Abstract
A knowledge of surface morphology is a useful element in microstructural characterization. Gaomiaozi Na-bentonite is the candidate buffer/backfill material for the Chinese repository. The results show that Gaomiaozi Na-bentonite is rough and mesoporous. The fractal dimension is 2.5870 by means of the F–H–H method compared with the Freundlich method. The correlation between the fractal dimensions and adsorption capacity has been observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deep geological disposal is internationally recognized as the most feasible and effective way to dispose of high-level radioactive waste (HLW). The deep geological repositories are generally designed on the basis of a multi-barrier, multi-phased system concept mainly composed by engineered and natural barriers between the HLW and the biosphere. The buffer/backfill material is one of the most important components in the engineered barrier system and the last line of defense between waste container and host rock [1]. The buffer/backfill material is designed to stabilize the repository excavations and the coupled thermo-hydro-mechanical-chemical conditions, and to provide low permeability and long-term retardation. A bentonite-based material is often proposed or considered as a possible buffer/backfill material for the isolation of the HLW. At the present stage, the Gaomiaozi Na-bentonite is considered as the candidate buffer/backfill material for the Chinese repository [2].
It is known that the irregularities and roughness of a surface are attributed to the existence of distributed pore sizes that contribute to the value of the fractal dimension (D S). The magnitude of the surface D S of mesoporous materials has a great influence on such important physico-chemical processes as adsorption, surface diffusion, and catalysis. The surface D S of Na-bentonite can also be used to estimate the maximum swelling strain and swelling pressure in the disposal system of nuclear waste. A complete physical description of the state of Gaomiaozi Na-bentonite is of considerable interest. Therefore, the aim of this study is to evaluate the morphology, pore size distribution, and fractal geometry of Gaomiaozi Na-bentonite using the F–H–H method and the Freundlich isotherm model method. The application of different dimension characterization methods is a prerequisite to get complementary information about Na-bentonite size and shape.
Experimental
Material
Na-bentonite obtained from Gaomiaozi county (Inner Mongolia Autonomous Region, China) possessed the following chemical composition (mass%): SiO2, 69.17; Al2O3, 14.43; Fe2O3, 3.12; CaO, 1.29; MgO, 3.31; Na2O, 1.95; K2O, 0.83; FeO, 0.02; MnO, 0.04; TiO2, 0.13; loss on ignition, 5.40.
Characterization
Scanning electron microscope (SEM) measurements were performed with a JSM-6360 instrument by Japan Electron Optics Laboratory, operated at an accelerating voltage of 20 kV.
The nitrogen sorption experiments were performed using Micromeritics Gemini, Shimadzu. The sample was first degassed under vacuum until no significant changes in the vacuum were observed. The adsorbed amount of nitrogen was measured by volume at standard temperature and pressure. The specific surface area of Na-bentonite was calculated by fitting the adsorption data to the linear range of the BET equation.
The stock suspension of Na-bentonite and Pb(II) solution were mixed in the glass tubes to achieve the desired solid/liquid ratio at the temperature of 10 °C. The pH values of the solution were adjusted by adding negligible volumes of 0.1 or 0.01 mol/l HCl or NaOH. After the suspensions were shaken for 2 h, the solid and liquid phases were separated by centrifugation at 15,000g for 5 min (Himac CF 15R High-speed Micro Centrifuge, Shimadzu). The concentration of Pb(II) in the supernatant was determined by using an atomic absorption spectrometer. The flame type was air-acetylene and the absorption wavelength was 283 nm for Pb(II).
Methods
The D S is a parameter used to assess quantitatively the fractal geometry, and it represents a measure of the irregularities on the surface of a solid. The value for this parameter may vary from 2 to 3, whereby the lowest value (2) corresponds to a perfectly smooth surface, while the upper limit (3) corresponds to a totally irregular or rough surface. It is known that the irregularities and roughness of a surface are attributed to the existence of distributed pore sizes that contribute to the value of the D S [3]. Pfeifer et al. [4] derived surface D S from an analysis of multilayer adsorption to a fractal surface using the F–H–H method:
where V is the volume of adsorbed gas at an equilibrium pressure P, V m is the volume of adsorbed gas in a monolayer, P is the adsorption equilibrium pressure of the gas, P o is the saturation pressure of the gas at the given temperature, C is the pre-exponential factor, and A is a power law exponent dependent on D S and the mechanism of adsorption.
The van der Waals forces and the effect of surface tension (or capillary condensation) represent limiting cases; however, in general, the adsorption forces are a mixture of the two. At the early stages of adsorption (lower P and lower surface coverage), the effect of surface tension is negligible and the interaction between adsorbate molecules and the particles is mainly due to van der Waals forces. When the van der Waals forces dominate, A varied from −1/3 to 0. The relationship between A and D S can be expressed as follows:
However, at the late stages of adsorption (higher P and higher surface coverage), the effects of surface tension (or capillary condensation) become more pronounced and A varied from −1 to −1/3. The relationship between A and D S changes to
According to Eq. 1, a plot of ln(V/V m) versus ln[ln(P o/P)] should produce a straight line with a negative slope A, from which D S can be deduced.
Results and discussion
F–H–H(Frenkel–Halsey–Hill) method
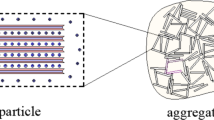
Scanning electron microscope photos of the rough Gaomiaozi Na-bentonite is shown in Fig. 1. As seen from Fig. 1, there was smaller contact area and larger average space between a particle and its neighborhood particles. The smaller contact area between particles proved that the particle surface is rough. The surface of Na-bentonite shows classical fractal characteristics.
The complete nitrogen isotherm is shown in Fig. 2. The isotherm belongs to a type IV isotherm according to IUPAC [5]. As usual, there is a hysteresis loop of the H3 type between adsorption and desorption. The isotherm gives useful information on the slit-shaped mesopore structure through its hysteresis loop. Hysteresis loops appear in the multilayer range of the physisorption isotherm. This is due to the fact that the interior pores are unable to empty during desorption unless the interior pores are connected to the exterior pores by a vapor path [6, 7]. The N2-BET surface area of the sample particles was 28.82 m2/g and the total pore volumes were up to 0.072 cm3/g.
The Na-bentonite was oven-dried overnight and the pore-size distribution by the Barrett–Joyner–Halenda method is given in Fig. 3. The total porosity is classified into three categories according to the pore diameter. The categories are: macropores (d > 50 nm), mesopores (2 < d < 50 nm), and micropores (d < 2 nm). Based on Fig. 3, Gaomiaozi Na-bentonite is mesoporous [8]. In general, the D S of the pore surfaces decreased, indicating a decrease in the complexity of the pore system.
Seen from Fig. 4, a plot of ln(V/V m) versus ln[ln(P o/P)] produces a straight line with a negative slope A, from which D S can be deduced. There is an excellent linear adjustment for the F–H–H method. The D S of 2.5870 agrees with the SEM of rough Na-bentonite [7]. Likewise, the surface D S by the F–H–H method is agreement with Xu’ data. The surface D S of Wyoming bentonite and Tsukinuno bentonite are 2.64 and 2.65, respectively, obtained from Xu et al. [9].
Freundlich isotherm model method
The Freundlich isotherm model is based on the adsorption of an adsorbate on a heterogeneous surface of an adsorbent and tested for predicting the equilibrium behavior of Pb(II) ions adsorption onto heterogeneous Na-bentonite. The Freundlich isotherm equation may be described as below:
where Q e is the amount of Pb(II) adsorbed per unit mass of Na-bentonite (mol/g) at the equilibrium, C e is the equilibrium concentration of Pb(II) (mol/l), and K F and n are Freundlich adsorption isotherm constants.
Fractal geometry provides fundamentals to describe the crude granule and its heterogeneity. The relationship between n of the Freundlich isotherm model and D S of porous Na-bentonite can be expressed as [10]:
The values of K F and n can be determined from the intercept and slope of the plot of log Q e versus log C e. D S can be deduced from n.
It is found that the Freundlich isotherm model describes the Pb(II) adsorption processes onto Gaomiaozi Na-bentonite relatively well, as shown in Table 1.
The Freundlich isotherm model shows that the adsorption–complexation reaction takes place in the adsorption processes. The Freundlich parameter n is a measure of the intensity of metal adsorption by the adsorbent. The value of n higher than 1 (n = 0.3921) indicates that the reaction for lead ions onto Na-bentonite is easily conducted. The value of n is very much in line with Karapinar’s data (1/2.56 = 0.3906) for cadmium ion on bentonite [11]. The value of n for zinc ion on Na-enriched bentonite and natural bentonite were 0.357 and 0.470, respectively, obtained by Kaya et al. [12].
The value of surface D S varies from 2 for a perfectly smooth surface to 3 for a very rough surface [5]. The D S of Na-bentonite of 2.3921 suggests that higher D S and the coarser surface of Na-bentonite can result in more spacious adsorption sites for metal ions. To some extent, the D S correlates with adsorption capacity.
Somewhat lower values are obtained by the Freundlich isotherm model than the F–H–H method. By comparison, the F–H–H method is more exact and the Freundlich isotherm model is more simple. This is because liquid-phase adsorption is a more complicated process than vapor-phase adsorption.
Conclusion
The porous structure of Gaomiaozi Na-bentonite was characterized quantitatively by both isothermal nitrogen adsorption and Pb(II) adsorption. The surface of Na-bentonite shows classical fractal characteristics. The fractal analysis is 2.5870 by means of the F–H–H method while the D S is 2.3921 by means of the Freundlich method.
References
A. Gens, L.D.N. Guimarães, S. Olivella, M. Sánchez, J. Rock. Mech. Geotech. Eng. 2, 97 (2010)
J. Wang, J. Rock. Mech. Geotech. Eng. 2, 1 (2010)
P.P. Srdan, M.V. Zorica, B.N. Tajana, P.N. Zoran, S.R. Ljikjan, J. Serb. Chem. Soc. 76, 1403 (2011)
P. Pfeifer, Y.J. Wu, M.W. Cole, Krim, J. Phys. Rev. Lett. 1989, 62 (1997)
P. Tang, Y.K.C. Nora, H.K. Chan, J.A. Raper, Langmuir 19, 2632 (2003)
K. Katsumi, J. Membr. Sci. 96, 59 (1994)
H. Qi, J. Ma, P.Z. Wong, Colloid Surf. A. 206, 401 (2002)
M.F. Cybelle, C.C. Kanb, M.L. Dalidac, K.J. Hsien, P. Chelo, W.W. Meng, Carbohydr. Polym. 83, 528 (2011)
Y.F. Xu, D.A. Sun, Y.P. Yao, Chaos Soliton Fract. 19, 347 (2004)
L.J. Xu, L.G. Gu, X.F. Xian, Coal Convers 23, 91 (2000). in Chinese with English abstract
N. Karapinar, R. Donat, Desalination 249, 123 (2009)
A. Kaya, A.H. Ören, J. Hazard. Mater. B125, 183 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Zr., Chen, Xs., Wang, Y. et al. Fractal dimension of Gaomiaozi Na-bentonite by different methods. Res Chem Intermed 41, 1683–1689 (2015). https://doi.org/10.1007/s11164-013-1303-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1303-0