Abstract
The ferrous ions (Fe2+) assistant air-bubble cavitation degradation of some organic dyes (Congo Red, Rhodamine B, Methyl Orange, and Methyl Violet) induced by air bubbles passing small glass balls was systematically investigated. Meanwhile, the influence of five operating factors, including air-bubbling time, FeSO4 ·7H2O amount, dye initial concentration, glass ball diameter, and gas flow rate, on the degradation of Congo Red dye were reviewed. Moreover, the degradation process of Congo Red is inspected by using UV–Vis, TOC, and HPLC techniques. It was found that the highest TOC removal ratio (81.42 %) was achieved under optimal conditions. On the other hand, the production and existence of ·OH radicals was testified through the oxidation of 1,5-diphenylcarbohydrazide (DPCI) forming diphenylcarbazone (DPCO). It can be inferred that the air-bubble cavitation induces ferrous ions to provide additional hydroxyl (·OH) radicals, which results in a rapid oxidation of organic dyes. Furthermore, the possible degradation process and mechanism were also discussed. All results demonstrate that this new degradation method has many advantages, such as high efficiency, energy savings, producing no secondary pollution, and being a simple device. Thus, it is expected to be feasible and promising as an advisable choice for the treatment of organic wastewater in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The textile dyeing industry consumes large quantities of water and, concurrently, produces large volumes of wastewater from different steps in the dyeing and finishing processes [1]. If the wastewaters are not treated properly, they can seriously contaminate our environment [2–4]. In general, commercial wastewater treatment methods utilize the combination of biological, physical, and chemical treatments. Biological treatment units tend to be very large due to the slowness of biological reactions. The physical methods only transfer waste components from one phase to another, which easily brings about secondary pollution. Also, the costs are considerably high for the needed equipment and operation. The chemical treatment of organic pollutants, such as chlorination, can result in the formation of chlorinated phenols and other by-products that have been reported to be toxic and non-biodegradable. Therefore, an alternative treatment method is required. Theoretically, cavitation technology should be an attractive alternative method for harnessing the refractory wastewater, as it not only removes toxic substances effectively but also has many advantages including effective degradation, energy savings, producing no secondary pollution, and also that it is a simple reaction device. Hence, as one of the physical–chemical water treatment methods, it has attracted more attention in recent years [5].
Cavitation is a term used to describe a special physical–chemical process that includes nucleation, growth, and implosion of vapor- or gas-filled cavities. These cavities are formed in a liquid when the pressure is reduced below the vapor pressure of the liquid in current temperature. When these cavities are carried to a higher-pressure region, they implode violently and result in 1,000-atm pressures and 5,000-K transient temperatures [6–8].
At present, all known cavitations are classified into four types based on the mode of generation, namely, acoustic, hydrodynamic, optic, and particle cavitations [9, 10]. Optic and particle cavitations are typically the single bubble cavitation, which fails to induce any physical or chemical change in the bulk solution [10]. Acoustic and hydrodynamic cavitations were two mainly used means among the four types of cavitations for treating wastewater in past studies [9, 11–15]. However, the efficiency of ultrasonic devices is limited by achieving cavitation in the form of a cloud of cavitation bubbles only in a relatively small region near the surface of the ultrasonic source, and the acoustic cavitation lacks comprehensive utilization of professional knowledge about related subjects and is a high-cost method. In a word, cavitation is effective in treating most liquid-phase pollutants but it is highly energy intensive and not economical or practically feasible when used alone [16, 17]. So, at the present stage, it is difficult to realize industrialization [8, 18]. Otherwise, the hydrodynamic cavitation technology is still in an infancy stage and its degradation efficiency is relatively low, so it cannot yet be applied in practice on a large scale [19].
In our previously work, the degradation effect of air-bubble cavitation was induced by air bubbles passing through small glass balls, and the possible degradation process and mechanism were also described [20, 21]. In the present work, in order to improve the effectiveness of air-bubble cavitation degradation, the ferrous ions were added into the system, and then the degradation of some organic dyes was investigated. The hydroxyl radical is an extremely powerful oxidant that is widely used for destruction of organic wastewater. Hydroxyl radicals can be formed by the effect of the ionizing radiation on water [22], but this requires a lot of energy. In this paper, it was found that the Congo Red in aqueous solution can obviously be destroyed using the method of air-bubble cavitation combined with ferrous ions. The production and existence of ·OH radicals generated by air-bubble cavitation combined with ferrous ions were also testified through the oxidation of 1,5-diphenylcarbohydrazide (DPCI) to 1,5-diphenylcarbazone (DPCO). Maybe this work sets up the foundation for fast and economic treatment of wastewater. It is wished that this method could become a practical and large-scale wastewater treatment technology on the basis of further study.
Experimental
Materials
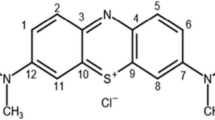
Congo Red (CG-R, analytical reagent, Aldrich Chemical Company, WI, USA) was purchased and chosen as a model compound. Its molecular structure is shown in Fig. 1. Other organic dyes (Rhodamine B (RM-B), Methyl Orange (MO) and Methyl Violet (MV), all analytical reagents, Guangcheng Chemical Reagent Limit Company, Tianjin, China) were adopted to compare the degradation ratio with Congo Red. Ferrous sulfate (FeSO4·7H2O, analytical reagent, Xinxing Chemical Reagent Factory, Shenyang, China) acted as a catalyst for enhancing the degradation effect. 1,5-diphenylcarbazide (DPCI, analytical reagents, Guangcheng Chemical Reagent Limit Company, Tianjin, China) and vitamin C (VC, analytical reagent, Sinopharm Group Chemical Reagent Limit Company, Shanghai, China) were bought to prove the existence of hydroxyl radicals (·OH). Glass balls (1.0–1.5, 2.0–2.5, 3.0–3.5, and 4.0–4.5-mm diameters, ordinary grinding glass ball, Yuyao Keyan Glass Ball Factory, Zhejiang, China) were marinated in 1.0 mol/l hydrochloric acid (HCl) solution for 2 days, and then taken out washing with secondary distilled water to achieve neutral. After that they were marinated in secondary distilled water for 1 day waiting for later utilization. The water used throughout in this experiment was purified by a Milli-Q water system (Millipore).
Apparatus
A UV–Vis spectrophotometer (Cary-50, Varian Company, USA) and TOC apparatus (Liqui, Elementar Analysensysteme GmbH Company, Germany) were used for the determination of Congo Red degradation process. High-performance liquid chromatography apparatus (HPLC, Pro-210, Varian Company, Palo Alto, CA, USA) was employed to inspect the mineralization and degradation process of Congo Red. The mobile phase was water and methyl alcohol (volume ratio = 10:90, chromatographically pure) and the flow rate of the solution was 0.8 ml/min. An air pump (EP-9000, Chuangxing Electronic Limit Company, Guangdong, China) was adopted to puff air into the Congo Red solution, operating at an output power of 5.6 W and a gas flow rate of between 2.25 and 9.00 l/min.
Procedure
Congo Red solution (700 ml) was put into a 2.5-l self-made glass reactor filled with small glass balls. The amount of glass balls was three-quarters of the reactor volume and the Congo Red solution was 3.0 cm higher than the height of glass balls. Air was puffed from bottom into the reactor. In order to compare the degradation effects under different conditions, UV–Vis spectra of Congo Red solutions treated by air-bubble cavitation alone (bubble + glass ball), air-bubbling combined with ferrous ions (bubble + FeSO4), and air-bubble cavitation combined with ferrous ions (bubble + glass ball + FeSO4) were determined by a UV–Vis spectrophotometer. Below 20 mg/l concentration, all the dye solutions were found to fully obey the Beer–Lambert law. The degradation efficiency is defined as:
where C0 and Ct are the initial and instant concentration (mg/l) of Congo Red solutions, respectively. In addition, for verifying the reliability of the calculated degradation efficiency based on the UV–Vis spectra, the total organic carbon (TOC) removal values of dyes treated by bubble + glass ball, bubble + FeSO4 and bubble + glass ball + FeSO4 were also determined. In order to track the degradation process, HPLC of Congo Red solutions treated by bubble + glass ball + FeSO4 at different bubbling times were also investigated at 498-nm channel wavelength. The experimental equipment is shown in Fig. 2.
Various factors influencing the degradation of Congo Red were studied by multifold methods. At first, the relationship between -ln (Ct/C0) and air bubbling time (t) was drawn and the corresponding degradation reaction kinetics was inferred. Then the influences of FeSO4·7H2O amount (0.0–0.5 g/l), initial Congo Red concentration (5.0–15.0 mg/l), glass ball diameter (1.0–1.5 to 4.0–4.5 mm), and gas flow rate (2.25–9.00 l/min) were investigated. For comparison, under the same conditions, the degradation ratios of several dyes, such as Rhodamine B (RM-B), Methyl Orange (MO), and Methyl Violet (MV), were also determined.
All experimental conditions such as initial dye concentration of 10.0 mg/l, glass ball diameter of 3.0–3.5 mm, gas flow rate of 4.50 l/min, temperature of 25.0 ± 0.2 °C, and bubbling time of 2.0 h were unaltered throughout the investigation except for those special studies.
Determination of hydroxyl radicals (·OH)
For detecting the existence of hydroxyl radicals (·OH), a series of similar experiments as mentioned above were performed. According to the oxidation of DPCI and extraction and photometry of diphenylcarbazone (DPCO), the existence and quantity of ·OH were proved and evaluated. Firstly, 15.00 ml DPCI solution (1.00 × 10−3 mol/l) was added to a separating funnel and then it was extracted by benzene and carbon tetrachloride (volume ratio = 1:1) mixed solution. Afterwards, they were diluted to 10.00 ml with the same benzene and carbon tetrachloride mixed solution. The experiments were performed for DPCI + bubble + FeSO4, DPCI + bubble + glass ball, DPCI + bubble + glass ball + FeSO4 and DPCI + bubble + glass ball + FeSO4 + VC at the same time. The UV–Vis spectra of all the solutions were determined.
Results and discussion
UV–Vis spectra, TOC, and HPLC of Congo Red solution during degradation
From Fig. 3, it can be found that the UV–Vis spectra of original Congo Red solutions gives two peaks at 343 and 498 nm, respectively, corresponding to the transitions of π → π* and n → π* of benzene ring and azo bond. Compared with the original solution, for air-bubble cavitation (bubble + glass ball), air bubbling combined with ferrous ions (bubble + FeSO4) and air-bubble cavitation combined with ferrous ions (bubble + glass ball + FeSO4), all maximal absorption peaks decrease more or less. This indicates that the benzene ring and azo bond can be destroyed greatly. Nevertheless, the best degradation effect is obtained by using bubble + glass ball + FeSO4 system. The degradation order is as follows: bubble + glass ball + FeSO4 > bubble + FeSO4 > bubble + glass ball. The corresponding degradation percentages are 83.35, 51.04, and 31.36 %, respectively, for 2.0-h bubbling time. All results illuminate that the method of air-bubble cavitation combined with ferrous ions could degrade Congo Red dye to a great extent.
Furthermore, the mineralization of corresponding treated Congo Red solutions is monitored by TOC determination. Experimentally, the degradation ratios calculated according to TOC are 77.54, 43.27, and 30.14 % for bubble + glass ball + FeSO4, bubble + FeSO4, and bubble + glass ball systems, respectively. These are comparable with the results calculated based on the UV–Vis spectra. Hence, it can clearly be seen again that the addition of ferrous sulfate is beneficial for the mineralization of Congo Red.
In order to explore the intermediates and degradation process of Congo Red solutions using the method of bubble + glass ball + FeSO4, HPLC of the treated solutions are determined at 498-nm channel wavelength for different bubbling times. As shown in Fig. 4, the maximal peak appears at 2.42-min retention time and the peaks decrease obviously along with the increase of bubbling time. Otherwise, for any bubbling time, one peak appears at about 2.42-min retention time, which shows that no new intermediates are formed during the degradation process. Therefore, it shows once again that the Congo Red could be degraded effectively using the bubble + glass ball + FeSO4 system.
Effect of bubbling time and reaction kinetics on degradation of Congo Red
Be aimed at discussing the influence of bubbling time on the degradation efficiency, a series of experiments through glass balls (3.0–3.5 mm diameter) within 2.0 h at 30.0-min intervals were carried out for 10.0 mg/l Congo Red solution, 0.30 g/l FeSO4·7H2O, and 4.50 l/min gas flow rate. The profiles for removal of Congo Red are shown in Fig. 5a. It can be seen that regardless of the method, the degradation efficiency all rose along with the increase of air bubbling time, which indicated that the Congo Red was gradually reduced. Moreover, the method using bubble + glass ball + FeSO4 system was the most effective one, and the maximum degradation efficiency (76.32 %) was obtained within 2.0 h bubbling time. In comparison, the degradation percentages were only 29.15 and 10.28 % corresponding with bubble + FeSO4 and bubble + glass ball systems, respectively. Therefore, a conclusion was drawn that ferrous ions could efficiently assist the air-bubble cavitation degradation of Congo Red.
Furthermore, for inferring the degradation efficiency of Congo Red in these three systems (bubble + glass ball, bubble + FeSO4 and bubble + glass ball + FeSO4), the reaction kinetics were studied. Here, the data of -ln (Ct/C0) for first-order reaction as a function of bubbling time (t) was calculated. As shown in Fig. 5b, a pseudo-first-order kinetic model was found to fit well most of the experimental data, and the reaction rate constants were 0.0213 h−1 (R 2 = 0.9993), 0.0591 h−1 (R 2 = 0.9676), and 0.2948 h−1 (R 2 = 0.9715) for bubble + glass ball, bubble + FeSO4, and bubble + glass ball + FeSO4, respectively. Obviously, the degradation of Congo Red using the bubble + glass ball + FeSO4 system was the most rapid among these three courses.
Effect of ferrous sulfate amount and initial concentration on the degradation of Congo Red
The amount of ferrous sulfate (FeSO4 ·7H2O) plays a crucial role in determining the overall efficacy of the degradation process. Adewuyi [23, 24] did a lot of research on the ferrous ion remove contaminants. Usually, the optimal catalyst amount depends on the nature of the organic pollutants and it itself is a property of the catalyst. Here, the influence of FeSO4 ·7H2O amount on the degradation efficiency of Congo Red is investigated in the range from 0.0–0.5 g/l. The obtained results are shown in Fig. 6a. It can be found that the degradation efficiency within 2.0-h bubbling time, for 10.0 mg/l initial concentration, 3.0–3.5 mm glass ball and 4.50 l/min gas flow rate rise with the increase of the amount of FeSO4 ·7H2O, and then decrease slightly after 0.30 g/l FeSO4 ·7H2O. This phenomenon may be explained as the more generated Fe3+ ions are hydrolyzed to the form of Fe(OH)3 precipitate as the FeSO4 ·7H2O amount increases. Because a great deal of Fe(OH)3 precipitation breaks away from the water phase, the number of generated ·OH decreases, which causes the degradation of Congo Red to diminish. Furthermore, when the iron ion concentration is too high, the reactor can be clogged up with ferric ion precipitates. So we chance the 0.30 g/l as the best ferrous sulfate amount for degradation. The ferrous iron ion concentration is very low, so it is a quite long way from hydrolysis conditions, almost have no hydrolysis.
The initial concentration of organic pollutants is an important parameter in wastewater treatment. If the initial concentration is too high, not only is more time needed but also the degradation is possibly incomplete. Contrarily, if the initial concentration is too low, it is difficult to degrade all organic pollutants, as they are far away from the reaction active field. Hence, the appropriate initial concentration is necessary for effective degradation [8]. Here, the influence of initial concentration on the degradation efficiency was investigated in the range of 5.0–15.0 mg/l, for 3.0–3.5-mm diameter glass balls, 2.0-h air-bubbling time, 0.30 g/l FeSO4·7H2O amount, and 4.50 l/min gas flow rate. As seen in Fig. 5b, the maximum degradation efficiency (approximately 73.26 %) is obtained at a low Congo Red concentration of 5.0 mg/l, and then it slightly decreases with the increase of Congo Red concentration. When the initial concentration is further increased, the degradation efficiency is greatly reduced. Under the same conditions, the degradation ratios are 71.15 and 48.32 %, respectively, for the initial concentrations of 10.0 and 15.0 mg/l. The observed results demonstrate that like most advanced oxidation technologies (AOTs), the slightly low concentration is propitious to the degradation of air-bubble cavitation combined with ferrous ions.
Effect of glass ball diameter and gas flow rate on degradation of Congo Red
The diameter of the glass balls decides both the size and number of air bubbles, and even influences the whole cavitation effect. Furthermore, it influences the catalytic activity of ferrous ions. As shown in Fig. 7a, for 10.0 mg/l Congo Red solution, 2.0-h bubbling time, 0.30 g/l FeSO4·7H2O, and 4.50 l/min gas flow rate, it was found that the degradation efficiency increases along with glass ball diameter rose at the beginning. When the glass ball diameter reaches 3.0–3.5 mm, the degradation efficiency begins to decrease slightly. This demonstrates that there is a dependent relationship between cavitation effect and glass ball diameter in a certain range. Small-diameter glass balls can produce more small-sized bubbles, but it goes against the cavitation effect, as there is not enough space for the bubbles to combine. Conversely, large-diameter glass balls produce less air bubbles. Because only a few bubbles can combine, there is not an obvious cavitation effect yet.
The gas flow rate not only determines the formation number and existence time of bubbles, but also their size and shape, which directly affect the air-bubble cavitation effect. As shown in Fig. 7b, when the gas flow rate is 9.00 l/min, the degradation efficiency is higher than the other two cases (2.25 and 4.50 l/min). This indicates that the high gas flow rate is a benefit for degradation. The reason for this may be that a high gas flow rate can produce more air bubbles in a unit of time and in this system it is sufficient to induce radial motion of the bubbles to generate OH radicals, which is good for the entire air-bubble cavitation effect. Of course, the degradation efficiency is not better if the gas flow rate is so quick that the bubbles are unable to break and syncretize adequately. Hence, an appropriate gas flow rate needs to be selected.
Comparison of degradation of some dyes
Figure 8 shows a comparison of the degradation efficiency of the organic dyes Rhodamine B (RM-B), Methyl Orange (MO), Methyl Violet (MV), and Congo Red (CG-R). Obviously, these dyes can all be degraded more or less through the air-bubble cavitation combined with ferrous ions. This indicates that this is a widespread treatment method for organic pollutants in wastewater under some optimized conditions. The order of degradation efficiency is as follows: Congo Red > Methyl Violet > Rhodamine B > Methyl Orange. This demonstrates that the dyes with an unstable structure, like Congo Red, are more easily to be degraded, whereas the dyes with a stable structure, like Methyl Orange, are considerably more difficult to be degraded.
Analysis of hydroxyl radicals (·OH)
The ·OH produced during air-bubble cavitation combined with ferrous ions can be detected through the oxidation of 1,5-diphenylcarbazide (DPCI). Figure 9 shows the UV–Vis absorption spectra of various DPCI solutions corresponding to bubble + glass ball + FeSO4 (e), bubble + glass ball (d), bubble + glass ball + FeSO4 + VC (c), bubble + FeSO4 (b), and pure DPCI (a) systems. All absorption peaks appear at 563 nm, which belong to diphenylcarbazone (DPCO), a product resulting from the oxidation of DPCI. However, the pure DPCI solution (a) gives little absorption around 563 nm. It can be seen that the DPCI solution with bubble + glass ball + FeSO4 condition exhibits the most obvious hyperchromic effect, being much higher than the ones with bubble + glass ball and bubble + FeSO4 conditions. Thus, it can be surmised that the DPCI are oxidized easily by air-bubble cavitation combined with ferrous ions, whereas, after adding VC, which acts as a quenching agent of ·OH, the absorption peak of DPCI solution with bubble + glass ball + FeSO4 condition is seriously weakened. This indicates that the DPCI is oxidized by ·OH in the bubble + glass ball + FeSO4 system, and this proves that the ·OH radicals are generated by using the method of air-bubble cavitation combined with ferrous ions (bubble + glass ball + FeSO4).
In addition, we found that a small amount of H2O2 was generated in cavitation or the air bubbles process. With the aid of bubbles, the H2O2 and ferrous iron as Fenton reagent has a better degradation effect. H2O2 added to the liquid medium as a Fenton reagent scavenges radicals produced by cavitation bubbles [25, 26]. In addition to the effects of chemicals in the actual process of degradation, physics effects are also very important. The radial motion of air bubbles is doing both jobs of generating H2O2 and providing convection and mixing in the system for the reaction between the dye molecules and the oxidant. All of the phenomena reveal that the method of air-bubble cavitation combined with ferrous ions can produce more ·OH than that of bubble + glass ball or bubble + FeSO4, which can degrade various organic pollutants effectively [20, 27].
Possible cavitation phenomenon and corresponding degradation mechanism of dyes during bubbling
The results mentioned above showed that the air-bubble cavitation combined with ferrous ions can effectively degrade organic dyes in an aqueous solution, such as Congo Red, Rhodamine B, Methyl Orange, and Methyl Violet. The concrete process can be divided into two steps. Firstly, the ascending bubbles having a certain velocity encounter some appropriate-diameter glass balls in solution, which are hampered, and then split into two or more smaller bubbles. As shown in Fig. 10, in order to reduce the surface tension and inner energy, these small bubbles have a recombination trend to form one bigger bubble. At this time, the interface between two bubbles becomes thinner and thinner until it ruptures, due to gradually increasing tension. Instantaneously, a large amount of energy is released along with the collapse of micro-bubbles locally. This phenomenon is similar to the ultrasonic or hydrodynamic cavitation effect in the water medium [28, 29]. Therefore, here, we called it the “air-bubble cavitation effect”.
The degradation of non-volatile organic pollutants in aqueous solution takes place in the bubble-bulk interface area due to the exposure to free radicals, high temperature, and high pressure. Being similar to the ultrasonic or hydrodynamic cavitation, the process of air-bubble cavitation can make water molecules become hydroxyl radicals (·OH) and hydrogen radicals (·H). These radicals are then mixed with the bulk, where they induce various chemical reactions [30, 31]. The ·H and oxygen (O2) dissolved in water react with each other to form the super oxygen radical anions (·O2 −). Because the ·OH and ·O2 − radicals have a strong oxidizing property, they naturally can degrade various organic pollutants in solution.
Secondly, the addition of Fe2+ ions can increase the yield of ·OH radicals and accelerate the degradation of Congo Red. Air-bubble cavitation not only breaks up water molecules to generate ·OH and ·H but also induces the catalytic activity of Fe2+ ions to provide additional ·OH. Hence, the Fe2+ ions can accelerate the degradation of organic pollutants. The possible process is shown in the following equations:
It may be considered that the achieved degradation of Congo Red is due to the attack of ·OH radicals, and the Fe2+ ions can help to produce more ·OH radicals, thereby resulting in high degradation of organic pollutants.
Conclusions
Some organic dyes in aqueous solution can be decomposed effectively adopting the novel method of air-bubble cavitation combined with ferrous ions. For Congo Red dye solution, at room temperature (25.0 ± 0.2 °C), with the bubbling time of 2.0 h, initial concentration of 10.0 mg/l, FeSO4 amount of 0.30 g/l, glass ball diameter of 3.0–3.5 mm, and gas flow rate of 4.50 l/min were adopted as experimental conditions, the degradation efficiency of 76.32 % was achieved. Under the optimization of experimental conditions, the degradation efficiency can be over 81.42 %. The corresponding degradation process was found to be described by pseudo first-order reaction kinetics. Moreover, it is speculated that the addition of ferrous ions can not only promote the collapse of the small bubbles but also induce H2O2 and H2O to produce more ·OH radicals. Therefore, the method of air-bubble cavitation combined with ferrous ions can be effectively used for the treatment of industrial wastewater samples.
References
B.B. Ramesh, A.K. Parande, S. Raghu, K.T. Prem, J. Cotton, Sci. 11, 141–153 (2007)
Y.X. Wang, J. Yu, Water Sci. Technol. 38, 233–238 (1998)
D. Georgiou, P. Melidis, A. Aivasidis, K. Gimouhopoulos, Dyes Pigments. 52, 69–78 (2002)
M.A.M. Martins, N. Lima, A.J.D. Silvestre, M.J. Queiroz, Chemosphere 52, 967–973 (2003)
I.K. Kim, O.J. Jung, Bull. Korean Chem. Soc. 23, 990–994 (2002)
Y.T. Didenko, W.B. Mcnamara, K.S. Suslick, J. Am. Chem. Soc. 121, 5817–5818 (1999)
W.B. Mcnamara, Y.T. Didenko, K.S. Suslick, Nature 401, 772–775 (1999)
X.K. Wang, Y. Zhang, J. Hazard. Mater. 161, 202–207 (2009)
P.R. Gogate, J. Environ. Manage. 85, 801–815 (2007)
P.R. Gogate, A.M. Kabadi, Biochem. Eng. J. 44, 60–72 (2009)
M.N. Patil, A.B. Pandi, Ultrason. Sonochem. 14, 519–530 (2007)
A.G. Chakinala, P.R. Gogate, A.E. Burgess, D.H. Bremner, Ultrason. Sonochem. 15, 49–54 (2008)
K.S. Kumar, V.S. Moholkar, Chem. Eng. Sci. 62, 2698–2711 (2007)
J.H. Perry, Mc-Graw Hill Publ. 5, 5–36 (1950)
V.S. Moholkar, P.S. Kumar, A.B. Pandit, Ultrason. Sonochem. 6, 53–65 (1999)
Y.G. Adewuyi, Environ. Sci. Technol. 39, 8557–8570 (2005)
Y.G. Adewuyi, Environ. Sci. Technol. 39, 3409–3420 (2005)
S. Arrojo, Y. Benito, Ultrason. Sonochem. 15, 203–211 (2008)
A.G. Chakinala, P.R. Gogate, R. Chand, D.H. Bremner, R. Molina, A.E. Burgess, Ultrason. Sonochem. 15, 164–170 (2008)
Z.H. Zhang, Y.H. Lv, J. Wang, Y.Q. Deng, Z. Jiang, W. Gao, J. Wang, Acta Scientiae Circumstantiae. 29, 955–959 (2009)
R. Xu, R.Z. Jiang, J. Wang, B. Liu, J.Q. Gao, B.X. Wang, G.X. Han, X.D. Zhang, Chem. Eng. J. 164, 23–28 (2010)
W.H. Glaze, J.W. Kang, Ind. Eng. Chem. Res. 28, 1573–1580 (1989)
Y.G. Adewuyi, N.Y. Sakyi, Ind. Eng. Chem. Res. 52, 14687–14697 (2013)
Y.G. Adewuyi, M.A. Khan, N.Y. Sakyi, Ind. Eng. Chem. Res. 53, 828–839 (2014)
N.N. Mahamuni, Y.G. Adewuyi, Ultrason. Sonochem. 17, 990–1003 (2010)
S. Chakma, V.S. Moholkar, AIChE J. 59, 4303–4313 (2013)
B. Song, G.L. Wang, J.L. Yuan, Talanta 72, 231–236 (2007)
R.A. Torres, F. Abdelmalek, E. Combet, C. Pétrier, C. Pulgarin, J. Hazard. Mater. 146, 546–551 (2007)
R.A. Torres, C. Pétrier, E. Combet, M. Carrier, C. Pulgarin, Ultrason. Sonochem. 15, 605–611 (2008)
J.S. Krishnan, P. Dwivedi, V.S. Moholkar, Ind. Eng. Chem. Res. 45, 1493–1504 (2006)
S. Kumar, V. Khanna, S. Moholkar, AIChE J. 58, 3858–3866 (2012)
Acknowledgments
The authors greatly acknowledge the National Science Foundation of China (21371084), Innovation Team Project of Education Department of Liaoning Province (LT2012001), Public Research Fund Project of Science and Technology Department of Liaoning Province (2012004001), Shenyang Science and Technology Plan Project (F12-277-1-15 and F13-316-1-51), and Science Foundation of Liaoning Provincial Education Department (L2011007) for financial support. The authors also thank our colleagues and other students for participating in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, C., Zhang, L., Wang, J. et al. Ferrous ions (Fe2+) assisted air-bubble cavitation degradation of organic pollutants. Res Chem Intermed 41, 6009–6022 (2015). https://doi.org/10.1007/s11164-014-1717-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1717-3