Abstract
The synthesis, characterization and self-assembly of a novel amphiphilic block copolymer containing a poly(N-vinylpyrrolidone) as a segment of hydrophilic and poly(4-vinyl benzene chloride) (PVBC) arms are reported. The copolymer was characterized by FT-IR spectroscopy 1H NMR. The composition and the molecular weights of the block copolymers were established using gel permeation chromatography and 1H NMR. The water-soluble fraction of poly(N-vinyl-2-pyrrolidone) (PVP)/PVBC block copolymers formed micelles which were investigated at 25 °C in water at 5 mg/ml concentration using a tensiometer. The morphology of micelles in aqueous solution was determined by the AFM, SANS, and SAXS methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amphiphilic block copolymers consist of alternating hydrophilic and hydrophobic segments and can auto-arrange in an aqueous environment into polymeric micelles [1]. In fact, polymeric micelles made of amphiphilic block copolymers are very appealing as drug carriers, since their ability to solubilize in water-soluble drugs is poor in the inner hydrophobic core as is their ability to transport them in the biological environment, protecting them from inactivation and preventing their sudden release within body compartments [2]. Furthermore, they have great application potential, e.g., in the stabilization of latex particles [3], emulsions [4], dispersions [5], separations [6], and also as drug and gene delivery vehicles [7, 8]. Generally, several studies have used poly(ethylene glycol) as a hydrophilic segment which is known for its water-solubility, lack of toxicity, and excellent biocompatibility. Sometimes, it is selected as a hydrophilic buoy block whose function is to provide a hydrated steric barrier [9] and to offer micellar and nano- and microsphere morphology [10–13].
As a versatile polymer soluble in both water and organic solvents, poly(N-vinyl-2-pyrrolidone) (PVP) has been the focus of interesting applications including in additives, cosmetics, coatings, and biomedicines [14–16]. The coating of PVP can evidently result in improved blood compatibility with no need for a tissue engineering technique such as endothelial cell seeding [14]. The principal reason for successful PVP applications is its excellent biocompatibility with living tissues and extremely low cytotoxicity. PVP has also been used to modify the surface properties of polymeric membranes [17].
The present study reports the synthesis, characterization, and micellization of novel block copolymers with poly(4-vinyl benzene chloride)-b-poly(N-vinylpyrrolidone) arms. The poly(4-vinyl benzene chloride) (PVBC) polymer is extensively employed/utilized as hydrophobic segment in amphiphilic block copolymers, since it is highly hydrophobic and crystalline polyester, and a suitable component of the micelle inner core that favors the loading of hydrophobic drugs.
Poly(N-vinylpyrrolidone) is classic water single-grouped soluble polymer, while PVBC is insoluble in this solvent. Therefore, in the copolymer structure, they form a novel hydrophilic and hydrophobic compound. This particular amphiphilic property is a fundamental condition for the occurrence of self-assembly to form polymer aggregates in an aqueous solution.
The synthesis of these block copolymers have been achieved by the direct method which consists to deactivate the macrocation obtained by the cationic polymerization of 4-vinyl benzene chloride using heterogenic catalysis with H2SO4 as initiator. Scheme 1 represented the structure of the amphiphilic block copolymers.
Experimental
Materials
The N-vinylpyrrolidone (NVP) monomer and 4-vinyl benzene chloride (VBC) monomer were purchased from Aldrich and distilled under reduced pressure. Solvents (chloroform and hexane) were dried before use by means of standard procedures.
Measurements
FT-IR spectra were produced and identified using a Nicolat Avatar 320 FT-IR spectrometer in a KBr pellets at 4,000–400 cm−1 in air atmosphere. 1HNMR and 13C NMR spectra were produced and recorded at room temperature using a 400 MHZ Bruker spectrometer, using the solvent CDCl3. Chemical shifts (δ) are given in ppm using tetramethylsilane as internal reference. The average molecular weights and the polydispersity index PI were determined from GPC (gel permeation chromatography) using a high pressure liquid chromatography pump with an HP 1050 system equipped with a vacuum degasser and a refractive index detector. Tetrahydrofuran was used as the eluting solvent. The molecular weight calibration curve was obtained using polystyrene standards. Samples for AFM measurements were prepared as follows. A droplet copolymer solution by dissolving the star block copolymers directly in double-distilled water was dropped on a freshly cleaved mica substrate, which was rapidly frozen. Then, the frozen micellar solution on the mica wafer was lyophilized for 48 h to remove water. AFM images were recorded with a Digital Instruments Nanoscope III operated in tapping mode in air using microfabricated Si (type NCH) cantilevers with a spring constant varying between 27 and 53 N/m, a resonance frequency in the range of 301–365 kHz, and scanning speeds of 1 and 2 Hz. SAXS analysis were performed at the Research Centre Paul Pascal Bordeaux, It included a copper anode tube that has an operating voltage of 40 kV and a current of 35 mA. Optics consists of two Gobels mirrors (Kα line λ = 1.54 A). The beam is collimated by three “Pinholes”. A detector sound “Histar” Bruker with dimensions of 22 × 22 cm was placed at a distance D of the sample which enables the acquisition of a two-dimensional spectrum which is then integrated. This gives the scattered intensity I (q) in arbitrary units as a function of scattering vector q. The range of scattering vectors is between 0.0096 and 0.2 A−1 to D = 1 m. Analyses were performed on samples in the form of film and powder. The films were placed directly on the sample holder, while the powders, obtained by freeze-drying, were contained in a 1-mm-thick sealed glass Lindman capillary. The procedure is different from the SANS.
Synthesis of amphiphilic blocks copolymer
Amphiphilic block copolymers synthesis was realized by means of the direct method, which consists of starting with vinyl benzene chloride (6,44 mol/l) and using H2SO4 (0.01 mol/l) as initiator. Deactivation of the macrocation obtained by poly(NVP) (5,13 mol/l) led to the formation of the copolymer block poly(4-vinyl benzene chloride)-b-poly(N-vinylpyrrolidone).
Characterization of amphiphilic blocks copolymer
FT-IR (cast film): 2,920,6 cm−1 (γ CH), 1,423 cm−1 (d CH), and 754 cm−1 (γ C–Cl), 1105,9 cm−1 (γ C–O–C)(Fig. 1).
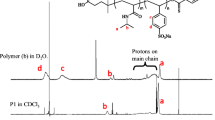
1H NMR (CDCl3): d = 3.7 ppm (CHCH2), 2.0 ppm (CH2CH2C–O), 1.7 ppm (CH2CH) and 1.1 ppm (CCH3), 7.26 ppm (CH benzene), 2.05 ppm (CH2Cl).(Fig. 2).
The mole fraction signals (spectra) of PVP in the PVP/PVBC copolymer was determined by 1H NMR according to the following equations [25]:
where I refers to the intensity of the peaks in the NMR spectrum, I
b is the intensity of the single peak of Hb and the overlapping peaks of Ha, c, e. The PVP content in the copolymer can go up to 52.2 %. 
The structure of the block copolymer was determined by means of 13C NMR analysis. The 13C NMR spectrum in CDCl3 is shown in Fig. 3. The peaks observed at 42.4–45.2, 35.7, and 31.7 ppm correspond to the –CH2– carbon atoms, and those observed at 173–178 ppm are attributed to carbonyl and –CH carbon atoms of the PVP segment.
Average molecular weights and the polydispersity index PI were determined from GPC analysis. The molecular weight calibration curve was obtained using polystyrene standards. The sample has Mw = 3,140, Mn = 1,795 and PDI = 1.075 (Fig. 4).
Given the presence of a hydrophilic–hydrophobic balance in the new block copolymer which has been prepared, we tried to exploit the amphiphatic characteristics of the copolymer in order to investigate the tensioactive properties of the copolymers.
The measurements were done by the technique of a ring on a CSC DuNOUY tensiometer. This equipment was operated in a range of 0 at 90 dyne/cm with a precision 0.05 dyne/cm at 25 °C. The ring used for measurements has a diameter of 60 mm, and it is made of platinuim.
Polymeric micelles formed by the water-soluble fraction of NVP/PVBC block copolymers were investigated at 25 °C in water at 5 mg/ml concentration. We used a tensiometer method to determine the critical micellar concentration of the sample.
The measurements of the relative superficial tension in the aqueous solution of the copolymer block show that there is a reduction in the superficial tension of water. A critical micellar concentration was found to be 0.593 mg/ml equal to (Fig. 5).
The results of the tensioactive properties allow us to conclude that this copolymer establishes a new class of non-ionic tensioactive compound. Further, to confirm the structure of the poly(VBC)-b-poly(NVP) micelle in aqueous solution, the sample was examined using AFM and by DRX 2D techniques. In this study, a thin NVP/PVBC copolymer layer on mica was prepared by in situ freeze-drying (Figs. 6, 7).
Conclusion
The synthesis and characterization of an amphiphilic novel block copolymer were performed. The purpose of the study is to make materials likely to have potential applications in some emerging fields and in particular with the aim of preserving the environment. The analysis by 1H NMR, FT-IR, and GPC confirmed the sequence copolymer of poly(4-vinyl benzene chloride)-b-poly(N-vinylpyrrolidone) that was made.
The results obtained may lead to a new route for the preparation of a series of amphiphilic block copolymers with sequences of poly(4-vinyl benzene chloride) and of poly(N-vinylpyrrolidone). The tensioactive properties of the copolymer allow us to conclude that the amphiphilic block copolymer established a new class of tensioactive non-ionic compounds. The AFM and SAXS analysis identified that the amphiphilic copolymer morphology has a cylindrical shape.
References
K. Kataoka, A. Harada, Y. Nagasaki, J. Adv Drug Deliv. Rev. 47, 113 (2001)
C. Allen, D. Maysinger, A. Eisenberg, J. Coll Surf. B 16, 3 (1999)
M. Gerst, M. Schuch, D. Urban, Assoc. polym. aqueous solut. media 765, 37 (2000). J.E. Glass (ed.)
R. Pons, B. Lindman (eds.), Self-assembly (Elsevier, Amsterdam, 2000), p. 409
K. Holmberg, in Self-assembly, ed. by P. Alexandridis, B. Lindman (Elsevier, Amsterdam, 2000), p. 305
M. Svenson, in Self-assembly, ed. by P. Alexandridis, B. Lindman (Elsevier, Amsterdam, 2000), p. 377
K. Kataoka, A. Harada, Y. Nagasaki, Adv. Drug Deliv. Rev. 47, 113 (2001)
A.V. Kabanov, E.V. Batrakova, V. Alakhov, J Control Release 82, 189 (2002)
T. Riley, C.R. Heald, S. Stolnik, M.C. Garnett, L. Illum, S. Davis, Langmuir 19, 8428 (2003)
V. Jeroen, F. Martin, L. Lizette, J.D. Teunis, A.T.W. Lia, L. Katja, J. Deinococcus geothermalis Polym. 4, 674 (2012)
V. Jeroen, F. Martin, L. Lizette, J.D. Teunis, A.T.W. Lia, L. Katja, J. Deinococcus geothermalis Polym. 4, 690 (2012)
Z. Chunfang, B. Yunxiang, S. Yuping, G. Jin, X. Youyi, J. Membr. Sci. 365(1), 216 (2010)
G. Ignacio, H. Marta, G. Ignacio, B. Jesús, M. Carmen López, J. Coll. Interface Sci. 359(2), 15,389 (2011)
G.M.R. Wetzels, L.H. Koole, J. Biomater. 20, 1879 (1999)
L.C. Lopergolo, A.B. Lugao, L.H. Catalani, Polymer 44, 6217 (2003)
G.J.M. Fechine, J.A.G. Barros, L.H. Catalani, Polymer 45, 4705 (2004)
S. Robinson, P.A. Williams, Langmur 18, 8743 (2002)
Acknowledgments
This work was performed in the laboratory of Chemical Physical Macromolecular, University of Oran, Algeria. The co-authors would like to thank CNRS of Marseille and Grenoble for helping with NMR and GPC measurements and Mullhose and Grenoble for helping with AFM and DRX (SAXS) measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nemiche, N., Sebba, F.Z. & Kada, S.O. Synthesis, characterization and self-assembly of amphiphilic block copolymer poly(4-vinyl benzene chloride)-b-poly(N-vinylpyrrolidone) in aqueous solution. Res Chem Intermed 40, 3127–3134 (2014). https://doi.org/10.1007/s11164-013-1158-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1158-4