Abstract
We have fabricated a three-dimensional (3D) nanostructured indium tin oxide (ITO) film in which the spaces were filled by use of a Cu, In, and Ga precursor solution. This solution has potential for use in bulk heterojunction CuIn x Ga1−x S2 (CIGS) thin-film solar cells. ITO nanorod films ~700 nm thick on glass substrates were synthesized by radio-frequency magnetron sputtering deposition. To ensure complete filling of the gaps in ITO nanorod films, a polymeric binder-free precursor solution was used. In addition, a two-step heating process (oxidation and sulfurization) was used after coating of the precursor solution to make a CIGS absorber film with a minimum of carbon impurities. Superstrate-type solar cell devices with 3D nanostructured films (CIGS–ITO) had a photovoltaic efficiency of 1.11 % despite the absence of a buffer layer (e.g. CdS) between the CIGS and ITO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crystalline Si-based solar cells have been successfully used for efficient conversion of sunlight into electricity, but there is little scope for reducing manufacturing costs, despite the demand for more cost-effective and efficient photovoltaic devices [1]. As an alternative, inorganic thin-film solar cells, for example CuIn x Ga1−x SySe2−y (CIGS) have attracted much attention because of their attractive optical and physical properties (e.g. high absorption coefficient, stability, etc). For manufacture of more cost-effective CIGS thin-film solar cells, solution processes have been suggested, including printing, spraying, etc. [2, 3], because CIGS absorber films are currently fabricated by use of a vacuum-based method.
With such thin-film technology, however, there is a common problem with solar cell efficiency arising as a result of poor material quality (e.g. small crystallites); this results in short photogenerated-carrier diffusion lengths and, hence, high recombination of charge carriers at grain boundaries [4]. One way of overcoming this problem is to make absorber films with large grains, which is a general approach in research on solution-processed CIGS thin-film solar cells. Very recently, we suggested an alternative solution to problems arising from poor material quality of solution-processed CIGS films—introduction of the bulk heterojunction concept to inorganic phases [5]. Such bulk heterojunctions, in which two interpenetrating nano-scale heterojunction networks for electrons and holes are formed, have already been found to enhance the power-conversion efficiency of organic solar cells [6, 7]. Similarly, the bulk heterojunction structure between two different semiconductor materials can be expected to reduce travel distance to the junction of photogenerated carriers; recombination of charge carriers can, therefore, be substantially reduced.
Among many issues involved in achieving highly efficient 3D nanostructured inorganic thin-film solar cells, complete gap filling is one of the most important, because solutions (e.g. inks) containing p-type semiconductor nanoparticles or precursor ions are not easily drawn into pores by capillary action. In addition, carbon impurities originating from organic solvents and/or additives in solution inks or pastes are frequently left in the film and may have a detrimental effect on solar cell operation. For example, in our previous study, CuInS2 film prepared using CuInS2 nanoparticle ink resulted in a substantial amount of residual carbon impurities (16 at. %) even after heat treatment at 350 °C in an Ar environment [8].
To obtain a 3D nanostructured inorganic film of CIGS–ITO nanorods with complete gap filling and a minimum of residual carbon impurities, we have used a binder-free precursor solution containing Cu, In, and Ga ions and a two-step heat-treatment process (oxidation and sulfurization). Complete filling of the spaces between ITO nanorods with CIGS material was verified by scanning electron microscopy (SEM). It was possible to remove residual carbon impurities by initial heat treatment under ambient conditions. Superstrate-type solar cell devices with 3D nanostructured inorganic films of CIGS–ITO nanorods were also fabricated; their power-conversion efficiency was 1.11 %.
Experimental
Preparation of the ITO nanorod film
As reported elsewhere [9], ITO nanorods were deposited on ITO thin film-coated glass substrates by use of radio-frequency magnetron sputtering. The ITO film-coated glass substrates were ultrasonically cleaned in acetone, ethanol, and de-ionized (DI) water for 10 min each and dried with N2 gas. They were then baked at 120 °C for 1 h in a vacuum box. Sputter deposition was conducted in an Ar atmosphere at a background pressure of 2 × 10−6 Torr, a working pressure of 7.8 × 10−3 Torr, and a radio frequency power of 30 W. Three small In metal disks (d ~3 mm) were placed on a 2-inch ITO target and used as a catalyst for growth of ITO nanorods. The Sn content (Sn/(In + Sn) atomic ratio) of the target pellet was fixed at 10 %. The sputter deposition process was performed at 500 °C for 1 h. After deposition, the sputtering chamber was left to cool to room temperature under vacuum. To improve the transparency of the ITO nanorods grown on the ITO film-coated glass substrate, post annealing was performed at 250 °C for 1 h.
Deposition of the dense TiO2 layer
A ~20 nm dense TiO2 film was deposited on the ITO nanorod film by atomic layer deposition (ALD) at 80 °C. Titanium(IV) isopropoxide and water vapor, respectively, were used as metal reactant and oxygen source. Ar was used as the carrier gas and for purging. The total flow rate of Ar was 100 cm3/min. The oxide layers were grown under pressure of 2.6 Torr.
Preparation of the precursor solution
The precursor solution of Cu, In, and Ga ions was prepared by dissolving Cu(NO3)2.xH2O (0.842 g), In(NO3)3.xH2O (1.043 g), and Ga(NO3)3.xH2O (0.449 g) in a 1:1 mixture of ethanol and 1,2-propanediol (10 mL). The total concentration of the solution was 0.9 M. After vigorous stirring, the solution became transparent with a bluish color. The solution was then filtered through a 0.2 μm syringe filter.
Fabrication of the solar cell device
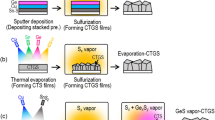
Solar cell devices with a superstrate-type configuration (ITO nanorods–dense TiO2–CIGS–Au) were fabricated. A superstrate-type CIGS solar cell in which the ITO nanorod film is filled by the CIGS absorber material is depicted schematically in Fig. 1. As mentioned above, the ITO nanorod and the dense TiO2 films were prepared by radio-frequency magnetron sputtering and ALD, respectively. The CIGS film was prepared by initial drop-casting with ultrasonification followed by spin-coating of the precursor solution. The film was then oxidized and sulfurized. Finally, an Au electrode film was deposited by use of a thermal evaporator.
Characterization
The morphology of the CIGS–ITO nanorod film was characterized by scanning electron microscopy (SEM; Hitachi S-4100). The crystalline structure of the CIGS absorber film was investigated by X-ray diffraction (XRD; Shimadzu XRD-6000). The optical properties of the CIGS absorber film were measured by use of a UV–visible spectrometer (Varian Cary 5000). The viscosities of the solutions were measured by use of a viscometer (Viscometer TV-22; Toki Sangyo). Solar cell efficiency was measured by use of a class AAA solar simulator (Sun 2000; Abet Technologies) under standard irradiation conditions (1.5 AM, 1 Sun).
Results and discussion
First, for the transparent conducting oxide layer, ITO nanorods were synthesized by radio-frequency magnetron sputtering deposition. As shown in the cross-sectional SEM image (Fig. 2a), the ITO nanorods were vertically aligned or slightly tilted from vertical alignment. The approximate thickness and length of the nanorods were 130 and 700 nm, respectively. The crystal structure of the ITO nanorod film was revealed to be cubic (bixbyite), and its sheet resistance and transmittance (at 550 nm) were 8 Ω/□ and 87 %, respectively. A dense layer of TiO2 was coated on the ITO nanorod film. The dense TiO2 layer was needed to prevent the formation of an internal shunt because of leakage currents from direct contact between the p-type semiconductor (e.g. CIGS) and the ITO. In this study, an atomic layer deposition technique was used to deposit a dense layer of TiO2 [10, 11]. The dense TiO2 layer was uniformly applied to the ITO nanorods with a thickness of 20 nm. We have already confirmed that the transmittance of ITO is maintained even after dense TiO2 coating.
Polymeric binders are usually added to precursor solutions to adjust the viscosity of the solution for the desired thickness of CIGS absorber film. For example, ethyl cellulose or poly(vinyl acetate) has been mixed with ethanol or methanol solutions of Cu, In, and Ga nitrate precursors. These solutions were confirmed to be suitable for use in planar type CIGS absorber films [12]. However, when a precursor solution with binder was used with the vertically grown ITO nanorod film, the space between the ITO nanorods was not successfully filled, as shown in the cross-sectional SEM image in Fig. 2b; therefore, the solar cell device with this film could not be properly operated. To solve this gap-filling problem we prepared a precursor solution with no polymeric binder. First, we tried to prepare the precursor solution with only the solvent (e.g. ethanol). The solution was found to be drawn into the deep spaces between the ITO nanorods but it was extremely difficult to fill the entire thickness of the ITO nanorods (~700 nm) by use of the spin coating method, because of the very low viscosity of the solution (~5.3 mPas at 21 °C). To increase the viscosity without addition of binder we tried mixing ethanol with a viscous solvent, for example 1,2-propanediol. We confirmed that Cu, In, and Ga nitrates dissolved completely in 1:1 ethanol–1,2-propanediol, and that the viscosity of the solution was substantially increased to ~17.0 mPas at 21 °C. To further enhance gap-filling, ultrasonification was applied for 2 min after drop-casting of the solution. The solution was then spin-coated again to obtain the desired thickness of the CIGS film.
Figure 2c shows a cross-sectional SEM image of the ITO nanorod film filled with the precursor solution, using 1:1 ethanol–1,2-propandiol as solvent, after the first heat treatment at 300 °C under ambient conditions. Very different from the situation shown in Fig. 2b, the spaces between the ITO nanorods were completely filled by the mixed oxides of Cu, In, and Ga. Notably, because the film was formed after air-annealing, almost all residual carbon impurities were removed, which was verified by electron probe microanalyzer (EPMA) composition analysis.
In the next step, the mixed oxide film formed on the ITO nanorod film was sulfurized at 450 °C under H2S (1 %) in Ar gas, to synthesize the CIGS alloy film. Figure 3a, b show the top view and cross-sectional SEM images of CIGS–ITO films. It is clear that the CIGS film consists of larger grains than the Cu, In, and Ga mixed oxide phase. To investigate the crystalline structure of the CIGS film, XRD measurement was performed. As shown in Fig. 4, the XRD pattern contained a peak at 2θ = 27.8°, with weak peaks at 2θ = 17.7°, 32.2°, 46.3°, 55.0°, and 57.6°. The most intense peak at 2θ = 27.8° is indicative of polycrystalline CIGS alloy with (112) orientation. The other prominent peaks at 2θ = 46.3° and 55.0° correspond to the (220)/(204) and (116)/(312) phases. In addition to these peaks commonly observed in CIGS, several weak peaks, such as (101) at 17.7° and (004)/(200) at 32.2°, were also present in the XRD patterns. The presence of these peaks clearly indicates the perfect chalcopyrite structure of CIGS, which is in good agreement with JCPDS reference #27-0159.
The optical properties of the CIGS film were also investigated, by UV–visible absorption spectroscopy. As is apparent from Fig. 5, the CIGS film absorbs photons with the wavelenth below 815 nm. The estimated direct optical band-gap of the film was 1.52 eV, almost identical with the value for a planar film of the same material [13].
Because of CIGS alloy formation by sulfurization, the film morphology was changed, as is apparent from Fig. 3a, b. The grain size was found to have increased, and the surface of the film became rough. However, the spaces between the ITO nanorods were still filled with CIGS material. To determine whether the CIGS–ITO film worked in solar cell applications, solar cell devices were constructed using this film by depositing an Au electrode on top of the CIGS–ITO nanorod film. The J–V characteristics revealed good power-conversion efficiency of 1.11 % with V oc, J sc, and FF values of 409 mV, 7.70 mA/cm2, and 0.353, respectively (Fig. 6).
Our solar cell device structure is a superstrate-type [8], which is different from that of conventional substrate-type planar CIGS thin-film solar cells (Mo–CIGS–CdS–i-ZnO–n-ZnO–Ni–Al). In superstrate-type CIGS solar cells, transparent conducting glass is usually used as substrate, and an n-type buffer layer (e.g. CdS) is prepared on the transparent conducting glass substrate, followed by deposition of a CIGS absorber layer. The light can, therefore, be transmitted through the glass substrate. It is well known that the presence of a buffer layer in CIGS thin-film solar cells is very important because significant improvement in electronic contact between a p-type semiconductor and n-type oxide semiconductor can be achieved [14, 15]. In addition, it is also well known that a buffer layer can prevent shunting of the photocurrent by suppressing the back flow of electrons. In our solar cell device the CdS buffer layer was absent. The CdS buffer layer should be formed earlier than the CIGS absorber layer; it is, therefore, strongly affected by the conditions used for synthesis of the CIGS absorber layer, because CdS is very vulnerable to high-temperature heat treatment, particularly under ambient conditions. In our synthetic method for preparation of the CIGS absorber film, an air-annealing step is included to remove residual carbon impurities; therefore, we were not able to incorporate a CdS buffer layer into our solar cell; this may be the main reason for the quite low solar cell efficiency of our device compared with that of substrate-type planar CIGS thin-film solar cells. Interestingly, despite the absence of a CdS buffer layer we were able to achieve over 1 % solar to electricity conversion efficiency. We assume this may be the result of the presence of an extremely thin TiO2 layer coated on the ITO nanorods; this layer acts as an n-type semiconductor and forms a p–n junction between the CIGS and TiO2. To achieve greater efficiency, incorporation of a thermally stable buffer layer in the solar cell device is necessary; this task is now under investigation in our laboratory.
Conclusion
A 3D nanostructure inorganic thin film solar cell with a bulk heterojunction between CIGS and TiO2 was fabricated by a solution process using a polymeric binder-free precursor solution. SEM measurements confirmed that the CIGS absorber material completely filled the space in the ITO nanorod film. A photovoltaic response of 1.11 % efficiency was obtained from a solar cell with no CdS buffer layer, which shows the possibility of enhanced power-conversion efficiency of the device if the proper buffer layer is incorporated.
References
S.W. Glunz, Adv. Optoelectron. 2007, 1 (2007)
S. Hegedus, Prog. Photovolt: Res. Appl. 14, 393 (2006)
M. Kemell, M. Ritala, M. Leskelä, Crit. Rev. Solid State Mater. Sci. 30, 1 (2005)
V.A. Akhavan, B.W. Goodfellow, M.G. Panthani, D.K. Reid, D.J. Hellebusch, T. Adachi, B.A. Korgel, Energy Environ. Sci. 3, 1600 (2010)
J.W. Cho, S.J. Park, J.H. Kim, W. Kim, H.K. Park, Y.R. Do, B.K. Min, ACS Appl. Mater. Interfaces 4, 849 (2012)
P. Peumans, S. Uchida, S.R. Forrest, Nature 425, 158 (2003)
R. Po, M. Maggini, N. Camaioni, J. Phys. Chem. C 114, 695 (2010)
J.W. Cho, S.J. Park, W. Kim, B.K. Min, Nanotechnology 23, 265401 (2012)
J.H. Park, H.K. Park, J. Jeong, W. Kim, B.K. Min, Y.R. Do, J. Electrochem. Soc. 158, 131 (2011)
B.D. Yuhas, P.J. Yang, Am. Chem. Soc. 131, 3756 (2009)
R. O’Hayre, M. Nanu, J. Schoonman, A. Goossens, Nanotechnology 18, 055702 (2007)
E. Lee, S.J. Park, J.W. Cho, J.H. Gwak, M.K. Oh, B.K. Min, Sol. Energy Mater. Sol. Cells 95, 2928 (2011)
S. Merdes, R. Mainz, J. Klaer, A. Meeder, H. Rodriguez-Alvarez, H. Shock, M.C. Lux-Steiner, R. Klenk, Sol. Energy Mater. Sol. C. 95, 846 (2011)
F. Lenzmanna, M. Nanub, O. Kijatkinac, A. Belaidid, Thin Solid Films 451–452, 639 (2004)
M. Valdésa, M.A. Frontinia, M. Vázqueza, A. Goossens, Appl. Surf. Sci. 254, 303 (2007)
Acknowledgments
This work was supported by a National Research Foundation of Korea grant (NRF-2009-C1AAA001-0092935 and 2011-0017449) and partly by a National Research Foundation of Korea grant funded by the Korean Government (MEST)” (2012, University–Institute Cooperation Program).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, V.B., Cho, J.W., Park, S.J. et al. Use of a precursor solution to fill the gaps between indium tin oxide nanorods, for preparation of three-dimensional CuInGaS2 thin-film solar cells. Res Chem Intermed 40, 49–56 (2014). https://doi.org/10.1007/s11164-013-1454-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1454-z