Abstract
Carbon-based solid acid efficiently catalyzes diastereoselective ring opening of α-epoxyketones in the presence of methanol to produce the corresponding α-hydroxy-β-methoxyketones in excellent yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxides and epoxyketones have attracted the attention of many organic chemists because of the effect of ring strain on chemical reactivity and the potential of derivatives of these compounds to act as precursors of more elaborate heterocyclic compounds. Among these transformations, ring opening reactions of α-epoxyketones have been recognized as important in thermal and photochemical transformations. These reactions are also of substantial interest synthetically and mechanistically. C–O and C–C bond cleavage in these compounds can occur via photochemical, thermal, and electrochemical processes [1–18].
Several methods have been reported for ring opening of epoxides in the presence of a catalyst, for example aluminium triflate [14], CsF [16], ZrCl4 [17], boron trifluoride [18], vanadium(IV) tetraphenylporphyrin [19], montmorillonite K 10 [20], and (C4H12N2)2[BiCl6]Cl·H2O [21]. There are, however, no reports of ring opening of α-epoxyketones in the presence of a Lewis or Bronsted acid.
The use of heterogeneous and reusable catalysts in organic reactions has proved useful to chemists in the laboratory and in the industrial context. These reactions are effected by reagents immobilized on solid supports and have such advantages as operational simplicity, environmental compatibility, non-toxicity, reusability, low cost, and ease of isolation [22].
To the best of our knowledge, ring opening reactions of α-epoxyketones catalyzed by carbon-based solid acid (CBSA) with a high density of sulfonic acid (SO3H) have not been reported in the open literature. Herein, in continuation of our interest in the use of heterogeneous catalysts in organic reactions [23, 24], we report use of CBSA as a reusable heterogeneous catalyst for diastereoselective nucleophilic opening of α-epoxyketone rings in the presence of methanol (Scheme 1).
Experimental
Chemicals were purchased from Fluka and Merck. All products obtained are known compounds and were identified by comparing their physical and spectral data with those reported in the literature. 1H NMR spectra were recorded on an Ultra Shield spectrometer (400 MHz) using TMS as internal standard. FT-IR spectra were obtained as potassium bromide pellets in the range 400–4,000 cm−1 with a Varian 4300 spectrometer. Information about the phase of CBSA was obtained by X-ray powder diffractometry (Philips, Cu Kα, λ = 1.54178 Å) with the Bragg angle ranging between 10° and 50°.
Catalyst preparation
Carbon-based solid acid (CBSA) was prepared as reported by Hara et al. [25]. In a typical synthesis, naphthalene (20 g) was heated in concentrated sulfuric acid (98 %, 200 ml) at 523 K under a flow of N2. After heating for 15 h, excess sulfuric acid was removed from the dark brown tar by vacuum distillation at 523 K for 5 h, which resulted in a black solid. The solid was then ground to a powder and washed in boiling water until impurities, for example sulfate ions, were no longer detected in the wash water. The SO3H group density was measured by using NaOH (0.01 mol l−1) as titrant in a neutralization titration. The amount of SO3H attached to the polycyclic aromatic carbon was 4.87 mmol g−1.
Typical procedure for the ring opening reaction
CBSA (0.1 g) was added to a stirred solution of α-epoxyketone (1 mmol) and MeOH (20 ml) and the mixture was stirred at room temperature for the time given in Table 1. The reaction was monitored by TLC (petroleum ether–EtOAc, 10:1). After completion of the reaction, the heterogeneous catalyst was separated by centrifugation and the solvent of resulting mixture was evaporated to give pure product (Table 1). The catalyst can be recycled by washing it with ethyl acetate and drying in an oven at 45 °C for 3 h.
Results and discussion
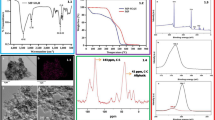
Synthesis of CBSA with a high density of sulfonic acid (SO3H) was reported by Hara et al. [25]. Such CBSAs can be readily prepared by heating aromatic compounds such as naphthalene in sulfuric acid at 523 K. In this synthesis, sulfonation of the aromatic compounds is the first stage of the reaction. The resulting sulfonated aromatic compounds are completely carbonized, which results in the formation of a solid with nominal sample composition of CH0.35O0.35S0.14. The resulting black powder is insoluble in such solvents as water, methanol, ethanol, and hexane even at boiling temperatures [26]. The X-ray diffraction (XRD) spectrum of CBSA is shown in Fig. 1. The two broad and weak peaks (2θ = 15°–30°, 35°–50°) are attributed to amorphous carbon.
To illustrate use of the CBSA catalyst, ring opening reaction of α-epoxyketone 1e was performed in the absence of the catalyst. In that case the product was not produced after stirring the reaction mixture in methanol for 10 h. Thus, it could be concluded that CBSA is an important component participating in this reaction. The best results were obtained by use of 0.1 g CBSA. Use of less catalyst resulted in lower yields whereas use of more catalyst did not affect reaction times and yields.
Reaction of α-epoxyketone 1e with 2 in methanol solution produced a mixture of diastereomeric products 3e and 4e. The 1H NMR spectrum of 3e + 4e is presented in Fig. 2.
This spectrum consists of two sharp lines for two methyl groups on phenyl rings (δ = 2.34 ppm for Hi′ and 2.36 ppm for Hi), two singlets for methoxy groups (δ = 3.12 ppm for Hh and 3.25 ppm for Hh′), a doublet for the benzylic proton (δ = 4.51 ppm for He′, 3 J He′Hd′ = 4.4 Hz), a doublet for the benzylic proton (δ = 4.56 ppm for He, 3 J HeHd = 3.2 Hz), a doublet for the –CO–CHOH– proton (δ = 5.19 ppm for Hd, 3 J HeHd = 3.2 Hz), a doublet for the –CO–CHOH– proton (δ = 5.42 ppm for Hd′, 3 J He′Hd′ = 4.4 Hz) and four doublets, two triplet-like peaks, and four doublet of triplets for the aromatic protons (δ = 7.03 ppm for Hg, d, 3 J HgHf = 8 Hz; δ = 7.10 ppm for Hg′, d, 3 J Hg′Hf′ = 8 Hz; δ = 7.18 ppm for Hf′, d, 3 J Hg′Hf′ = 8 Hz; δ = 7.26 ppm for Hf, d, 3 J HgHf = 8 Hz; δ = 7.49 ppm for Hb′, t-like, 3 J Hb′Ha′ = 8 Hz, 3 J Hb′Hc′ = 7.6 Hz;, δ = 7.53 ppm for Hb, t-like, 3 J HbHa = 8 Hz, 3 J HbHc = 7.2 Hz; δ = 7.62 ppm for Ha, dt, 3 J HbHa = 7.2 Hz, 4 J HaHc = 1.2 Hz; δ = 7.66 ppm for Ha′, dt, 3 J Hb′Ha′ = 7.2 Hz, 4 J Ha′Hc′ = 1.2 Hz; δ = 7.89 ppm for Hc′, dt, 3 J Hb′Hc′ = 7.2 Hz, 4 J Ha′Hc′ = 1.2 Hz; δ = 7.97 ppm for Hc, dt, 3 J HcHa = 7.2 Hz, 4 J HbHc = 1.2 Hz). The 3e/4e ratio was determined by comparison of the integral ratios of the hydrogens on C-2 (Hd and Hd′). Then, all reactions were performed under these conditions and the ratios of diastereomers were determined by the method mentioned above. The results are presented in Table 1.
As shown in Table 1, the rate and diastereoselectivity of the catalytic ring opening of α-epoxyketones 1a–h are dependent on not only the nature of the additional substituents on the parent molecule but also their location. Whereas electron donor groups, for example methyl and methoxy, on the phenyl ring directly attached to the epoxide ring (1b, 1d and 1f) facilitate ring opening, the same substituents on the phenyl ring of the benzoyl moiety (1g and 1h) have less effect. Also, for 1b, 1d and 1e, the ratios of the diastereomeric products are inverted in comparison with 1a, 1c, 1f, 1g, and 1h.
On the basis of the results obtained, we propose that activation of 1a–h by 2 could lead to three different intermediates 5, 6, and 7 in the catalytic ring opening of α-epoxyketones. These intermediates are trapped by nucleophilic attack of methanol on the less hindered side, forming 3a–h and 4a–h via SN1 and SN2 processes [18] (Scheme 2).
The ratios of the diastereomeric products lead us to assume that the inductive effect of the p-methyl group (1e) and the resonance effect of the o- and p-methoxy groups (1b and 1d) on the phenyl ring directly attached to the epoxide ring increase the contribution of the intermediates 6 and 7 because of stabilization of the carbocation. Nucleophilic attack of methanol on intermediate 7 produces predominantly 3b, 3d, and 3e (Scheme 2). On the other hand, intermediates 5 and 6 can be proposed for 1a, 1c, 1f, 1g, and 1h, in which the nucleophilic attack of methanol on intermediate 5 produces 4a, 4c, 4f, 4g, and 4h (Scheme 2).
We also studied recycling of the used CBSA in repeated reactions. After the first run, the catalyst was recovered by filtration, washed with ethyl acetate, and reused. The catalyst can be reused ten times in sequence without loss of activity (Fig. 3).
Conclusion
We have developed a simple, efficient, and green procedure for the ring opening reaction of α-epoxyketones with CBSA as catalyst in methanol solution. The rate and diastereoselectivity of catalytic ring opening of α-epoxyketones depend not only on the nature of additional substituents on the parent molecule but also on their location. Whereas electron donor groups on the phenyl ring directly attached to the epoxide ring facilitate ring opening, the same substituents on the phenyl ring of the benzoyl moiety have a smaller effect. Simple work-up in isolation of the products and high purity are features of this procedure. Further, the catalyst can be easily recovered and reused without much loss of its activity.
References
H.R. Memarian, A. Saffar-Teluri, M.K. Amini, Heterocycles 68, 1861 (2006)
E. Hasegawa, K. Ishiyama, T. Fujita, T. Kato, T. Abe, J. Org. Chem. 62, 2396 (1997)
E. Hasegawa, A. Yoneoka, K. Suzuki, T. Kato, T. Kitazume, K. Yanagi, Tetrahedron 55, 12957 (1999)
E. Hasegawa, T. Kato, T. Kitazume, K. Yanagi, K. Hasegawa, T. Horaguchi, Tetrahedron Lett. 37, 7079 (1996)
E. Hasegawa, K. Ishiyama, K. Kato, T. Horaguchi, T. Shimizu, S. Tanaka, Y. Yamashita, J. Org. Chem. 57, 5352 (1992)
E. Hasegawa, M. Kamata, in CRC Handbook of Organic Photochemistry and Photobiology, ed. by W.M. Horspool, F. Lenci (CRC Press, Boca Raton, 2004), pp. 1–17
K. Okada, E. Hasegawa, T. Mukai, Chem. Lett. 3, 305 (1983)
E. Hasegawa, K. Ishiyama, H. Kashiwazaki, T. Horaguchi, T. Shimizu, Tetrahedron Lett. 31, 4045 (1990)
C. Hardouin, F. Chevallier, B. Rousseau, E. Doris, J. Org. Chem. 66, 1046 (2001)
L. Lopez, L. Troisi, Tetrahedron Lett. 30, 3097 (1989)
J. Kagan, P.Y. Juang, B.E. Firth, J.T. Przybytek, S.P. Singh, Tetrahedron Lett. 18, 4289 (1977)
C. Huo, X. Jia, W. Zhang, L. Yang, J. Lu, Z.L. Liu, Synlett 2, 251 (2004)
J. Delaunay, A. Lebouc, A. Tallaec, J. Sinonet, J. Chem. Soc. Chem. Commun. 387 (1982)
D. Bradley, G. Williams, M. Lawton, Org. Biomol. Chem. 3, 3269 (2005)
F. Fringuelli, F. Pizzo, L. Vaccaro, J. Org. Chem. 69, 2315 (2004)
V. Polshettiwar, M.P. Kaushik, Catal. Commun. 5, 515 (2004)
A.K. Chakraborti, A. Kondaskar, Tetrahedron Lett. 44, 8315 (2003)
J. Izquierdo, S. Rodriguez, F.V. Gonzalez, Org. Lett. 13, 3856 (2011)
S.A. Taghavi, M. Moghadam, I. Mohammadpoor-Baltork, S. Tangestaninejad, V. Mirkhani, A.R. Khosropour, V. Ahmadi, Polyhedron 30, 2244 (2011)
A.K. Chakraborti, A. Kondaskar, S. Rudrawar, Tetrahedron 60, 9085 (2004)
H.F. Lu, L.L. Sun, W.J. Le, F.F. Yang, J.T. Zhou, Y.H. Gao, Tetrahedron Lett. 53, 4267 (2012)
R.S. Varma, Green Chem. 1, 43 (1999)
A. Saffar-Teluri, S. Bolouk, Monatsh. Chem. 141, 1113 (2010)
A. Saffar-Teluri, Res. Chem. Intermed. (2012). doi:10.1007/s11164-012-0846-9
M. Hara, T. Yoshida, A. Takagaki, T. Takata, J.N. Kondo, S. Hayashi, K. Domen, Angew. Chem. Int. Ed. 43, 2955 (2004)
H. Toda, A. Takagaki, M. Okamura, J.N. Kondo, K. Domen, S. Hayashi, M. Hara, Nature 438, 178 (2005)
Acknowledgments
We gratefully acknowledge financial support from the Research Council of Islamic Azad University of Najafabad Branch.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saffar-Teluri, A. Carbon-based solid acid: an efficient and reusable catalyst for the diastereoselective ring opening reaction of α-epoxyketones. Res Chem Intermed 40, 523–529 (2014). https://doi.org/10.1007/s11164-012-0979-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0979-x