Abstract
In this review different methods of preparing lanthanum aluminate (LaAlO3) phosphors are discussed. The molten salt method, the combustion method, the sucrose method, and the coprecipitation technique are the best methods for preparing LaAlO3 phosphors with small particle size and high surface area by low-temperature synthesis. LaAlO3 usually has a rhombohedral structure. It has good dielectric properties and, hence, is regarded as an attractive alternative to SiO2 in microelectronic devices. LaAlO3 phosphors have excellent chemical and thermal stability, mechanical durability, and exploitable optical and electronic properties, leading to a wide range of potential applications. LaAlO3 phosphors doped with rare-earth ions have luminescence properties and can, hence, be used in optical display systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lanthanum aluminate (LaAlO3) with a perovskite-type structure has, because of its properties, attracted much attention in recent years for many applications. Indeed, the material has good thermal stability with a high melting point, 2,180 °C, which can minimize interfacial dislocations [1]. Traditionally, LaAlO3 has been prepared by conventional solid-state reaction of Al2O3 and La2O3 in the temperature range 1,500–1,700 °C [2, 3]. But this typical method suffers from many inherent shortcomings, for example high-temperature heat treatment, which has a detrimental effect on grain size, limited chemical homogeneity, and low sintering temperature.
Several low-temperature (750–900 °C) methods are used to prepare finer, more homogeneous powders of LaAlO3; examples include the poly(vinyl alcohol) (PVA) with metal nitrate synthesis [4], sol–gel processes [5–7], the EDTA gel route [8, 9], co-precipitation methods [10, 11], pyrolysis using triethanolamine (TEA) [12], and combustion synthesis with urea as fuel [13, 14].
Review of the literature
Adak et al. reported preparation of pure LaAlO3 powders by evaporation of PVA added to a mixed metal nitrate solution. Precursor powders and calcined powders were characterized by differential thermal analysis (DTA), thermogravimetric analysis (TGA), X-ray powder diffraction (XRD), and infrared (IR) spectroscopy. The crystallite size ranged between 10 and 20 nm. The dielectric properties of the material were also measured [4].
Taspinar et al. reported that a promising candidate for ferroelectric substrate materials, lanthanum monoaluminate (LaAlO3), could be successfully synthesized by two separate chemical powder preparation techniques: homogeneous precipitation from aqueous solutions containing urea (CH4N2O) in the presence of nitrate salts, and self-propagating combustion synthesis from aqueous solutions containing CH4N2O and the nitrate salts of lanthanum and aluminium. The submicrometer, spherical-like particles of the precursors were completely converted to pure LaAlO3 at 850 °C by homogeneous precipitation; the conversion temperature was observed to be 750 °C, the lowest ever reported for powder synthesis of a pure LaAlO3 phase. The materials were characterized by powder XRD, simultaneous TGA and DTA, scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy. Structure refinement by Rietveld analysis showed that LaAlO3 was isostructural with BaTbO3 and had the space group R-3C, in contrast with the R-3M space group previously assumed for this phase. The atomic positions in the structure of LaAlO3 were refined and presented for the first time in this space group [15].
Jacobs et al. used molecular dynamics simulations to study the bulk and (100) surface of LaA1O3. Bulk and surface structures and vibrational spectra are reported. AlO and LaO surface termination were both studied. LaO termination was clearly more stable than AlO termination [16].
Kakihana et al. reported synthesis of LaAlO3 powders with large surface areas by the polymerized complex technique based on in-situ polyesterification between citric acid (CA) and ethylene glycol (EG). Heating of a mixed solution of CA, EG, and the nitrates of lanthanum and aluminium at 130 °C gave a brown transparent gel without any precipitation. The gel was preheated at 350 °C to give a black powdery mass, which was subsequently used as a precursor for LaAlO3. Formation of pure perovskite LaAlO3 occurred when the precursor was heat treated in a furnace set at 700 °C for 8 h or at 750 °C for 2 h. No XRD evidence of the presence of crystalline impurities was obtained. The LaAlO3 powder prepared by the polymerized complex method had an very large surface area, in the range 13–16 m2 g−1, compared with 0.3 m2 g−1 for the conventional solid-state reaction powder of the same compound [17].
Spinicci et al. investigated methane coupling at 600–750 °C over LaAlO3, La1−x M x AlO3 (M = Na, K, Ca, Ba, x = 0.1) and LaAl1−x M x O3 (M = Li, Mg, x = 0.1) perovskite-type catalysts, prepared by calcining the citrate precursors at 800 °C for 5 h. Introduction of the alkali and alkaline earth metals produces oxygen vacancies and increases the bond strength of both lattice and surface oxygen species. Substitution of Al3+ with Li+ and Mg2+ increases both catalytic activity and selectivity for C2 hydrocarbons in comparison with unsubstituted LaAlO3 perovskite. Diffusional control is suggested for oxidation to carbon oxides, whereas methane coupling should occur under kinetic control. The overall process involves a complex series of reactions. The results were rationalized on the basis of the structural properties of the catalysts and their adsorptive behavior towards oxygen, investigated by means of temperature-programmed desorption [18].
Hayward et al. used high-resolution X-ray rocking diffraction to measure the spontaneous strain associated with the cubic–rhombohedral phase transition in LaAlO3 in the range 10 ≤ T ≤ 750 K. The results were consistent with a second-order Landau-like model at high temperatures, with T C = 834(2) K. At lower temperatures, the strain data display order parameter saturation, related to quantum saturation of the phonon modes. Comparison of the saturation temperature for the spontaneous strain (θ S = 95 K) with the saturation temperatures for independent measurements of the rotation (θ S = 260 K) and distortion (θ S = 150 K) of the AlO6 octahedra reveals that the phase transition consists of two coupled processes, and that coupling does not have the same effect in the classical and quantum saturation limits [19].
Deren et al. reported the absorption, time-resolved emission spectra, and decay times of LaAlO3:Eu3+. It was found that emission by LaAlO3:Eu3+ crystals is very efficient. Observed peaks were assigned to 5D3,2,1,0 → 7FJ transitions. The strongest emission observed was that from the 5D0 level to the 7F1,2,4 levels. Decay time of the 5D0 emission was 2.44 ms at 4.5 K, with weak dependence on temperature. Decay times of the 5D1 and 5D2 levels were short, and highly dependent on temperature. Energy levels of Eu3+ in LaAlO3 were assigned [20].
Xiang et al. reported epitaxial growth of LaAlO3 films on Si (100) substrates by inserting an SrO or SrTiO3 buffer layer by use of a computer-controlled laser molecular beam epitaxy system. Structural characterization indicated that the LaAlO3 films were two-dimensional (2D) layer-by-layer growth. Atomic force microscopy (AFM) observations revealed that the surfaces of the epitaxial LaAlO3 films were atomically smooth. The crystallinity of the LaAlO3 films determined by XRD and high-resolution transmission electron microscopy (HRTEM) was a single-crystalline structure. After annealing at 1,050 °C in N2 for 5 min, the crystallinity of the LaAlO3 film clearly improved. Successful LaAlO3–SrO–Si and LaAlO3–SrTiO3–Si epitaxial growth suggested the possibility of development of 3D heterostructures on Si in a new generation of microelectronics devices [21].
Deren et al. reported measurement of absorption, emission, and emission decay times of Ho3+ ions embedded in an LaAlO3 single crystal. The experimental results were analyzed on the basis of Judd–Ofelt (J–O) theory. Energy levels, oscillator strengths, and probabilities of radiative transition were determined. The results indicated non-radiative transitions occurred because of cross-relaxation down and up-conversion processes [22].
Fidancev et al. reported measurement of the absorption and emission of Er3+-doped LaAlO3 crystals at room and low temperature (10 K). Preliminary crystal-field calculations were performed on the basis of these experimental results. The energy levels of Er3+ in LaAlO3 were well reproduced [23].
Busani et al. reported use of grazing incidence X-ray reflectivity measurements to determine the density of sputter-deposited LaAlO3 and anodized LaAl films. The results, with refractive index and dielectric constant measurements, resulted in a coherent explanation of the low dielectric constant of the amorphous films (~13) compared with the single-crystal value (~26). The importance of the dependence of molecular volume on electronic and vibrational molecular polarizabilities was emphasized [24].
Chang et al. reported details of a process for preparation of an Eu2+-activated long-lasting Sr4Al14O25 nano-sized phosphor by the precipitation method. SEM, simultaneous differential scanning calorimetry (DSC)–TGA, XRD, photoluminescence spectroscopy (PLS), and thermal luminescence spectroscopy were used to characterize the phosphor. Nano-scale SrAl2O4:Eu2+Dy3+ and Sr4Al14O25:Eu2+Dy3+ phosphors were obtained by calcining the precipitated precursors at 1,200 and 1,300 °C, respectively. Both the low-temperature product SrAl2O4:Eu2+Dy3+ and the high-temperature product Sr4Al14O25:Eu2+Dy3+ emitted photoluminescence (PL) on ultraviolet (UV) illumination; emission peaks were at 480 and 505 nm, respectively. Compared with the emission spectrum of the powder obtained by use of the conventional method, a blue shift was observed for the nano-sized powders, because of the decrease in grain size. These two phosphors had a long-persistence afterglow; that of the Sr4Al14O25:Eu2+Dy3+ phosphor was better than that of the SrAl2O4:Eu2+Dy3+ phosphor, because of a deeper trap level and a higher trap concentration in the host material [25].
Hreniak et al. reported the preparation, morphology, and structural properties of Eu3+-doped LaAlO3 nano-crystallites prepared by Pechini’s (Pe) method. The effects of annealing temperature and Eu-ion content on the process of formation of the nano-crystallites were investigated. Preliminary studies were conducted on the effect of LaAlO3 nano-crystallite size on the luminescence properties of the Eu ions. Time-resolved luminescence spectra and decay times were recorded for powders of different grain size. It was found that nano-crystallite size affected radiative relaxation of Eu3+ luminescence [26].
Deren et al. reported observation of anti-Stokes intense green emission after direct excitation of the 5F5 level of Ho3+ in LaAlO3. Up-conversion was observed, because of strong excited state absorption (ESA) in which two intermediate levels the 5I7 and the 5I6, were involved. Power dependence of the anti-Stokes emission depends on excitation wavelengths. The mechanism of the ESA was discussed in detail [27].
Ishigaki et al. used a novel “melt synthesis technique”, rather than conventional solid-state reaction techniques, to synthesize a variety of perovskite ABO3-type compounds and their solid solutions. In the melt synthesis, the mixture of oxides or their precursors is rapidly (1–60 s) melted by irradiation with intense light in an arc-imaging furnace. A spherical molten sample in which cations were mixed homogeneously was directly solidified on a copper hearth with rapid cooling of 102 K/s. LaAlO3, GdScO3, ATiO3 (A = Ba, Sr and Ca), and their mixed solid solutions were synthesized by use of this technique [28].
Tian et al. reported preparation of pure LaAlO3 powder by combustion synthesis from a concentrated solution of the nitrates of lanthanum and aluminate as oxidizer and glycine acid as fuel, with the objective of obtaining nano-sized crystallites of the material with high specific area at relatively low temperature. Precursor powders and calcined powders were characterized by DTA, TGA, XRD, and TEM. The results showed that pure perovskite LaAlO3 powder of particle size 78–100 nm was formed by treatment at 700 °C for 2 h. The specimen sintered at 1,500 °C for 12 h had maximum bulk density and the best microwave dielectric properties: ε r = 23 and Q f = 38,000 GHz [29].
Kuo et al. reported preparation of nano-crystalline lanthanum monoaluminate (LaAlO3) powders by chemical coprecipitation and use of 25 % v/v NH4OH, 0.05 M La(NO3)3·6H2O, and 0.05 M Al(NO3)3·9H2O aqueous solutions as starting materials. Fourier-transform IR spectroscopy (FTIR), TGA–DTA, XRD, Raman spectrometry, specific surface area (BET) analysis, SEM, TEM, and electron diffraction (ED) were used to characterize the LaAlO3 powders obtained. The crystallization temperature of the LaAlO3 precursor gels precipitated at pH 9 was estimated to be 810 °C by TGA–DTA. The XRD patterns of LaAlO3 precursor gels precipitated at pH 8–12 and calcined at 700 °C for 6 h contained a broad arciform continuum between 24° and 32° and sharp peaks of LaAlO3, except for precursor gels precipitated at pH 9. When the LaAlO3 precursor gels were precipitated at pH 9 and calcined at 700 °C for 6 h the perovskite LaAlO3 phase was formed, and the presence of crystalline impurities was not observed. The crystallite size of LaAlO3 increased slightly from 37.8 to 41.5 nm when the calcination temperature was increased from 700 to 900 °C for 6 h. LaAlO3 powders prepared by chemical co-precipitation have a very large specific surface area of 30 m2 g−1. The relative density is >97 % when these nano-crystalline LaAlO3 powders are sintered at 1,550 °C for 2 h [30].
Li et al. reported synthesis of rhombohedral LaAlO3 powder by reacting equimolar La2O3 and Al2O3 in a molten KF–KCl eutectic salt for 3 h between 630 and 800 °C. The lowest synthesis temperature (630 °C) is approximately 1,000 degrees below that of conventional mixed oxide synthesis, and close to or lower than those used by most wet chemical methods. LaAlO3 particle size increased from <3 to 3–7 μm when the temperature was increased from 630 to 700 °C, but changed little on further increasing the temperature to 800 °C. Particle size decreased when the salt-to-oxide weight ratio was increased from 1:1 to 6:1. The “dissolution–precipitation” mechanism is important in the molten salt synthesis of LaAlO3 [31].
Behera et al. reported that LaAlO3 ceramic powders could be prepared from metal chlorides by a combined gel precipitation process using ammonia. The conventional gel precipitation technique was slightly modified by introducing an ultrasonication step followed by centrifugal washing of the gel. The dried gels produced pure-phase LaAlO3 powders on calcination of the combined gel-precipitated (GP) powders at 1,100 °C and calcination of the washed gel (WG) at 600 °C. The phase evolution was studied and it was found that the delay in obtaining monophasic LaAlO3 in the combined GP powder was because of crystallization of an impure phase, LaOCl. This phase was not detected in the WG powders. TEM micrographs revealed uniform morphology of the calcined WG powders, in contrast with the irregular particles in the GP powders. The uniform morphology was attributed to ultrasonic effects during washing of the gel [32].
Ran et al. reported successful synthesis of LaAlO3 powders by pyrolysis of complexes of lanthanum and aluminum with TEA. The precursors and the derived powders were characterized by simultaneous TGA and DSC analysis, XRD, specific surface area measurements, and TEM. Pure LaAlO3 phase was obtained at 775 °C for 2 h or 750 °C for 4 h, without formation of any intermediate phase. TEM images revealed pores in LaAlO3 powders prepared at 800 °C for 2 h [12].
Luo et al. reported investigation of the stable structure, phase transition, and elastic properties of LaAlO3 by use of first principles linearized augmented plane wave calculations within density functional theory. Calculation reveals that at low temperatures the rhombohedral R-3C phase is the most energetically stable of the three proposed structures: R-3C (No. 167), R-3M (No. 166), and R-3C (No. 161). It was found that LaAlO3 is transformed from the rhombohedral R-3C phase to the cubic PM-3M phase, with a volume change of 1 %, when the applied hydrostatic pressure is 15.4 GPa; this is consistent with experimental results. The elastic constants, shear modulus, bulk modulus, and Poisson’s ratio of LaAlO3 were calculated and compared with corresponding experimental data. The results showed that rotation of the AlO6 octahedra in LaAlO3 substantially affects the anisotropic elastic constants. From the calculated Debye temperature and elastic constants, the R-3C phase of LaAlO3 is predicted to be more thermostable and have greater fracture toughness than the high-pressure-generated PM-3M phase [33].
Deren et al. investigated LaAlO3 single crystals doped with Tm3+ ions. After continuous-wave excitation of the 3F2 level (at approx. 15,115 cm−1) strong violet and UV light were observed, corresponding to the 1D2 → 3F4 (22,000 cm−1), 1D2 → 3H6 (27,700 cm−1), and 3P0 → 3F4 (28,600 cm−1) transitions. Emission and excitation spectra of the Stokes and anti-Stokes emission were measured. The excitation and absorption spectra were compared and the power dependence of the anti-Stokes emission was recorded and analyzed. Possible mechanisms of ESA and cross-relaxation involving three or more photons are discussed [34].
Luo et al. investigated the electronic structure, chemical bonding, and optical properties of rhombohedral LaAlO3 by use of the full potential linearized augmented plane wave (FP-LAPW) method with the generalized gradient approximation (GGA). Analysis of the electronic density profile, Mulliken charge, and bond population revealed both covalent and ionic nature of the chemical bonding. The calculated complex dielectric function is consistent with experimental results from UV spectroscopic ellipsometry measurement. The optical spectra were assigned to the interband transition from O valence to La conduction bands in the low-energy region. The absorption spectrum, the electron energy-loss spectrum, optical conductivity, reflectivity, and refractive index were, furthermore, derived from the complex dielectric function. The absorption spectrum is indicative of an optical band gap of 6.1 eV, which is consistent with several other experimental measurements [35].
Singh et al. prepared holmium-doped LaAlO3 powder phosphors at furnace temperatures as low as 500 °C by combustion without further calcination. Powder XRD and FTIR spectrometry measurements were used to characterize the products, and their optical properties were studied by use of UV–visible–NIR and PLS. The J–O model was used to obtain oscillator strengths (f) and three phenomenological intensity values. By use of J–O values (Ω 2, Ω 4 and Ω 6) the radiative transition probabilities (A ab), radiative lifetimes (τ R), and branching ratios were calculated for some excited states of Ho3+. By use of the Fuchtbauer–Ladenberg formula, the stimulated emission cross-sections (semi) for some interesting transitions, for example 5S2 → 5I8 and 5F5 → 5I8, of Ho3+ in LaAlO3, were determined and discussed [36].
Deren et al. reported the spectral and laser properties of an Nd3+-doped (1 % w/w) LaAlO3 single crystal. The energy levels of the Nd3+ ion in the LaAlO3 matrix were assigned. The J–O values Ω 2 = 1.346, Ω 4 = 4.490, and Ω 6 = 5.168 (all ×10−20 cm−2) were evaluated. The absorption σ abs and emission σ emi cross-sections at the respective pumping and emission wavelengths were calculated to be σ abs(789.7 nm) = 2.42 × 10−20 cm2, σ emi(909 nm) = 5.02 × 10−20 cm2, and σ emi(1,080 nm) = 7.03 × 10−20 cm2. Continuous-wave laser action at 1,080 nm was obtained for LaAlO3:Nd3+ [37].
Gocalinska et al. reported results from spectroscopic research on an LaAlO3 crystal doped with Tm3+ ions. The host has the perovskite structure. The investigated sample was a single crystal grown by the Czochralski method. Absorption, emission, and decay profiles were measured in the visible and IR regions at room and low (77 K) temperature. By excitation matching emission from several excited states of the 1D2 (27,700 cm−1), 1I6 + 3P0 (28,600 cm−1), 3H4 (12,400 and 6,800 cm−1), and 3F4 (5,800 cm−1) levels was obtained; with these strong bands other weaker transitions were also recorded. The decay times were short for the violet and blue emission and significantly longer for the red and IR emission (at room temperatures the values were 22 ls and 5.8 ms for the 1D2 and 3F4 levels, respectively). The system is discussed in terms of Tm3+ energy level structure. Further investigations are being considered [38].
Kharlamova et al. reported preparation of Al and Sr-doped apatite-type lanthanum silicates (ATLS) by mechanochemical activation (MA) and use of the Pe method; some structural and electrical properties of the doped samples were studied. MA results in ATLS formation even at room temperature after 20–35 min activation. Synthesis by the Pe method occurs via a solid-state reaction mechanism. MA of carbonate precursors obtained by use of the Pe method results in the formation of a single-phase ATLS after annealing at 900 °C. For Al-doped apatite samples, formation of LaAlO3 as a secondary phase is observed at high substitution levels, depending on sample stoichiometry, which affects the properties of ceramics obtained [39].
Deren et al. reported calculation of rates of multiphonon non-radiative transitions, W MNR, from rates of radiative transitions and measured emission decay times for praseodymium, neodymium, erbium, holmium, and thulium ion-doped LaAlO3. Radiative transition rates were determined on the basis of J–O theory. The results obtained were plotted as a function of energy gap DE and fitted by use of an exponential function W MNR = B·exp(−α·ΔE), where B = 1.02×109 and α = 3.61 × 10−3. Fitting results showed that rates of multiphonon non-radiative transition were low compared with those of other oxide crystals, and were similar to those of YAG [40].
Chang et al. reported that LaAlO3 had potential as a gate dielectric for future very-large-scale integration devices. In this work, metal-oxide–semiconductor capacitors and transistors were fabricated with LaAlO3 gate dielectric and the electron mobility degradation mechanisms were studied. The LaAlO3 films were deposited by radiofrequency magnetron sputtering. The LaAlO3 films were examined by XRD, secondary ion mass spectroscopy, and X-ray PLS. The temperature dependence of metal-oxide–semiconductor field-effect transistor characteristics was studied from 11 to 400 K. The rate of threshold voltage change with temperature (ΔV T/ΔT) was −1.51 mV/K. Electron mobility, which is limited by surface roughness, is proportional to E −0.66eff in electric fields of 0.93 < E eff < 2.64 MV/cm at 300 K and phonon scattering is proportional to T −5.6 between 300 and 400 K. Soft optical phonon scattering was used to explain the extra source of phonon scattering in LaAlO3-gated n-channel metal-oxide–semiconductor field-effect transistors [41].
Liu et al. reported preparation of nano-crystalline LaAlO3:Sm3+ phosphors by a Pe-type sol–gel process. XRD, field emission SEM (FESEM), PL, and cathodoluminescence (CL) spectra were used to characterize the synthesized phosphors. XRD results revealed that the sample begins to crystallize at 600 °C, and the pure LaAlO3 phase can be obtained at 700 °C. FESEM images indicate that the Sm3+-doped LaAlO3 phosphors are composed of aggregated spherical particles with sizes ranging from 40 to 80 nm. On excitation of the Sm3+-doped LaAlO3 phosphors by UV light (245 nm) or low-voltage electron beams (1–3 kV) the characteristic yellow emission of Sm3+ (4G5/2–6H5/2, 6H7/2, 6H9/2 transitions) is observed. The CL intensity (brightness) of the Sm3+-doped LaAlO3 phosphor is greater than that of the commercial product [Zn (Cd) S:Ag+] (yellow) [42].
Chandradass et al. reported synthesis of pure LaAlO3 nano-powders by use of an emulsion precursor derived from a mixed-metal and oleic acid solution. The precursors and derived oxide powders were characterized by DTA, TGA, IR spectroscopy, XRD, and TEM. The pure LaAlO3 phase was synthesized at 800 °C for 2 h, in air, directly from amorphous precursors, without formation of an intermediate phase. The average particle size determined by TEM was 60 nm. FTIR analysis was used to monitor elimination of the oleic acid from the emulsion-derived precursor and calcined powder [43].
Yu et al. reported synthesis of pure LaAlO3 nanoparticles by use of a citrate-precursor technique. La(NO3)3, Al(NO3)3, and C3H4(OH)(COOH)3, in the molar ratio 1:1:4.5, were dissolved in deionized water. The pH of the aqueous solution was adjusted by use of aqueous NH3. After drying, the citrate precursors were charred at 350 °C, then calcined at different temperatures. The thermochemical behavior of the charred citrate precursor in the formation of LaAlO3 was investigated by use of XRD, IR spectroscopy, TGA, and DTA. Whereas the charred specimen obtained at pH 2 (without addition of aqueous NH3) was composed of LaAl(OOCH2)3 the charred specimens obtained at pH > 2 were composed of LaAlO3−x−y(CO3) x (OH)2y . All these metallic salts were decomposed at temperatures between 600 and 780 °C to form crystalline LaAlO3 but calcination of the specimens in air at ≥800 °C was required to remove all residual charring and produce pure LaAlO3. At 900 °C, the citrate-derived particles obtained at pH > 2 were composed of LaAlO3 crystallites of average size ~30 nm [44].
Tian et al. reported preparation of spherical LaAlO3 nanoparticles in a reverse microemulsion consisting of solution (water phase), Tween-80 and Span-80 (surfactant), n-butanol (cosurfactant), and cyclohexane (oil phase). Precursor powders and calcined powders were characterized by DTA, TGA, XRD, and TEM. Pure perovskite LaAlO3 was formed when the precursor hydroxides were calcined at 800 °C for 2 h. Particle size was approximately 50 nm and the monodisperse particles were spherical. By use of the reverse microemulsion process the crystallization temperature of LaAlO3 can be dramatically reduced by approximately 700 °C compared with that used in the classical solid-state reaction method [45].
Chandradass et al. reported synthesis of lanthanum monoaluminate (LaAlO3) nanoparticles by use of microreactors containing poly(oxyethylene) nonylphenyl ether (Igepal CO-520)–water microemulsions. Control of particle size was achieved by varying the water-to-surfactant molar ratio. The synthesized and calcined powders were characterized by TGA–DTA, XRD analysis, SEM, TEM, and FTIR spectroscopy. DTA showed that LaAlO3 phase transformation decreased with increasing water-to-surfactant ratio. The pure LaAlO3 phase was synthesized, without formation of intermediate phase, by direct annealing of the amorphous precursors at 800 °C for 2 h in air. The average particle size was found to increase with increasing water-to-surfactant ratio. FTIR analysis was used to monitor elimination of residual oil and surfactant phases from the microemulsion-derived precursor and calcined powder [46].
Negahdari et al. reported synthesis of nano-crystalline LaAlO3 powder by calcination of a precursor prepared by evaporation of an aqueous solution of sucrose, PVA, and stoichiometric amounts of the desired Al and La nitrates. Phase evaluation (XRD) in conjunction with thermal analysis (DTA–TGA) showed that pure nano-crystalline LaAlO3 phase powder was obtained at temperatures between 600 and 700 °C. The average crystallite size of the synthesized powder, obtained by Rietveld analysis and TEM, was approximately 30 nm. Average particle size, as determined by FESEM, was <100 nm. The average specific surface area of the powder was very high ~43 m2 g−1. According to the electrokinetic behavior of synthesized LaAlO3 power, it had a point of zero charge (pzc) at approximately pH 9.9 [47].
Boudali et al. studied the structural, elastic, and electronic properties of perovskite LaAlO3 by use of two different methods—the FP-LAPW method and the pseudopotential plane wave scheme with the GGA. They evaluated ground-state quantities, for example lattice properties, bulk modulus, and pressure derivative, and the elastic constants. They also reported results from measurement of band structure, densities of states, and charge densities. These results were in good agreement with previous theoretical work and other experimental results. To complete determination of the fundamental characteristics of this compound they analyzed the thermodynamic properties by use of the quasi-harmonic Debye model [48].
Deren et al. studied the symmetry of LaAlO3 nano-crystals as a function of crystallite size. Properties of LaAlO3 nano-crystallites obtained by the precipitation method; doped praseodymium and chromium ions were examined spectroscopically. By use of Raman spectroscopy, HRTEM, XRD, and electronic spectroscopy they proved that the symmetry of LaAlO3 crystallites depends on their size. At room temperature the smallest crystals obtained (average size ~5 nm) have cubic symmetry whereas the largest (average size >110 nm) have rhombohedral symmetry. Possible explanations for this phenomenon were discussed [10].
Zhang et al. reported construction of the complete d 3 energy matrix, including the cubic crystal field, coulombic interactions, spin–orbit coupling, and the low-symmetry crystal field, for LaAlO3:Cr3+ on the basis of the strong scheme of ligand field theory and non-coupling trigonal bases. By diagonalizing the complete d 3 energy matrix, the energy levels, wavefunctions, and crystal-field data were calculated for LaAlO3:Cr3+ at normal pressure. The g factor of the ground state under normal pressure and low temperature was calculated on the basis of these results and proved to be consistent with experiment data. By taking into account the wavefunctions and thermal shift theory, the thermal shifts of the R 1 line of LaAlO3:Cr3+ were calculated and the related values were determined. All the results were in good agreement with experimental results. The results also revealed more completely the physical origin and micro-mechanism of R 1 line thermal shifts [49].
Mao et al. used the PL and lifetime decay properties of Eu of different valence to investigate the mechanism of emission of green luminescence at ~515 nm for full color emission LaAlO3 phosphors co-doped with Eu2+ and Eu3+. The green emission was assigned to enhanced 5D2 → 7F3 transition emission of Eu3+. Energy transfer between Eu2+ and the 5D2 level of Eu3+, resulting in enhancement of the 5D2 → 7F3 transition emission, was proposed. In addition, energy-transfer relationships between host Eu and charge transfer state-Eu were also discussed, in relation to PLE (PL of excitation) spectra and band schemes [50].
Liu et al. motivated by the recent discovery of superconductivity at the LaAlO3–SrTiO3 heterointerface, conducted a theoretical investigation of impurity-induced resonance states with coexisting spin singlet s-wave and triplet p-wave pairing symmetries by considering the effect of Rashba-type spin–orbit interaction (RSOI). Because of the nodal structure of the mixed gap function, single non-magnetic impurity-induced resonance peaks occur in the local density of state. They also analyzed, by point contact tunneling and scanning tunneling microscopy, the evolution of density of states and local density of states with the weight of the triplet pairing component determined by the strength of the RSOI, which will be widely observed in thin films of superconductors with surface or interface-induced RSOI or different non-centrosymmetric superconductors, and thus shed light on the admixture of the spin singlet and RSOI-induced triplet superconducting states [51].
Deren et al. reported the preparation, by the precipitation method, and spectroscopic properties of nano-sized crystallites of LaAlO3 doped with Pr3+ (1 mol%) and traces of Cr3+ ions. A new material was obtained with spectroscopic properties differing nominally from those of the same bulk of LaAlO3:Pr3+. It was observed that when the diameter of the nano-LaAlO3 sample was >110 nm the perovskite structure changed from cubic to rhombohedral. As a result, the intensity of emission by larger samples was two orders of magnitude higher than that for smaller samples [52].
Malinowski et al. investigated the concentration-dependent emission spectra and fluorescence dynamic profiles of Pr x La1−x AlO3 single crystals to better understand the processes responsible for concentration quenching of the praseodymium 3P0 and 1D2 emissions. The rates of cross-relaxation transfer were experimentally determined as a function of Pr3+ concentration. Decays were modeled and nearest-neighbor trapping rates were calculated [53].
Mortada et al. studied initial Si growth mechanisms on LaAlO3 (001), a crystalline oxide with a high dielectric constant (high-k material). The clean LaAlO3 (001) substrate has a c(2 × 2) structure that can be attributed to surface O vacancies. Deposition of Si by molecular beam epitaxy was studied as a function of both deposition temperature and thickness. Epitaxy was obtained only above 550 °C. In this case, a Volmer–Weber mode is observed. The associated nano-dots are relaxed and formed by pure Si, as ascertained by monitoring the Si2s XPS peak, which remains for 1 and 10 ML at the binding energy corresponding to Si–Si bonds. Moreover the islands have an abrupt interface with the LaAlO3 (001) substrate without formation of silicate or silica. A unique epitaxial relationship between LaAlO3 and the crystallized Si islands, in which the Si (001) planes are parallel to the LaAlO3 (001) planes, but rotated by 451 in the (001) direction, is indicated by RHEED (reflection high-energy ED) and confirmed by HRTEM. This orientation leads to mismatch and strain minimization of the Si film [54].
Dudek et al. reported the luminescence properties of Y2O3 and LaAlO3 nano-powders doped with Pr3+ ions and of PMMA (poly(methyl methacrylate))-based composite materials doped with these powders. Active nano-powders differing in praseodymium ion concentration were prepared by use of a sol–gel method and their emission properties in the visible spectral range were carefully characterized. In particular, the excitation and emission spectra were measured, with their fluorescence decay profiles, and the differences between the optical properties of these materials were discussed and compared with data available for the bulk materials. Finally, PMMA-based composite materials doped with Pr3+:Y2O3 and Pr3+:LaAlO3 nano-powders were manufactured and characterized. The results obtained showed that polymer composites doped with active nano-powders tended to retain the luminescence properties of the original nano-powders [55].
Yu et al. reported the synthesis of crystalline LaAlO3 nanoparticles at relatively low temperatures by use of a citrate-precursor technique. La(NO3)3, Al(NO3)3, and C3H4(OH)(COOH)3, in the molar ratio 1:1:1, were dissolved in deionized water. Aqueous NH3 was used to adjust the aqueous solution to pH 7. After drying, the citrate precursors were charred at 350 °C, followed by calcination at different temperatures, in air or oxygen atmosphere. The thermochemical properties of the resulting particles were analyzed by use of TGA and DTA, XRD, IR spectroscopy, SEM, and TEM. Effects of calcination temperature and heating atmosphere on the formation of crystalline LaAlO3 nanoparticles were investigated. In O2 atmosphere, calcination of the citrate-derived charred solid precursor at 700 °C for 3 h decomposed all intermediates, producing pure LaAlO3 nanoparticles (particle size ≤100 nm) with an average crystallite size of approximately 24 nm and high sinterability [56].
Liu et al. studied the pulsed laser deposition and growth of a high-k dielectric lanthanum aluminate LaAlO3 (LAO) thin film on an indium tin oxide–glass substrate at different oxygen partial pressure. On the basis of a pulsed laser deposition growth mechanism we can explain how different oxygen partial pressures affect surface roughness, formation of an interfacial layer, and the transparent resistive switching characteristics of LAO thin films. The micro-structure and oxygen concentration difference inside LAO thin films may be the main reason for the different electrical and resistive switching properties. Films grown at higher oxygen partial pressure had more reliable resistive switching performance, because of formation of the interfacial layer and a lower concentration of oxygen vacancies. The interfacial layer serves as a good oxygen reservoir and the involvement of more oxygen ions ensures switching reliability. Migration of oxygen ions between the interfacial layer and the LAO film under the applied bias may be the switching mechanism [57].
Yamasaka et al. reported that, by measuring electron spin resonance (ESR) spectra at the X-band frequency and absorption spectra from the visible to UV region at room temperature, they confirmed that perovskite single-crystal LaAlO3 contains Cr and Fe as impurities. When LaAlO3 is exposed to photons with energy >4.5 eV, the intensities of ESR signals from Cr3+ and Fe3+ decrease, which indicates that electrons released by the photon irradiation are captured by Cr3+ and Fe3+. Concurrently with this, a broad optical absorption band at approximately 3.0 eV and two new broad and weak ESR signals appear. The former was attributable to a combination of a hole and an La3+ (or Al3+) vacancy, and the two weak ESR signals were assigned to the O− center and F+ center [58].
Maczka et al. reported the synthesis of LaAlO3, La0.9Dy0.1AlO3, La0.9Er0.1AlO3, and La0.8Dy0.1Er0.1AlO3 nano-crystalline powders by a two-step process combining a mechanically induced metathesis reaction and molten salt synthesis. The proposed two-step method gives ready access to pure and/or doped perovskite-type LaAlO3 nano-powders at remarkably low temperatures, i.e., even at 350 °C although firing at 500 °C is needed to obtain the pure phases. The samples obtained were characterized by XRD, TEM, Raman, IR, and luminescence methods. These methods showed that mean crystallite size is approximately 50–60 nm and the LaAlO3 nano-crystallites have the R\( \overline{3} \)c structure, the same as for bulk LaAlO3. The Raman spectrum of nano-crystalline LaAlO3 is very similar to that of bulk. In contrast with this behavior, IR spectra of the synthesized compounds were significantly different from the IR spectrum of bulk LaAlO3. The origin of this behavior was discussed. Luminescence study showed that cross-relaxation processes quench the emission of samples doped with Dy3+ and Er3+ [59].
Khamkar et al. reported obtaining nano-structured LaAlO3 by self-combustion synthesis using lanthanum nitrate and aluminium nitrate as precursors and glycine as fuel, without subsequent heat treatment after synthesis. The effect of temperature variation was investigated for a sample of constant molar ratio. The crystallinity (phases present and crystallite size: estimated by the single-line method) of the product obtained was determined by XRD measurement, TGA–DTA, SEM, and TEM. This synthetic method facilitated production of perovskite LaAlO3 with crystallite size between 40 and 70 nm [60].
Djoudi et al. reported the synthesis and characterization of lanthanum monoaluminate LaAlO3 by the method of co-precipitation. The powder was successfully synthesized by use of NaOH, La(NO3)3·6H2O, and Al(NO3)3·9H2O as raw materials and calcination at different temperatures. It was characterized by several techniques: FTIR, TGA–DTA, XRD, and laser diffusion. All the results from physicochemical characterization showed that the crystallization temperature of the precipitated LaAlO3 precursor gels was 790 °C. The XRD pattern showed that calcination of the LaAlO3 precursor gels at 700 °C for 6 h results in a rhombohedral hexagonal phase with the perovskite structure; the presence of crystalline impurities was not detected. The crystallite size of LaAlO3 increased slightly from 31 to 44.5 nm when the calcination temperature was increased from 700 to 1,000 °C, again for 6 h [61].
Mendoza et al. reported the preparation of perovskite-type LaAlO3 nanoparticles by a facile, rapid, and environmentally benign molten-salts method in which alkali metal nitrates were used as low-temperature fluxes. Starting from hydrated lanthanum and aluminum nitrates and alkali metal hydroxides, the proposed method consists of two steps—a mechanically induced metathesis reaction then brief firing at temperatures above the melting points of the nitrates. The purpose of the first step is twofold—in situ generation of the alkali metal nitrate flux and formation of an La and Al-containing precursor material suitable for synthesis of bulk LaAlO3 nanoparticles in molten nitrates. Different alkali metal nitrates and eutectic mixtures were used to investigate the effect of melt basicity in the reaction outcome. Single-phase LaAlO3 was obtained directly, without any purification step, by use of three molten media: LiNO3, NaNO3, and their mixture; use of KNO3 as flux, either alone or as part of eutectic compositions, prevents complete conversion, and the material is obtained mixed with additional crystalline phases, for example lanthanum hydroxynitrates and carbonates. As-prepared LaAlO3 powders are composed of loosely agglomerated nanoparticles with very fine crystallite size (32–45 nm). This method results in substantially reduced synthesis times and temperatures compared with other methods used to prepare this material [62].
Li et al. reported that the gap states of the oxygen vacancy in LaAlO3 and related high-dielectric constant (high-k) perovskite oxides can be passivated by fluorine, or by substitution with nitrogen or alkaline earth metal atoms at adjacent sites. The mechanism is completion of the electron shell by the substitutions, and repulsion of the now empty vacancy gap state into the conduction band by relaxation of the adjacent Al and La ions away from the vacancy because it is locally +2 charged [63].
Murtaza et al. reported, for the first time, investigation of the structural and optoelectronic properties of LaAlO3 under pressure by use of the highly accurate all electrons FP-LAPW method. The calculated lattice parameter at zero pressure was in excellent agreement with the experimental results. Furthermore, with increasing external pressure the lattice constant and bond lengths decrease in accordance with the experimental results. The compound at zero pressure is an indirect band gap semiconductor; interestingly the indirect nature shifts to direct with increasing pressure. The bonding in the material is of mixed covalent and ionic nature. The frequency-dependent optical properties, for example the real and imaginary parts of the dielectric function, refractive index, reflectivity, optical conductivity, absorption coefficient, and sum rules were calculated under pressure [64].
Jang et al. reported successful growth of epitaxial LaNiO3 (1u.c.)–LaAlO3 (1u.c.) superlattices on single-crystal LaAlO3 (001) substrates by use of the pulsed laser deposition method. Specular RHEED intensity oscillations were repeated continuously throughout the entire growth. Large angle θ–2θ X-ray scans showed only peaks from the superlattices and substrates. These results confirm the highly qualified crystal structure of the superlattices. The temperature dependence of the resistivity has semiconducting behavior in the entire temperature range studied. These observations indicated that the semiconducting characteristics of the superlattice can be attributed to radical alteration of the electronic structure of the NiO2 layers [65].
Maczka et al. synthesized LaAlO3 nanoparticles doped with Eu3+ and Er3+ ions, at 500 °C, by a two-step process which combined a mechanically induced metathesis reaction with molten salt synthesis. The samples obtained were characterized by XRD and TEM, which showed the mean crystallite size was ~45 and ~57 nm, respectively. Furthermore, excitation and luminescence spectra and decay profiles were measured for the synthesized samples. These studies suggested that the Eu3+ ions are located at three different sites without inversion symmetry, and also revealed up-conversion emission in the samples doped with Er3+ ions. The up-conversion mechanism was discussed [66].
Li et al. reported preparation of LaAlO3 powders by use of a simple polymer-complexing plus combustion method with PVA or PEG (poly(ethylene glycol)) as complexing agent and fuel. The effect of different polymers on phase purity, powder morphology, and sintering performance was investigated. Trace amounts of the impurity La2O3 were present in the PEG powder, but could be eliminated after high temperature sintering. The pure LaAlO3 phase was readily obtained by calcination of PVA powders at 950 °C, although severe aggregation was always observed. PEG has advantages over PVA in terms of densification and microstructure control during the sintering process. High relative density of 97.0 % and homogeneous fine microstructure with grain size <3 μm can be obtained for PEG-derived samples sintered at 1,600 °C for 5 h. To obtain better quality LaAlO3 powders by combustion, PEG is preferred to PVA [67].
Dhahri et al. reported synthesis of Eu3+-doped LaAlO3 nano-phosphors by a combustion process. They used a concentrated solution of lanthanum nitrates and aluminate as oxidizer, and glycine acid as fuel. The powders were characterized by IR spectroscopy, XRD, SEM, TEM, and fluorescence spectroscopy. The pure LaAlO3 phase was obtained by heating at 800 °C for 4 h, without formation of any intermediate phase, with an average crystal size, as determined by TEM, of 60 nm. Intense PL emission was reported at 616 nm, enabling use of this material as a red phosphor [68].
Watras et al. measured the optical properties of two series of perovskites (first: LaAlO3, GdAlO3 and YAlO3 and second: LaAlO3, LaGaO3 and LaScO3) doped with 1 % Ce3+ ions. The results obtained enabled estimation of values of the centroid shift (ε c) and crystal-field splitting (ε cfs). The effects on ε c and ε cfs of ionic radii, electronegativity of cations, and distortion of structure were determined [69].
Comparative study of the literature
A comparative study of the literature is given in Table 1.
Characterization
X-ray diffraction
XRD is a technique used to measure the structural properties of a material, for example strain, epitaxy, phase composition, preferred orientation, and defect structure. XRD is non-destructive and can be used in most environments, making it advantageous over other techniques that can also be used for analysis of crystalline phases, for example TEM, which is a destructive technique. XRD can be used to determine the thickness of thin films and multilayers. It is important in many technological applications because of its ability to determine strain states. For magnetic thin films, it can be used to uniquely identify phases and preferred orientations, because these can determine magnetic properties. Figure 1 shows the XRD pattern of LaAlO3 prepared by chemical coprecipitation; it shows that the calcined LaAlO3 precursor powders are representative of LaAlO3, and no reflections from La2O3 and Al2O3 are observed as distinct intermediate phases during formation of LaAlO3 by thermal decomposition of the precursor powders, even at 1,000 °C [61] (Fig. 2).
XRD patterns of the LaAlO3 precursor powders calcined at different temperatures for 6 h: (a) 500 °C, (b) 600 °C, (c) 700 °C, (d) 800 °C, (e) 900 °C, and (f) 1,000 °C [61]
XRD patterns of LaAlO3:Eu3+ a temperature variation and b doping concentration variation [68]
TGA and DTA
TGA involves measurement of the weight of a sample under investigation as the temperature is increased at a pre-determined rate. The sample may either lose weight to the atmosphere or gain weight by reaction with the atmosphere. The TGA record is generally in the form of an integral curve, with absolute weight (W) as the Y axis and time (t) or temperature (T) as the X axis. The shape of the thermogravimetric curve is affected by several factors, the most important being heating rate, sample, and atmosphere.
DTA is measurement of the difference in temperature between a sample and a reference as heat is applied to the system. It is a fingerprint technique which provides information about the chemical reactions, phase transformations, and structural changes that occur in a sample during a heating or cooling cycle. The DTA technique is especially suited to studies of structural changes within a solid at elevated temperatures, where few other methods are available. TGA and DSC curves of the hydroxide precursor of LaAlO3 (prepared by the reverse microemulsion process) are shown in Fig. 3. TGA and DSC were performed at a heating rate of 10 °/min under static air on SDT Q600 instruments. The DSC curve indicates the presence of one exothermic peak at 479 °C and two endothermic peaks at 46 and 315 °C, respectively. The endothermic peak at 46 °C corresponds to elimination of residual water and solvent. The endothermic peak at 315 °C represents the decomposition of Al(OH)3 and La(OH)3. The exothermic peak at 479 °C may be associated with combustion of the residual surfactant or cosurfactant. The TGA curve is in agreement with DSC peaks and shows distinct weight-loss in regions corresponding to the temperature regions in DSC.
TGA–DSC curves of the hydroxide precursor [45]
FTIR spectroscopy
FTIR spectroscopy is rapidly becoming a common feature in modern spectroscopy laboratories. The Fourier-transform technique depends upon the basic principle that any wave function can be represented as a series of sine and cosine functions with different frequencies. IR analysis of synthesized samples is important for control both of the reaction process and of the properties of the materials obtained. The FTIR absorption spectra of LaAlO3 precursor powders calcined at different temperatures for 6 h are shown in Figs. 4 and 5.
FTIR absorption spectra of LaAlO3 precursor powders calcined at different temperatures for 6 h: (a) room temperature, (b) 200 °C, (c) 500 °C, (d) 600 °C, (e) 700 °C [61]
IR spectra of the LaAlO3 system prepared by the 2.5 PVA method. (a) Precursor powder, and after calcination of the precursor powder for 2 h at (b) 500 °C, (c) 700 °C, and (d) 800 °C [4]
Transmission electron microscopy
Historically, TEM has been a complementary tool for X-ray structural crystallographers owing to the insufficient image resolution at the time (which has since been overcome) and in the poor quality of diffraction data, as a consequence of multiple (dynamic) scattering. Today however, the electron microscope has the advantage of being able to form a fine probe for nano-scale characterization while simultaneously enabling collection of improved diffraction patterns with kinematic or near-kinematic data. TEM enables a variety of experiments for characterization of materials, not only to determine the properties of the bulk materials but also to obtain surface information. For example, surface morphology, surface unit cell dimensions, surface atomic positions, and even surface charge density and charge transfer can be observed. TEM micrographs of LaAlO3 powder calcined at 800 °C are shown in Fig. 6. From the TEM images it can be seen that individual particles are spherical in shape, with particle size of approximately 50 nm, and are loosely agglomerated.
TEM micrograph of LaAlO3 particles calcined at 800 °C for 2 h [45]
Scanning electron microscopy
An SEM obtains topographic images of organic and inorganic materials and enables elemental analysis. Its usefulness stems from its capability of obtaining 3D-like images of the surfaces of different samples and specimens. Most elements can be identified by use of the electron dispersive spectrometer portion of the SEM. The SEM’s instrumental resolution is generally approximately 10–50 Å; it also has a large depth of field, which is responsible for the 3D appearances of sample imaging. Overall the SEM’s most important use is for structural analysis and elemental analysis. An SEM micrograph of powder calcined at 700 °C is shown in Fig. 7. The synthesized powder is partially agglomerated and its particle size is smaller than 100 nm. The rough surface indicates that these particles are essentially secondary agglomerates of finer particles.
FESEM micrograph of the calcined LaAlO3 powder at 700 °C for 1 h [47]
Photoluminescence
Photoluminescence (abbreviated “PL”) is a process in which a substance absorbs photons (electromagnetic radiation), hence the prefix “photo”, and then re-radiates photons. Quantum mechanically, this can be described as excitation to a higher energy state and then a return to a lower energy state accompanied by emission of a photon. This is one of many forms of luminescence (light emission). The period between absorption and emission is typically extremely short, of the order of 10 ns. In special circumstances, however, this period can be extended to minutes or hours. The normalized PL spectra of single (Eu3+) and co-doped (Eu2+, Eu3+) samples on excitation with UV light are presented in Fig. 8a, b, respectively. The inset in Fig. 8a shows the partial PL spectral range from 380 to 560 nm for the single-doped sample. It is found that strong red emission at ~592 and ~618 nm and several sub-emission peaks located in the blue–green region are observed for the single-doped sample. The emission intensity of sub-emission peaks is so weak it can be neglective compared with the strong red emission, as shown in Fig. 8a. Full color emission is recorded for the mixed-valence Eu co-doped sample, as shown in Fig. 8b. The red emission arises from the same transitions as for the above Eu3+ single-doped sample whereas the blue emission band at approximately 445 nm is assigned to the transition emission of Eu2+. In addition, emission of green light at 515 nm is clearly apparent in the PL spectrum of the co-doped sample.
Normal PL spectra of a Eu3+ single-doped and b mixed-valence Eu co-doped samples on excitation of UV light. The inset is the partial PL range from 380 to 560 nm for the Eu3+ single-doped sample [50]
Atomic force microscopy
AFM or scanning force microscopy is a very high-resolution type of scanning probe microscopy, with demonstrated resolution of the order of fractions of a nanometer, more than 1,000 times better than the optical diffraction limit. The precursor to the AFM, the scanning tunneling microscope, was developed by Gerd Binnig and Heinrich Rohrer in the early 1980s at IBM Research, Zurich, a development that earned them the Nobel Prize for Physics in 1986. Binnig, Quate, and Gerber invented the first atomic force microscope (also abbreviated as AFM) in 1986. The first commercially available AFM was introduced in 1989. The AFM is one of the foremost tools for imaging, measurement, and manipulation of matter on the nano-scale. The information is gathered by “feeling” the surface with a mechanical probe. Piezoelectric elements that facilitate tiny but accurate and precise movements on (electronic) command enable very precise scanning. In some variations, electric potentials can also be scanned by use of conducting cantilevers. In more advanced versions, currents can be passed through the tip to probe the electrical conductivity or transport of the underlying surface, but this is much more challenging with few research groups reporting consistent data. Figure 9 shows the 3D AFM image of an LAO–SrO–Si sample. The surface of the LAO film is atomically smooth, and no particles or islands have been found.
3D AFM image of LAO thin film for determination of LAO–SrO–Si structure [21]
Electrokinetic behavior
The electrokinetic behavior of nano-sized LaAlO3 powder calcined at 800 °C is depicted in Fig. 10. It has a pzc at approximately pH 9.9. It is obvious from the zeta-potential curve that the surface charge of the LaAlO3 particles dispersed in an aqueous solvent depends on the pH of the suspension. At low pH LaAlO3 is positively charged and at pH above pzc it is negatively charged.
Potential behavior of the synthesized nano-crystalline LaAlO3 at 800 °C in 1 M KCl solution (solid portion of suspension = 6 % w/w) [47]
Dielectric behavior
Figure 11 below shows the dielectric behavior of LaAlO3 as a function of temperature (from room temperature to 300 °C). A strong dielectric assembly was found at 66 °C. The dielectric constant (ε) increases sharply from room temperature to its maximum value of 35 at 66 °C and then decreases gradually to 180 °C and ultimately becomes constant at approximately 6. The room temperature dielectric constant was strongly supported by the reported value. The dielectric loss (tan δ), which is related to the complex part of the dielectric constant, has a diffuse peak at the same temperature and has a loss of 1.13, which indicates that conduction of the carriers was performed at that temperature. At higher temperature (>260 °C), the mode of loss increases, which is because of the space charge effect.
Variation of dielectric constant (ε) and dielectric loss (tan δ) as a function of temperature at 10 kHz [4]
Perovskite crystal structure
The perovskite crystal structure is shown in Fig. 12.
The ideal ABX3 cubic perovskite structure illustrating the octahedral coordination of the B-site (aluminium here) cations (for LaAlO3) [70]
Discussion
Many methods of preparation of LaAlO3 nano-phosphors have been reported in this review. Although conventional solid-state reaction is a very simple method for synthesis of LaAlO3, this process is not entirely satisfactory because of several serious drawbacks, for example introduction of impurities during milling, high reaction temperature, limit of complete oxide reaction and chemical homogeneity, large particle size, and low sintering ability. Extensive research has therefore been performed to prepare finer and more homogeneous powders at lower temperatures by use of a variety of chemical processes. Methods with lower synthesis temperature are preferred as they lead to a high-surface-area material, i.e. material with improved catalytic activity. Pure LaAlO3 phase with average crystallite size of ~60 nm can be obtained by combustion synthesis, in which the temperature of synthesis is reduced to 800 °C [29, 60, 68]. From Table 1 it is apparent that chemical coprecipitation using aqueous NH3 as a basic precipitant is a simple method for synthesis of nano-powders of pure perovskite LaAlO3. The calcination temperature (700 °C) is, so far, the lowest process temperature used for complete LaAlO3 formation; nano-crystallite size increases from 31 to 44.5 nm with increasing calcination temperature from 700 to 1,000 °C [61]. Synthesis time and/or temperature are reduced in the molten salt method also (350 °C) and particle size is less than 100 nm; this technique has the advantages of being a facile, rapid, simple, cost-effective, environment-friendly, and high yielding method with no special handling precautions required. LaAlO3 powders of loosely agglomerated nanoparticles with very fine crystallite size (32–45 nm) are obtained in this method [62]. For LaAlO3 prepared by such methods as emulsion combustion, reverse microemulsion, and the citrate precursor technique, or by combination of mechanically induced metathesis with molten salt synthesis, average crystallite size is 50–60 nm [43, 45, 56, 59]. The combined gel precipitation process yields LaAlO3 powders with fairly spherical morphology and size ~25 nm [32]. Pure single-phase nano-crystalline LaAlO3 powder with a high specific average surface area of ~43 m2 g−1 and average crystallite size ~30 nm can be prepared by the sucrose method [47].
Irrespective of the method chosen for preparation of LaAlO3 phosphors, XRD shows that no intermediate Al2O3 or La2O3 phase is present. Pure LaAlO3 has a rhombohedral structure and occurs as a single phase. Sometimes a hexagonal structure [29] is also observed, and some samples have an orthorhombic [15] or cubic structure [21]. LaAlO3 doped with transition metal impurities has luminescence properties.
Pure nano-crystalline LaAlO3 powders prepared by combustion synthesis using a concentrated solution of nitrates of lanthanum and aluminate as oxidizer, and glycine acid as fuel have a hexagonal structure. Particle size is 78 nm and surface area is 11 m2 g−1. TEM micrography shows that the powder obtained has faceted polyhedral morphology. Samples sintered at 1,500 °C have the best microwave dielectric properties (permittivity, ε r, = 23 and quality factor, Q f, = 38,000 GHz). Figure 14 shows ε r and Q f as a function of sintering temperature [29] (Fig. 13).
Microwave dielectric properties of LaAlO3 ceramics sintered at different temperature [29]
Color online excitation (a) and emission (b) spectra of LaAlO3:0.5 at.% Sm3+ annealed at 800 °C [42]
LaAlO3 films grown on Si by laser molecular beam epitaxy have a single cubic crystalline structure. The surface of the epitaxial LaAlO3 films were atomically smooth. Figure 10 shows the 3D AFM image of a thin film of LAO–SrO–Si. This successful growth of LaAlO3 on Si suggests the possibility of development of 3D heterostructures on Si in a new generation of microelectronics devices [21]. LaAlO3 with an orthorhombic structure has also been prepared by homogeneous precipitation from aqueous solutions containing urea [15].
LaAlO3 has excellent chemical and thermal stability (mp ~2,110 °C), mechanical durability, and exploitable optical and electrical properties, leading to a wide range of potential applications. LaAlO3 has an excellent lattice match and good thermal expansion matching with many materials with the perovskite structure and, hence, is frequently used as a substrate and buffer layer for deposition of high-temperature superconductor and ferroelectric thin films. Because of its elegant dielectric properties, and high quality factor (Q f) and relative permittivity (ε r), LaAlO3 is also a promising candidate for low-loss microwave and dielectric resonance applications; LaAlO3 is, thus, widely used in superconducting microwave devices. Experimental work also demonstrates that LaAlO3 is a potential candidate for SiO2 replacement because of its high k value. At elevated temperature LaAlO3 has the cubic perovskite structure but transforms into a trigonal rhombohedral form at approximately 800 K. The capabilities of perovskite oxide materials just as remarkable dielectric, piezoelectric, ferroelectric, optical, electro-optic, ferromagnetic, superconducting and catalytic properties have close relation with the energy spectrum. It has been also used as a support for combustion catalysts or even as a catalyst itself for oxidative coupling of methane and hydrogenation of hydrocarbons. Finally, with La3+ and/or Al3+ partially replaced by Sr2+ and/or Mg2+, respectively, it becomes a good oxygen ion-conducting material at low oxygen partial pressures and high temperatures, changing to a mixed ionic and p-type electronic conductor at high oxygen pressures. Phosphors are important materials in modern technology because of their ability to convert incident electromagnetic radiation into light.
Rare-earth (RE) ions have been widely used as activators in different host matrices because of their highly efficient emission performance. The development of RE-ion-doped novel luminescent phosphors is therefore of interest because of their potential applications in different optical display systems. LaAlO3 has been chosen as a host material because it has a reasonably large band gap of >5 eV and high thermal stability up to 2,100 °C. RE-doped LaAlO3 also has remarkable properties which can be exploited, for example, to enhance solar efficiency, laser action, etc.
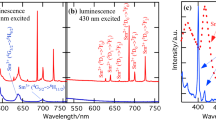
LaAlO3:Sm3+ phosphors have been prepared by a Pe-type sol–gel process. On excitation with UV light or low-voltage electron beams, the phosphors emit yellow luminescence with good chromaticity coordinates (Fig. 14). The CL properties of LaAlO3:Sm3+ are, to some extent, comparable with those of the corresponding commercial low-voltage phosphors. Because of their excellent CL, good CIE chromaticity, stability, and environmentally friendly properties, LaAlO3:Sm3+ phosphors have potential applications in FED devices [42]. Dy3+ in the LaAlO3 host lattice emits light from the blue to red region. The most intense is yellow–greenish emission (Figs. 15, 16). The emission of LaAlO3:Er3+ is weaker than that of LaAlO3:Dy3+ [59]. Eu3+-doped LaAlO3 nano-phosphors are prepared by combustion synthesis. Typically the Eu3+ ion emits characteristic red light with a number of narrow lines. The PL emission intensity increases when the Eu3+ concentration is increased from 2 %, reaching a maximum when the concentration of Eu3+ is 5 % and then decreasing slightly for higher concentrations (Figs. 17, 18) [68].
Emission spectra of La0.9Dy0.1AlO3 and La0.8Dy0.1Er0.1AlO3 [59]
Emission spectra of La0.9Er0.1AlO3 and La0.8Dy0.1Er0.1AlO3 [59]
Excitation spectra of LaAlO3:Eu (5 %) [68]
Emission spectra of LaAlO3:Eu (2 %, 5 %, 10 %) obtained at 800 °C [68]
Conclusion
From the discussion above we conclude that the combustion, chemical coprecipitation, sucrose, and molten salt methods are best for low-temperature synthesis of LaAlO3 phosphors. LaAlO3 prepared by use of the combustion method is reported to have the best microwave properties. Among all the methods used to prepare LaAlO3 nano-crystalline phosphors, only methods which give single-phase pure perovskite LaAlO3 of small particle size, small average crystallite size, and high specific surface area are preferred. The rhombohedral structure of LaAlO3 has been reported most often. Orthorhombic, hexagonal, and cubic structures are occasionally reported. LaAlO3 has exploitable optical and electrical properties and is widely used in superconducting microwave devices. RE-doped-LaAlO3 has potential applications in optical display systems. LaAlO3 doped with transition elements emits luminescence, as discussed in detail above. These RE ions act as activators in LaAlO3 phosphors. The chemical and thermal stability and mechanical durability of LaAlO3 phosphors are excellent, and their exploitable optical and electronic properties lead to a wide range of potential applications.
References
R.K. Simon, C.E. Platt, K.P. Daly, A.E. Lee, M.K. Wager, Appl. Phys. Lett. 53, 2677 (1988)
B. Jancar, D. Suvorov, M. Valant, G. Drazic, J. Eur. Ceram. Soc. 23, 1391 (2003)
I. Zvereva, Y. Smirnov, V. Gusarov, V. Popova, J. Choisnet, Solid State Sci. 5, 343 (2003)
A.K. Adak, P. Pramanik, Mater. Lett. 30, 269 (1997)
M. Chroma, J. Pinkas, I. Pakutinskiene, A. Begankiene, A. Kareiva, Ceram. Int. 31, 1123 (2005)
S.N. Koc, F. Oksuzomer, E. Yasav, S. Akturk, M.A. Gurkaynak, Mater. Res. Bull. 41, 2291 (2006)
A. Barrera, S. Fuentes, M. Viniegra, M. Avalos-Borja, N. Bogdan Chikova, J.C. Molina, Mater. Res. Bull. 42, 640 (2007)
Y. Xu, G. Huang, H. Long, Ceram. Int. 29, 837 (2003)
D. Zhou, G. Huang, X. Chen, J. Xu, S. Gong, Mater. Chem. Phys. 84, 33 (2004)
P.J. Deren, K. Lemanski, A. Gagor, M. Watras, M. Malecka, M. Zawadzki, J. Solid State Chem. 183, 2095 (2010)
R. Pazik, G.A. Seisenbaeva, R.J. Wiglusz, L. Kepinski, V.G. Kessler, Inorg. Chem. 50, 2966 (2011)
S. Ran, L. Gao, Ceram. Int. 34, 443 (2008)
J.J. Kingsley, K.C. Patil, Mater. Lett. 6, 427 (1988)
M.D. Shaji Kumar, T.M. Srinivasan, P. Ramasamy, C. Subramanian, Mater. Lett. 25, 171 (1995)
E. Taspinar, A. Cuneyt Tas, J. Am. Ceram. Soc. 80(1), 133 (1997)
J.P. Jacobs, M.A.S. Miguel, L.J. Alvarez, J. Mol. Struct. (Theochem) 390, 193 (1997)
M. Kakihana, T. Okubo, J. Alloys Compd. 266, 129 (1998)
R. Spinicci, P. Marini, S. De Rossi, M. Faticanti, P. Porta, J. Mol. Catal. A 176, 253 (2001)
S.A. Hayward, S.A.T. Redfern, E.K.H. Salje, J. Phys. Condens. Matter 14, 10131 (2002)
P.J. Deren, J.C. Krupa, J. Lumin. 102, 386 (2003)
W.F. Xianga, H.B. Lua, Z.H. Chena, X.B. Lub, M. Hea, H. Tiana, Y.L. Zhoua, C.R. Lia, X.L. Mac, J. Cryst. Growth 271, 165 (2004)
P.J. Deren, J.C. Krupa, J. Alloys Compd. 380, 362 (2004)
E.A. Fidancev, P.J. Deren, J.C. Krupa, J. Alloys Compd. 380, 376 (2004)
T. Busani, R.A.B. Devine, J. Appl. Phys. 96, 6642 (2004)
C. Chang, Z. Yuan, D. Mao, J. Alloys Compd. 415, 220 (2006)
D. Hreniak, W. Strek, P. Deren, A. Bednarkiewicz, A. Lukowiak, J. Alloys Compd. 408, 828 (2006)
P. Deren, Ph. Goldner, O.G. Noel, J. Lumin. 119, 38 (2006)
T. Ishigaki, K. Seki, E. Nishimura, T. Watanabe, M. Yoshimura, J. Alloys Compd. 408, 1177 (2006)
Z.Q. Tian, H.T. Yu, Z.L. Wang, Mater. Chem. Phys. 106, 126 (2007)
C.L. Kuo, C.L. Wang, T.Y. Chena, G.J. Chenb, I.M. Hung, C.J. Shih, K.Z. Funga, J. Alloys Compd. 440, 367 (2007)
Z. Li, S. Zhang, W.E. Lee, J. Eur. Ceram. Soc. 27, 3201 (2007)
S.K. Behera, P.K. Sahu, S.K. Pratihar, S. Bhattacharyya, J. Phys. Chem. Solids 69, 2041 (2008)
X. Luo, B. Wang, J. Appl. Phys. 104, 073518 (2008)
P.J. Deren, Ph. Goldner, O.G. Noel, J. Alloys Compd. 461, 58–60 (2008)
X. Luo, B. Wang, J. Appl. Phys. 104, 053503 (2008)
V. Singh, D.T. Naidu, R.P.S. Chakradhar, Y.C. Ratnakaramd, J.J. Zhu, M. Soni, Physica B 403, 3781 (2008)
P.J. Dereń, A. Bednarkiewicz, Ph. Goldner, O. Guillot-Noël, J. Appl. Phys. 103, 043102 (2008)
A. Gocalinska, P.J. Deren, P. Głuchowski, Ph. Goldner, O. Guillot-Noel, Opt. Mater. 30, 680 (2008)
T. Kharlamova, S. Pavlova, V. Sadykov, O. Lapina, D. Khabibulin, T. Krieger, V. Zaikovskii, A. Ishchenko, A. Salanov, V. Muzykantov, N. Mezentseva, M. Chaikina, N. Uvarov, J. Frade, Chr. Argirusis, Solid State Ion. 179, 1018 (2008)
P.J. Deren, R. Mahiou, Ph. Goldner, Opt. Mater. 31, 465 (2009)
I.Y.K. Chang, S.W. You, M.G. Chen, P.C. Juan, C.H. Chen, J.Y. Lee, J. Appl. Phys. 105, 104512 (2009)
X. Liu, J. Zou, J. Lin, J. Electrochem. Soc. 156(2), P43 (2009)
J. Chandradass, K.H. Kim, J. Alloys Compd. 481, L31 (2009)
H.F. Yu, J. Wang, S.S. Wang, Y.M. Kuo, J. Phys. Chem. Solids 70, 218 (2009)
Z. Tian, W. Huang, Y. Liang, Ceram. Int. 35, 661 (2009)
J. Chandradass, H.K. Kim, J. Cryst. Growth 311, 3631 (2009)
Z. Negahdaria, A. Saberi, M.W. Poradaa, J. Alloys Compd. 485, 367 (2009)
A. Boudali, B. Amrani, M.D. Khodja, A. Abada, K. Amara, Comput. Mater. Sci. 45, 1068 (2009)
P. Zhang, J.-P. Zhanga, Eur. Phys. J. B 78, 1 (2010)
Z.Y. Mao, Y.C. Zhu, Q. Fei, D. Wang, J. Lumin. 131, 1048 (2011)
B. Liu, F. Yuan, X. Huc, J. Phys. Chem. Solids 72, 380 (2011)
P.J. Deren, K. Lemanski, J. Lumin. 131, 445 (2011)
M. Malinowski, M. Kaczka, S. Turczynski, D. Pawlak, Opt. Mater. 33, 1004 (2011)
H. Mortada, D. Dentel, M. Derivaz, J.L. Bischoff, E. Denys, R. Moubah, C.U. Bouillet, J. Werckmann, J. Cryst. Growth 323, 247 (2011)
M. Dudek, A. Jusza, K. Anders, L. Lipinska, M. Baran, R. Piramidowicz, J. Rare Earths 29(12), 1123 (2011)
H.F. Yu, Y.M. Guo, J. Alloys Compd. 509, 1984 (2011)
K.C. Liu, W.H. Tzeng, K.M. Chang, J.J. Huang, Y.J. Lee, P.H. Yeh, P.S. Chen, H.Y. Lee, F. Chen, M.J. Tsai, Thin Solid Films 520, 1246 (2011)
D. Yamasaka, K. Tamagawa, Y. Ohki, J. Appl. Phys. 110, 074103 (2011)
M. Maczka, E.M. Mendoza, A.F. Fuentes, K. Lemanski, P. Deren, J. Solid State Chem. 187, 249 (2012)
K.A. Khamkar, S.V. Bangale, S.R. Bamane, V.V. Dhapte, Der Chem. Sin. 3(4), 891 (2012)
L. Djoudi, M. Omari, N. Madoui, EPJ. Web Conf. 29, 16 (2012)
E.M. Mendoza, S.M. Montemayor, J.I.E. Garcı, A.F. Fuentes, J. Am. Ceram. Soc. 95(4), 1276 (2012)
H. Li, J. Robertson, J. Appl. Phys. 112, 034108 (2012)
G. Murtaza, I. Ahmad, J. Appl. Phys. 111, 123116 (2012)
A.N. Jang, S.K. Seung, K.H. Choi, J.H. Song, Ceram. Int. 38S, S627 (2012)
M. Maczka, A. Bednarkiewicz, E.M. Mendoza, A.F. Fuentes, L. Kepinski, J. Solid State Chem. 194, 264 (2012)
S. Li, B. Bergman, Z. Zhao, Mater. Chem. Phys. 132, 309 (2012)
A. Dhahri, K.H. Naifer, A. Benedetti, F. Enrichi, M. Ferid, Opt. Mater. 34, 1742 (2012)
A. Watras, R. Pazik, P.J. Deren, J. Lumin. 133, 35 (2013)
J.M. Rondinelli, Model Catalytic Oxide Surfaces: A Study of the LaAlO 3 (001) Surface (Northwestern University, Evanston, 2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, J., Singh, D., Dubey, V. et al. Review of the synthesis, characterization, and properties of LaAlO3 phosphors. Res Chem Intermed 40, 2737–2771 (2014). https://doi.org/10.1007/s11164-013-1126-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1126-z