Abstract
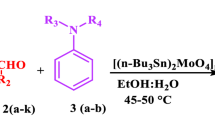
An efficient single-pot strategy for 2-amino nicotinonitrile derivatives 5 has been developed by multi-component reaction of arylaldehydes 1, methylketones 2, malononitrile 3, and ammonium acetate 4 using tetrabutyl ammonium bromide as catalyst in aqueous medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyridine-based molecules exhibit diverse pharmacological and biological activities useful as HIV-1 inhibitors [1], IKK-B inhibitors [2, 3], and adenosine receptor antagonists [4], and were recently recognized as potential targets for the development of new drugs. Among pyridine derivatives, 2-amino nicotinonitriles have attracted considerable attention because of their promising bio-activity [5–10]. A recent report [11] describes the preparation of 2-amino nicotinonitriles by using ytterbium perfluorooctanoate as catalyst. Most of the existing methods involves multiple steps [12], long reaction times and usage of hazardous solvents [13, 14]. A mild, convenient and high yield procedure for the preparation of 2-amino nicotinonitriles using inexpensive catalyst and eco-friendly solvent would be valuable.
In the recent past, tetrabutyl ammonium bromide (TBAB) has emerged as a mild, water-tolerant, inexpensive, and environmentally compatible homogenous catalyst in various organic transformations [15–17]. Biginelly-type compounds [18] and biscoumarine and 3,4-dihydro pyrano[c]chromone derivatives [19] were prepared by using TBAB as catalyst. In continuation of our interest in the development of new synthetic methods [20, 21], we report here a convenient and novel approach for the synthesis of 2-amino nicotinonitriles by multi-component reaction of aryl aldehydes, ketones, malononitrile, and ammonium acetate using Bu4N+Br− (TBAB) as catalyst in aqueous medium.
Experimental
Melting points were recorded on a Casia-Siamia (VMP-AM) melting point apparatus and are uncorrected. IR spectra were recorded on a Perkin-Elmer FT-IR 240-C spectrophotometer using KBr discs. 1H NMR spectra were recorded on Bruker AV spectrometers at 300 MHz and INOVA 400 MHz in CDCl3 using TMS as internal standard. 13C NMR spectra were recorded on a Bruker AV 75 MHz in CDCl3. Electron impact (EI) mass spectra were recorded on a VG 7070 H instrument at 70 eV. All the reactions were monitored by thin layer chromatography (TLC) on precoated silica gel of 60–120 mesh; spots were visualized with UV light. Merck silica gel (60–120 mesh) was used for column chromatography. CHN analyses were recorded on a vario EL analyzer.
Synthesis of 2-aminonicotinonitrile derivatives (5a–5o)
General procedure
A mixture of aldehyde (1 mmol), ketone (1 mmol), malononitrile (1 mmol), ammonium acetate (1.5 mmol) and tetrabutyl ammonium bromide (TBAB) (10 mol%) was stirred in one-pot in water (5 mL) at refluxing temperature for 2 h. The mixture was allowed to cool to room temperature and was extracted with ethyl acetate twice (2 × 20 ml). Combined extracts were washed with distilled water, dried over anhydrous Na2SO4 and concentrated under vacuum. The crude product was purified by passing through a column packed with silica gel.
2-Amino-4,6-diphenyl-nicotinonitrile (5a) [14], 2-amino-4-(4-chloro-phenyl)-6-phenyl-nicotinonitrile (5b) [14], 2-amino-6-phenyl-4-p-tolyl-nicotinonitrile (5c) [14], and 2-amino-4-(4-fluoro-phenyl)-6-phenyl-nicotinonitrile (5d)
I.R. (KBr, cm−1): 3,364, 3,313 (–NH2), 2,209 (–CN); 1H NMR (CDCl3, 300 MHz): δ 5.34 (br., s, 2H, NH2), 7.15 (s, 1H, Py–H), 7.18–7.23 (m, 2H, Ar–H), 7.42–7.46 (m, 3H, Ar–H), 7.60–7.65 (m, 2H, Ar–H), 7.95–7.99 (m, 2H, Ar–H); MS (EI, 70 eV); 13C NMR (CDCl3, 75 MHz): δ 87.6, 113.7, 114.5, 116.0, 127.3, 127.6, 129.2, 130.7, 134.6, 139.0, 154.5, 156.2, 159.2, 161.4; MS (EI, 70 eV): m/z M+., 289; Anal. Calcd. for C18H12FN3: C 74.73, H 4.18, N 14.52 %. Found: C 74.45, H 4.19, N 14.42 %.
2-Amino-4-(4-methoxy-phenyl)-6-phenyl-nicotinonitrile (5e) [12], 2-amino-6-phenyl-4-(2-trifluoromethyl-phenyl)-nicotinonitrile (5f)
I.R. (KBr, cm−1): 3,492, 3,351 (–NH2), 2,222 (–CN); 1H NMR (CDCl3, 300 MHz): δ 5.31 (br., s, 2H, NH2), 7.11 (s, 1H, Py–H), 7.37–7.46 (m, 4H, Ar–H), 7.58–7.70 (m, 2H, Ar–H), 7.82–7.87 (m, 1H, Ar–H), 7.93–7.98 (m, 2H, Ar–H); 13C NMR (CDCl3, 75 MHz): δ 88.9, 112.3, 113.8, 123.9, 125.4, 125.7, 127.5, 127.8, 129.2, 129.4, 129.6, 131.2, 132.5, 139.0, 156.1, 157.6, 161.9; MS (EI, 70 eV): m/z M+., 339; Anal. Calcd. for C19H12F3N3: C 67.25, H 3.56, N 12.38 %. Found: C 67.19, H 3.52, N 12.29 %.
2-Amino-6-phenyl-4-(2,4,6-trifluoro-phenyl)-nicotinonitrile (5g)
I.R. (KBr, cm−1): 3,473, 3,368 (–NH2), 2,207 (–CN); 1H NMR (CDCl3, 300 MHz): δ 5.36 (br., s, 2H, NH2), 7.11 (s, 1H, Py–H), 7.28–7.35 (m, 1H, Ar–H), 7.42–7.47 (m, 3H, Ar–H), 7.49–7.55 (m, 1H, Ar–H), 7.92–7.99 (m, 2H, Ar–H); 13C NMR (CDCl3, 75 MHz): δ 88.2, 104.3, 111.3, 113.7, 115.9, 127.4, 127.7, 129.5, 139.1, 156.2, 156.6, 161.4, 166.1; MS (EI, 70 eV): m/z M+., 325; Anal. Calcd. for C18H10F3N3: C 66.46, H 3.10, F, N 12.92 %. Found: C 66.35, H 3.05, N 12.79 %.
2-Amino-4-(4-isopropyl-phenyl)-6-phenyl-nicotinonitrile (5h)
I.R. (KBr, cm−1): 3,498, 3,375 (–NH2), 2,205 (–CN); 1H NMR (CDCl3, 500 MHz): δ 1.32 (d, 6H, J = 8.01 Hz, –(CH3)2), 2.96–3.02 (m, 1H, –CH–), 5.31 (br., s, 2H, NH2), 7.17 (s, 1H, Py–H), 7.35 (d, 2H, J = 9.0 1 Hz, Ar–H), 7.40–7.46 (m, 3H, Ar–H), 7.56 (d, 2H, J = 8.01 Hz, Ar–H), 7.96–8.00 (m, 2H, Ar–H); 13C NMR (CDCl3, 75 MHz): δ 23.8, 33.9, 88.1, 111.2, 117.3, 127.0, 127.3, 128.1, 128.7, 130.1, 134.3, 138.0, 150.8, 155.1, 159.6, 160.2; MS (EI, 70 eV): m/z M+., 313; Anal. Calcd. for C21H19N3: C 80.48, H 6.11, N 13.41 %. Found: C 80.50, H 6.01, N 13.45 %.
2-Amino-4-(furan-2-yl)-6-phenyl-nicotinonitrile (5i) [4], 2-amino-6-phenyl-4-(thiophen-2-yl)-nicotinonitrile (5j) [4], 2-amino-6-(4-fluoro-phenyl)-4-phenyl-nicotinonitrile (5k) [22], 2-amino-6-(4-methoxy-phenyl)-4-phenyl-nicotinonitrile (5l) [22], 2-amino-6-(4-bromo-phenyl)-4-phenyl-nicotinonitrile (5m) [22], 2-amino-6-(4-hydroxy-phenyl)-4-phenyl-nicotinonitrile (5n) [23], and 2-amino-6-(3-methoxy-phenyl)-4-phenyl-nicotinonitrile (5o)
I.R. (KBr, cm−1): 3,478, 3,306 (–NH2), 2,203 (–CN); 1H NMR (CDCl3, 400 MHz): δ 3.88 (s, 3H, –OCH3), 5.36 (br., s, 2H, NH2), 6.96 (d, 1H, J = 7.40 Hz, Ar–H), 7.17 (s, 1H, Py–H), 7.34 (t, 1H, J = 7.40 Hz, Ar–H), 7.42–7.45 (m, 1H, Ar–H), 7.48–7.55 (m, 4H, Ar–H), 7.60–7.64 (m, 2H, Ar–H); 13C NMR (CDCl3, 75 MHz): δ 55.9, 87.8, 112.9, 113.7, 114.8, 119.9, 127.4, 127.8, 129.2, 129.6, 130.5, 139.0, 141.2, 154.7, 156.3, 161.2, 161.9; MS (EI, 70 eV): m/z M+., 301; Anal. Calcd. for C19H15N3O: C 75.73, H 5.02, N 13.94 %. Found: C 75.65, H 4.98, N 13.89 %.
Results and discussion
In our preliminary studies, we have investigated the multi-component reaction of benzaldehyde (1 mmol), acetophenone (1 mmol), malononitrile (1 mmol), and ammonium acetate (1.5 mmol) using various catalysts such as CuF2, TlF2, ZnF2, NiF2, TiF4, and Bu4N+Br− in ethanol and obtained the product in 5 h. The Bu4N+Br− (TBAB) was found to be the best catalyst at 10 mol% concentration furnishing 86 % yield of the product with good purity. The details of the catalysts screened are set out in Table 1.
In order to have a choice of solvent, the same reaction was carried out in different solvents using TBAB as catalyst at specific time intervals, with water being found to be the best solvent which gave high yield of 92 % of product 5a. The details of solvents screened are outlined in Table 2.
After having identified the suitable catalyst and suitable solvent, the reaction was performed with substituted aryl aldehydes, methyl ketones, malononitrile, and ammonium acetate to investigate the role of substituents on the rate of reaction and yield of products and to establish the conditions. In order to further improve the scope of the reaction, the same reaction was repeated with aliphatic aldehyde such as n-heptanal and alternatively with methyl isobutyl ketone; however, this could not give the product. This may be attributed to the reduced stability of aliphatic imine and the product from aliphatic aldehyde–malononitrile. The details of the reaction and the possible mechanism are outlined in Schemes 1 and 2, respectively. The sequence of the reaction is mainly the formation of imine by the reaction of methyl ketone with ammonium acetate followed by the activation of imine with the catalyst. The activated imine generated in situ was reacted with arylidene malononitrile, formed from aryl aldehyde and malononitrile, followed by cyclization, isomerization, and by aerial oxidation resulting in product 5. The yields of the products are shown in Table 3.

Conclusion
In conclusion, an efficient and green process has been developed for the synthesis of 2-amino nicotinonitrile derivatives via one-pot condensation of aryl aldehydes, ketones, malononitrile, and ammonium acetate using TBAB as catalyst in aqueous medium, and will be useful for the synthesis of a wide range of compounds.
References
J. Deng, T. Sanchez, L.Q. Al-Mawsawi, R. Dayam, R.A. Yunes, A. Garofalo, M.B. Bolger, N. Neamati, Bioorg. Med. Chem. 15, 4985 (2007)
T. Murata, M. Shimada, S. Sakakibara, T. Yoshino, H. Kadono, T. Masuda, M. Shimazaki, T. Shintani, K. Fuchikami, K. Sakai, H. Inbe, K. Takeshita, T. Niki, M. Umeda, K.B. Bacon, K.B. Ziegelbauer, T.B. Lowinger, Bioorg. Med. Chem. Lett. 13, 913 (2003)
T. Murata, M. Shimada, H. Kadono, S. Sakakibara, T. Yoshino, T. Masuda, M. Shimazaki, T. Shintani, K. Fuchikami, K.B. Bacon, K.B. Ziegelbauerb, T.B. Lowingera, Bioorg. Med. Chem. Lett. 14, 4013–4017 (2004)
M. Mantri, O. De Graaf, J. Van Veldhoven, A. Goblyos, J.K. Von Frijtag Drabbe Kunzel, T. Mulder-Krieger, R. Link, H. De Vries, M.W. Beukers, J. Brussee, A.P. Ijzerman, J. Med. Chem 51, 4449 (2008)
T. Indumathi, V.S. Jamal Ahamed, M. Surk-Sik, F.R. Fronczek, K.J. Rajendra Prasad, Eur. J. Med. Chem. 46, 5580–5590 (2011)
T. Kobayashi, S. Sasaki, N. Tomita, S. Fukui, N. Kuroda, M. Nakayama, A. Kiba, Y. Takatsu, T. Ohtaki, F. Itoh, A. Baba, Bioorg. Med. Chem. 18, 3841–3859 (2010)
F. Zhang, Y. Zhao, L. Sun, L. Ding, Y. Gu, Ping Gong, Eur. J. Med. Chem. 46, 3149–3157 (2011)
A. Lauria, I. Abbate, C. Gentile, F. Angileri, A. Martorana, A.M. Almerico, J. Med. Chem. 56, 3424–3428 (2013)
T. Murata, M. Shimada, S. Sakakibara, T. Yoshino, T. Masuda, T. Shintani, H. Sato, Y. Koriyama, K. Fukushima, N. Nunami, M. Yamauchi, K. Fuchikami, H. Komura, A. Watanabe, K.B. Ziegelbauer, K.B. Baconb, T.B. Lowingera, Bioorg. Med. Chem. Lett. 14, 4019–4022 (2004)
O.M. Abdelhafez, K.M. Amin, R.Z. Batran, T.J. Maher, S.A. Nada, S. Sethumadhavan, Bioorg. Med. Chem. 18, 3371–3378 (2010)
J. Tang, L. Wang, Y. Yao, L. Zhang, W. Wang, Tetrahedron Lett. 52, 509–511 (2011)
A.S. Girgis, A. Kalmouch, H.M. Hosni, Amino Acids 26, 139 (2004)
S. Kambe, K. Saito, Synthesis 5, 366 (1980)
S. Khaksar, M. Yaghoobi, J. Fluorine Chem. 142, 41–44 (2012)
A. Mobinikhaledil, M.A. Bodaghi Fard, Acta Chim. Slov. 57, 931–935 (2010)
D. Amantini, F. Fringuelli, F. Pizzo, L. Vaccaro, J. Org. Chem. 66, 6734–6737 (2001)
C. Kurumurthy, G. Santhosh Kumar, G. Malla Reddy, P. Nagender, P. Shanthan Rao, B. Narsaiah, Res. Chem. Intermed. 38, 359–365 (2012)
A.K. Bahar Ahmed, R. Habibullah, M. Keshari, Tetrahedron Lett. 50, 2889–2892 (2009)
M.K. Jitender, S. Kumar, Tetrahedron Lett. 50, 4125–4127 (2009)
G. Santhosh kumar, C. Kurumurthy, P. Sambasiva Rao, B. Veeraswamy, P. Shanthan Rao, B. Narsaiah, Helv Chim Acta 94, 1692–1696 (2011)
P. Sambasiva Rao, K. Rathnakar Reddy, P. Nagender, R. Naresh Kumar, P. Shanthan Rao, B. Narsaiah, Chem. Lett. 42, 604–605 (2013)
L. Prakash, Shaihla, S.S. Verma, T. Erra, R.L. Mital, J. Fluorine Chem. 41, 303–310 (1988)
N. Latif, F.M. Asaad, N.S. Girgis, Indian J Chem Sect B 20, 463–466 (1981)
Acknowledgments
Authors thank Dr. M. Lakshmikantham, Director, IICT, for her constant encouragement. Authors C. Kurumurthy and R. Naresh Kumar thank CSIR, New Delhi, Govt. of India, for providing senior research fellowships and contingency grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurumurthy, C., Naresh Kumar, R., Yakaiah, T. et al. Novel Bu4N+Br− catalyzed one-pot multi-component synthesis of 2-amino nicotinonitriles in aqueous medium. Res Chem Intermed 41, 3193–3199 (2015). https://doi.org/10.1007/s11164-013-1424-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1424-5