SDS-PAGE using 12.5 % acrylamide gels with 6 M urea followed by immunoblotting using antibodies purchased from Agrisera (Vännäs, Sweden).
Generation and verification of the CyanoP mutant (the ∆P strain). A The position of the primers used to amplify the CyanoP gene. B PCR verification of segregation of the interrupted CyanoP gene. C Immuno-detection of the D2 and CyanoP proteins in thylakoid membranes of wild-type (WT) and the ∆P cells (ΔP) separated by SDS-PAGE. Each lane contains proteins corresponding to 2 µg Chl. Km r: kanamycin-resistance cassette
Effect of detergents on the association of CyanoP in thylakoid membranes
Thylakoid membranes of 1 mg chlorophyll (Chl)/ml were solubilized for 30 min on ice in buffer containing 50 mM Mes-NaOH, pH 6.0, 10 mM MgCl2, 5 mM CaCl2, and 25 % glycerol containing 0.8 % n-dodecyl-β-d-maltoside (β-DM) or 0.7 % n-dodecyl-α-d-maltoside (α-DM) or 2.5 % n-heptyl-β-d-thioglucoside (HTG) (Dojindo Laboratories, Kumamoto, Japan). After centrifugation for 10 min at 20,000×g, solubilized membranes in supernatant were mixed with 15 % PEG 1,540 and centrifuged for 30 min at 4 °C and 100,000×g to precipitate the large protein complexes.
Gel-filtration analysis
Thylakoid membranes of the HT3 strain at 1 mg Chl/ml were solubilized with 0.7 % α-DM for 30 min on ice in buffer containing 50 mM Mes-NaOH, pH 6.0, 10 mM MgCl2, 5 mM CaCl2, and 10 % glycerol. After centrifugation for 10 min at 20,000×g, the solubilized membranes were filtered (Ultrafree-MC Filter Devices 45 µm; Merck Millipore, Billerica, MA, USA) and subjected to a gel-filtration on a Superdex 200 column, with use of an Äkta purifier system (Healthcare, Buckinghamshire, UK), at 4 °C and a flow rate of 0.35 ml/min. For SDS-PAGE, the 1–13 fractions were concentrated by use of an Amicon Ultra 0.5 ml Centrifugal Filter (Merck Millipore), and the proteins in fractions 14–30 were precipitated by addition of 20 % trichloroacetic acid.
Results and discussion
Characterization of the Synechocystis mutant lacking CyanoP (∆P)
Three research groups have produced Synechocystis mutant strains lacking CyanoP and observed somewhat different phenotypes [8–10]. Although they did not detect trace amounts of CyanoP by immunoblotting using a peptide antibody against its N-terminus, it was suggested that some parts of the relatively hydrophobic region at the C-terminus of CyanoP may be expressed and affect mutant phenotypes [11]. We therefore created a mutant in which entire region of the CyanoP gene was replaced with a kanamycin-resistant gene (∆P, Fig. 1A). Complete segregation of the interrupted CyanoP gene was confirmed by PCR (Fig. 1B) and no CyanoP protein was detected in the ∆P strain by immunoblotting (Fig. 1C).
The ∆P cells grew photoautotrophically in normal BG-11 medium, at a rate similar to that observed for the wild-type, as reported elsewhere [8–10] (Fig. 2). In CaCl2-depleted medium, growth of the cells was slightly reduced, but photoautotrophic growth was not significantly different for the wild-type and ∆P cells during the first subculture (Fig. 2A). However, photoautotrophic growth of the ∆P cells was clearly delayed after the third subculture in CaCl2-depleted medium (Fig. 2B). It has been reported that mutants lacking CyanoP were less fit than wild-type in competitive growth experiments [11]. Our results further suggest functional significance of CyanoP in the cells, even in the separated culture and under laboratory growth conditions.
Photoautotrophic growth of the Synechocystis cells during the first (A) and third (B) subcultures. Growth curves of wild-type (open circles) and ∆P (open squares) cells in BG11 medium, and those of wild-type (filled circles) and ∆P (filled squares) cells in CaCl2-deficient medium. The data are averages ± SD from three independent experiments. Error bars not visible are smaller than the symbols
The effect of CyanoP removal on PSII function was measured for the cells. Figure 3 shows the PSII-specific oxygen-evolving activity of wild-type and ∆P cells in the presence of K3Fe(CN)6 and 2,6-dichloro-p-benzoquinone (DCBQ) as electron acceptors. It is apparent that removal of CyanoP significantly reduced the rate of oxygen evolution, particularly under CaCl2-depleted conditions in the first subculture of the cells (Fig. 3A). This result is consistent with that reported by Thornton et al. [8]. However, the difference between the PSII activity of wild-type and ∆P cells became insignificant in the third subculture of the cells under CaCl2-depleted conditions (Fig. 3B), because the activity of wild-type cells was significantly reduced by CaCl2 depletion. This suggests the presence of CyanoP can delay the PSII damage caused by CaCl2 depletion, which is relevant to its proposed function in stabilizing the oxygen-evolving complex of PSII [11].
Effect of CaCl2 depletion on the O2 evolution activity of wild-type and ∆P cells. Wild-type and ∆P cells were harvested from (A) first subculture or (B) third subculture grown in BG11 or BG11 (− CaCl2). The cells were resuspended in a fresh BG11 or BG11 (− CaCl2) at 5 µg Chl/ml for measurements. Oxygen-evolving activity was measured with a Clark-type electrode at 30 °C in BG11 (open bars) or BG11 (− CaCl2) (filled bars); 0.5 mM DCBQ and 1 mM K3Fe(CN)6 were used as electron acceptors. The O2 evolution activity of wild-type cells in the first subculture was 297 ± 7 µmol O2 mg Chl−1 h−1; that in the third subculture was 166 ± 21 µmol O2 mg Chl−1 h−1. Error bars represent SD (n = 3) and asterisks indicate a significant difference (Student’s t test: *p < 0.05, **p < 0.01)
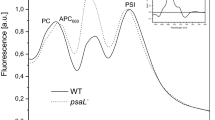
The effects of CaCl2 depletion and removal of CyanoP on PSII function were sensitively detected by 77 K fluorescence emission spectroscopy using 580 nm excitation of the phycobilisome (PBS). As shown in Fig. 4A, increased emission at 685 nm was observed for wild-type and ∆P cells in CaCl2-depleted medium, even for cells in the first subculture, indicating enhanced fluorescence emission from the terminal emitter of the PBS. Moreover, fluorescence emission at 685 nm by the ∆P cells was more prominent than that by the wild-type. The 77 K fluorescence emission spectra obtained by 430 nm excitation, which is preferentially absorbed by chlorophyll, did not suggest a decrease of the amount of PSII in the ∆P cells (data not shown). These data suggest that energy transfer from the phycobilisome to PSII was perturbed under CaCl2-depleted conditions, particularly in ∆P cells. For cells in the third subculture, the effect of CyanoP removal can be observed as an increase in fluorescence at 685 nm, even under normal BG-11 conditions (Fig. 4B). These results again indicate a constitutive role of CyanoP in maintaining functional integrity around the PSII structure.
The 77 K fluorescence emission spectra of wild-type and ∆P cells in BG11 or BG11 (− CaCl2) medium. Wild-type (black lines) and the ∆P cells (gray lines) from (A) the first subculture and (B) the third subculture were harvested four days after each subculture, and resuspended in fresh BG11 (solid lines) or BG11 (− CaCl2) (dotted lines) at 5 µg Chl/ml for measurements. Spectra were collected by use of a FP-8600 fluorescence spectrophotometer (Jasco, Tokyo, Japan), using excitation at 580 nm, and fluorescence signals were normalized to the PSI emission peak at 725 nm
Association of CyanoP with detergent-solubilized thylakoid membranes
A previous study suggested CyanoP would be present in thylakoid membranes with the same stoichiometry as other PSII components [9]; however, only small amounts were detected in detergent-purified PSII complexes [8, 9]. Nevertheless, the fact that no CyanoP protein was detected in mutants lacking PSII indicates that association with PSII is essential for stable accumulation of CyanoP in vivo [9]. To characterize the PSII complexes that bind CyanoP, we tested use of different detergents to solubilize thylakoid membranes (Fig. 5). Thylakoid membranes of wild-type, ∆P, and the HT3 strain having a 6× histidine tag on CP47 were solubilized with three different detergents, n-dodecyl-β-d-maltoside (β-DM), n-dodecyl-α-d-maltoside (α-DM), and n-heptyl-β-d-thioglucoside (HTG). Solubilized membranes were treated with PEG 1,540, and large protein complexes were precipitated by centrifugation. CyanoP was detected in the PEG precipitates from the membranes of wild-type and the HT-3 cells solubilized by α-DM and HTG but not by β-DM (Fig. 5). CyanoP cannot be detected in the supernatant fraction, indicating that free CyanoP might be degraded in the solution. This indicates that α-DM and HTG would be better for examining the association of CyanoP with protein complexes in thylakoid membranes.
Effect of different detergents on the association of CyanoP in thylakoid membranes. Thylakoid membranes of wild-type (WT), ∆P, and HT3 cells were solubilized by use of 0.8 % β-DM or 0.7 % α-DM or 2.5 % HTG. The solubilized membranes (m) were subsequently treated with 15 % PEG 1,540 to separate the supernatant (s) and the large protein complexes (p). Proteins corresponding to 0.8 µg Chl in each fraction were analyzed by SDS-PAGE and immunoblotting using anti-D2 and CyanoP antibodies
Gel-filtration profiles of HT3 thylakoid membranes and immunoblot analysis. Thylakoid membranes were solubilized by use of 0.7 % α-DM and subjected to gel filtration on a Superdex 200 column. The elution profiles measured by absorbance at 670 nm (black solid line), 620 nm (black dotted line), and 280 nm (gray dotted line) are shown at the top. Eluted fractions were subjected to immunoblot analysis with anti-PsaB, D2, and CyanoP antibodies. Numerals above the gels indicate fraction numbers
To separate the PSII complexes that bind CyanoP, thylakoid membranes of the HT3 strain were solubilized with 0.7 % α-DM and subjected to gel-filtration analysis. Immunoblot analysis showed that a large part of the CyanoP was detected in the low-molecular-mass fraction (fractions 16–19 in Fig. 6), and some CyanoP could be detected in the large-molecular-mass fraction (fractions 3–5 in Fig. 6). The fractionated pattern of CyanoP in the large-molecular-mass fractions was similar to that of D2 protein in PSII, which is consistent with the absence of CyanoP in the D2-less mutant [9]. However, our attempt to isolate PSII complexes having CyanoP from the membrane solubilized by use of α-DM using the His-tag on CP47 in the HT3 strain was not successful (results not shown). Because a substantial part of the CyanoP was dissociated from the protein complexes in our gel filtration analysis, further optimization of the membrane-solubilization or separation method is obviously required to elucidate the exact binding site and function of CyanoP in the cyanobacterial PSII complex.
Conclusion
It has been suggested that removal of CyanoP affects the photosynthetic activity of Synechocystis 6803 cells, but the effect detected is usually marginal and differs between reports [8–10]. Here we suggest that bacterial growth conditions are critical to detection of the specific phenotype resulting from CyanoP deletion. After repeated subculture in CaCl2-depleted medium, growth retardation was clearly observed for the CyanoP knockout mutant of Synechocystis sp. PCC 6803 (∆P), and the ∆P mutant had lower PSII-mediated O2-evolution activity in CaCl2-depleted medium than the wild-type in the first subculture only. Analysis of 77 K fluorescence emission using 580 nm excitation of PBS was a sensitive means of detection of changes of PSII function caused by CaCl2 depletion and removal of CyanoP. Our results are basically consistent with the suggestion by Sveshnikov et al. [11] that CyanoP of Synechocystis 6803 constitutively stabilizes the functionality of the oxygen-evolving complex of PSII, rather than having a transient regulatory function. An in-silico model of the hypothetical position of docking of CyanoP with the PSII complex has recently been proposed [14]; however, direct biochemical evidence is still required to support the model. We have shown that membrane solubilization using α-DM or HTG partly preserves CyanoP binding to the PSII complex; further research is required to more fully understand its binding site and function in the PSII complex.
References
T.J. Wydrzynski, K. Satoh (eds.), Photosystem II: The Light-Driven Water: Plastoquinone Oxidoreductase (Springer, Dordrecht, 2005)
Y. Umena, K. Kawakami, J.R. Shen, N. Kamiya, Nature 473, 55 (2011)
G. Renger, in Photosynthesis: Plastid biology, Energy Conversion and Carbon Assimilation, ed. by J.J. Eaton-Rye, B.C. Tripahty, T.D. Sharkey (Springer, Dordrecht, 2012), pp. 359–414
K. Ifuku, K. Ido, F. Sato, J. Photochem. Photobiol. B 104, 158 (2011)
T.M. Bricker, J.L. Roose, R.D. Fagerlund, L.K. Frankel, J.J. Eaton-Rye, Biochim. Biophys. Acta 1817, 121 (2012)
Y. Kashino, W.M. Lauber, J.A. Carroll, Q. Wang, J. Whitmarsh, K. Satoh, H.B. Pakrasi, Biochemistry 41, 8004 (2002)
J. De Las Rivas, M. Balsera, J. Barber, Trends Plant Sci. 9, 18 (2004)
L.E. Thornton, H. Ohkawa, J.L. Roose, Y. Kashino, N. Keren, H.B. Pakrasi, Plant Cell 16, 2164 (2004)
Y. Ishikawa, W.P. Schröder, C. Funk, Photosynth. Res. 84, 257 (2005)
T.C. Summerfield, R.T. Winter, J.J. Eaton-Rye, Photosynth. Res. 84, 263–268 (2005)
D. Sveshnikov, C. Funk, W.P. Schröder, Photosynth. Res. 93, 101–109 (2007)
N. Inoue-Kashino, Y. Kashino, K. Satoh, I. Terashima, H.B. Pakrasi, Biochemistry 244, 1221 (2005)
T.M. Bricker, J. Morvant, N. Masri, H.M. Sutton, L.K. Frankel, Biochim. Biophys. Acta 1409, 50 (1998)
R.D. Fagerlund, J.J. Eaton-Rye, J. Photochem. Photobiol. B 104, 191 (2011)
Acknowledgments
We thank Dr K. Ido, Mr S. Matsui, and Mr T. Nishimura from Kyoto University for their help with protein analysis and for stimulating discussion. This work was supported in part by a grant from JST Presto (to K.I.) and by a Grant-in-Aid for Young Scientists (B) from JSPS (Grant No. 18770032 to K.I.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aoi, M., Kashino, Y. & Ifuku, K. Function and association of CyanoP in photosystem II of Synechocystis sp. PCC 6803. Res Chem Intermed 40, 3209–3217 (2014). https://doi.org/10.1007/s11164-014-1827-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1827-y