Abstract
Inhibition of the corrosion of mild steel in sulfuric acid by extracts of Anacyclus pyrethrum L. (leaves and stems, AP-LS; flowers AP-F; roots, AP-R) has been studied by use of electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization. Anacyclus pyrethrum L. inhibited the corrosion of mild steel in 0.5 M H2SO4 solution. Polarization curves show that the different parts of plants act as anodic type inhibitors. Changes in impedance data (charge transfer resistance, R t, and double layer capacitance, C dl) were indicative of adsorption of the extracts on the metal surface, leading to the formation of protective films. The extent of surface coverage by the inhibitors was determined by measurement of ac impedance; it was found that adsorption of these inhibitors on the mild steel surface obeys the Langmuir adsorption isotherm. Activation energies in the presence and absence of AP-LS and AP-F were obtained by measuring the temperature dependence of the corrosion current.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Corrosion of metals is a very common problem with economic implications costing billions of dollars each year. Corrosion inhibition is required by many industries, for example oil and gas exploration and production, petroleum refining, chemical manufacture, and the product additives [1]. Inhibition of corrosion is achieved by use of inhibitors that prevent corrosion from occurring on metal surface. Inhibitors are chemicals that often work by becoming adsorbed on the metal surface, protecting the metal surface by forming a film. The scientific and technical corrosion literature has descriptions and lists of numerous chemical compounds with inhibitive properties [2–5]. Considerations of cost, toxicity, availability, and environmental friendliness are of substantial importance when assessing the current state of the art in so-called “green chemistry”. By definition, green chemistry is the design, development, and implementation of chemical products and processes to reduce or eliminate the use and generation of substances hazardous to human health and the environment. A variety of natural products have been reported to be very good inhibitors [6–10].
This study was conducted to gain insight into the corrosion of mild steel in 0.5 M H2SO4 in the presence, as a corrosion inhibitors, of extracts of different parts (leaves, stems, flowers, and roots) of Anacyclus pyrethrum L. Inhibition of the corrosion of mild steel in 0.5 M H2SO4 by these naturally occurring biological molecules was investigated by potentiodynamic polarization and electrochemical impedance.
Experimental
Plant material
Anacyclus pyrethrum L. was harvested from the mountains of Tlemcen (north-west of Algeria). Methods for identification of the plant, extraction, and phytochemical screening are similar to those reported elsewhere [10].
Solution and material
Experiments were performed in solutions of 0.5 M sulfuric acid (uninhibited and inhibited) on mild steel containing (wt%): C ≤ 0.1 %, Si ≤ 0.03 %, Mn ≤ 0.2 %, P ≤ 0.02 %, Cr ≤ 0.05 %, Ni ≤ 0.05 %, Al ≤ 0.03 %, and the remainder iron. Specimens in the form of discs were polished successively with different grades of emery paper up 1200 grade.
Electrochemical measurements
Electrochemical measurements were performed as described elsewhere [10].
Inhibition efficiency was evaluated from measured I corr values by use of the relationship:
where I 0corr and I icorr are the corrosion current density in the absence and presence of inhibitor, respectively.
Inhibition efficiency was calculated from charge-transfer resistance values by use of the equation:
where R 0t and R it are the charge transfer resistance in the absence and presence of the inhibitor, respectively.
Results and discussion
Phytochemical screening
Results from phytochemical screening of aqueous extracts of the plant are listed in Table 1.
The results from phytochemical analysis showed that the aqueous extracts contained reducing compounds—catechol tannins, saponins, alkaloids, flavonoids, and amino acids. It is also apparent that gallic tannins are absent from all parts of this plant and flavonoids are absent from the roots. The number of positive signs is proportional to the intensity of reactions that reflect the quantity available.
EIS measurements
Figure 1 shows Nyquist plots recorded for mild steel in 0.5 M H2SO4 in the absence and presence of the optimum concentrations of different parts of Anacyclus pyrethrum L. In general the impedance diagrams for the solutions are almost semicircular in appearance, indicates that corrosion of steel is mainly controlled by a charge-transfer process [11, 12].
The equivalent circuit consists of a constant phase element (CPE) Q, in parallel with a resistor R t. Use of a CPE-type impedance has been extensively described elsewhere [13–15]:
This equation provides information about the non-ideality of the capacitance behavior. It makes it possible to differentiate between the behavior of an ideal capacitor (n = 1) and of a CPE (n < 1). Considering that a CPE may be regarded as a parallel combination of a pure capacitor and a resistor that is inversely proportional to the angular frequency, the value of capacitance, C dl, can thus be calculated for a parallel circuit composed of a CPE (Q) and a resistor (R t), according to the formula [16, 17]:
The impedance spectra of mild steel in 0.5 M H2SO4 with and without inhibitor were analyzed by use of the circuit in Fig. 2, and the double layer capacitance (C dl) was calculated by use of Eq. 4. Values of elements of the circuit corresponding to different corrosion systems, including values of C dl and inhibition efficiency, are listed in Table 2.
The results in this table indicate:
-
The value of R t increases with increasing concentrations of the extracts, indicating that the rate of corrosion decreases in the presence of the inhibitor molecules.
-
The value of C dl decreases on addition of inhibitors, indicating a decrease in the local dielectric constant and/or an increase in the thickness of the electrical double layer, suggesting that the inhibitor molecules function by formation of a protective layer on the metal surface [18].
-
Overall, the aerial parts are more efficient than the roots. This pronounced effectiveness of the aerial parts is because their phytochemical constitution is different from that of the roots.
Polarization measurements
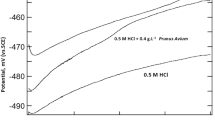
Figure 3 shows the effect of the optimum concentrations of the extracts on the anodic and cathodic polarization curves for mild steel in 0.5 M H2SO4. It was observed that extracts of different parts of plant increase both the anodic and cathodic overpotentials but the effect on the cathodic side is much less obvious (i.e. the extracts act predominately as anodic inhibitors).
All polarization data, i.e. corrosion potential (E corr), cathodic Tafel slope (b c), corrosion current density (I corr), and the corresponding inhibition efficiency (P %) values for mild steel corrosion in the absence and presence of different concentrations of the plant extracts were estimated, and are listed in Table 3. From this table it can be concluded that I corr values decrease with increasing inhibitor concentration. The cathodic Tafel slopes were found to vary over the range 285–200 mV dec−1. Therefore, the cathodic slope changed with increasing concentration of plant extract in 0.5 M H2SO4. This result indicates the effect of the inhibitor on the kinetics of hydrogen evolution [11]. Inhibition efficiency (P %) increases with inhibitor concentration, reaching a maximum (84.3 %) at 350 mg L−1 for the extract of the flowers. The aerial parts are usually more effective than the roots.
Adsorption isotherm
It is known that adsorption isotherms are very important for understanding of the mechanism of corrosion inhibition [19]. The most frequently used are the Langmuir, Freundlich, Temkin, and Frumkin isotherms. Assuming a direct relationship between inhibition efficiency and surface coverage, θ, of the inhibitor, electrochemical impedance spectroscopy data were used to evaluate surface coverage, which is given by Eq. 5
where R it and R 0t are the charge-transfer resistance with and without inhibitor, respectively.
By far the best fit was obtained with the Langmuir isotherm. According to this isotherm θ is related to concentration inhibitor C via:
with
where K is the adsorptive equilibrium constant and \( \Updelta G^\circ_{\text{ads}} \) is the free energy of adsorption.
Plots of C/θ versus C (Fig. 4) were straight lines for all part of the plant, with slopes close to unity. This result indicates that adsorption of the compounds at the mild steel–acid solution interface at all temperatures follows the Langmuir adsorption isotherm.
Thermodynamic data are important for study of the mechanism of inhibition. The values of \( \Updelta G^\circ_{\text{ads}} \) for the different systems were estimated from the values of K and by use of Eq. (7).
The values of the equilibrium adsorption constant obtained from these isotherms are approximately 2.3 × 104, 3.8 × 104, and 5.85 × 104 L kg−1 for leaves and stems (LS), flowers (F), and roots (R), respectively. Moreover, the largest negative values of ΔGads, i.e. 35.41 kJ mol−1 for LS, 36.74 kJ mol−1 and 37.77 kJ mol−1 for R extracts indicate that these extracts are strongly adsorbed by the mild steel surface. Literature survey reveals that negative values of ΔGads of approximately −20 kJ mol−1 or lower are consistent with electrostatic interaction between charged molecules and the charged metal (physisorption) whereas those of approximately −40 kJ mol−1 or higher involve charge sharing or transfer from organic molecules to the metal surface to form co-ordinate-type bonds (chemisorption) [20]. The results obtained indicate that the values of ΔGads for all the systems indicate spontaneous adsorption of the additives by the mild steel surface via chemisorption.
Effect of temperature
To investigate the mechanism of inhibition and to calculate the activation energies of the corrosion process, polarization measurements were obtained at different temperatures in the absence and presence of 350 mg L−1 of the aerial part. In the studied temperature range (25–40 °C) the corrosion current density increased with increasing temperature both in uninhibited and inhibited solutions. The corrosion current density of steel increases more rapidly with temperature in the absence of the inhibitor; these results confirm that extracts of the aerial parts (LS and F) act as efficient inhibitors in the range of temperature studied. The activation data for the corrosion process were calculated by use of Arrhenius-type plots (Fig. 5) by use of Eq. (8):
where E a represents the apparent activation energy, R is the universal gas constant, T is the absolute temperature, and A is the pre-exponential factor.
The apparent activation energies for the corrosion in the absence and presence of 350 mg L−1 LS and F extracts can be calculated from the slopes of the regression and are, respectively:
We draw attention to the decrease in activation energy in the presence of the inhibitors studied. The effect of temperature on corrosion inhibition has been studied by several authors [21–23], who reported that the activation energy is lower for the inhibited than for the uninhibited reaction. This was attributed to increased surface coverage of the metal surface with the inhibitor molecule with increasing temperature. On the other hand, the decrease in E a in the inhibited solution supports the assumption of chemisorption of the inhibitor on the metal surface [24].
Conclusions
The results obtained show that extracts of the different parts of Anacyclus pyrethrum L. (LS, F, and R) are good corrosion inhibitors for mild steel under acidic conditions. Maximum inhibition efficiency was 87.01 % for leaves and stems (LS), 88.88 % for the flowers (F), and 79.30 % for the roots (R). Good agreement was obtained between calculated inhibition efficiencies and those obtained by use of different techniques. Adsorption of the organic inhibitors by the mild steel surface was characterized by decreases in:
-
1
the cathodic and anodic current densities calculated from potentiodynamic polarization curves obtained in the presence of Anacyclus pyrethrum L. extracts;
-
2
the charge transfer resistance (R t) of solutions containing the inhibitors; and
-
3
the double-layer capacitance computed from electrochemical impedance spectroscopy experiments.
Adsorption of LS, F, and R on the mild steel surface in sulfuric acid obeys the Langmuir adsorption isotherm model and leads to formation of a protective film. Analysis of the experimental results suggests chemisorption of the inhibitors by the metal surface. The apparent activation energy of corrosion is lower in the presence of the inhibitors than in their absence; this and the higher values of the free energy of adsorption verify the chemisorptive character of the adsorption.
References
G. Schmitt, K. Bedbur, Werkstoffe und Korrosion 36, 273 (1985)
O. Benali, L. Larabi, M. Traisnel, L. Gengembre, Y. Harek, Appl. Surf. Science 253, 6130 (2007)
S. Merah, L. Larabi, O. Benali, Y. Harek, Pig. Res. Technology 37(5), 291 (2008)
O. Benali, L. Larabi, Y. Harek, J. Appl. Electrochemistry 39, 769 (2009)
O. Benali, L. Larabi, Y. Harek, J. Saudi Chem. Society 14(2), 231 (2010)
E.E. Oguzie, Mater. Chem. Phys. 99, 441 (2006)
P.C. Okafor, M.E. Ikpi, I.E. Uwah, E.E. Ebenso, U.J. Ekpe, S.A. Umoren, Corros. Sci. 50, 2310 (2008)
A. Bouyanzer, B. Hammouti, L. Majidi, Mater. Lett. 60, 2840 (2006)
F.S. de Souza, A. Spinelli, Corros. Sci. 52, 1845 (2009)
C. Selles, O. Benali, B. Tabti, L. Larabi, Y. Harek, J. Mater. Environ. Sci. 3(1), 206 (2012)
O. Benali, L. Larabi, B. Tabti, Y. Harek, Anti-Corr Meth. Mater. 52, 280 (2005)
O. Benali, L. Larabi, S.M. Mekelleche, Y. Harek, J. Mater. Sci. 41, 7064 (2006)
M. Hukovic-Metikos, R. Babic, Z. Grutac, J. Appl. Electrochem. 32, 35 (2002)
F. Mansfeld, Corrosion 37, 301 (1981)
E. McCafferty, Corros. Sci. 39, 243 (1997)
X. Wu, H. Ma, S. Chen, Z. Xu, A. Sui, J. Electrochem. Soc. 146, 1847 (1999)
H. Ma, S. Chen, B. Yin, S. Zhao, X. Liu, Corros. Sci. 45, 867 (2003)
L. Larabi, Y. Harek, O. Benali, S. Ghalem, Prog. Org. Coatings 54, 256 (2005)
N. Hackerman and E. McCafferty. in Proceedings of the fifth international congress on metallic corrosion, Houston, 1974, p 542
R.F.V. Villamil, P. Corio, J.C. Rubin, S.M.L. Agostinho, J. Electroanal. Chem. 535, 75 (2002)
I.N. Putilova, S.A. Balezin, V.P. Barannik. in Metallic corrosion inhibitors. (Pergamon, New York, 1960)
A.E. Stoyanova, E.I. Sokolova, S.N. Raicheva, Corros. Sci. 39, 1595 (1997)
M. Lagrenée, B. Mernari, M. Bouanis, M. Traisnel, F. Bentiss, Corros. Sci. 44, 573 (2002)
S. Sankarapapavinasam, F. Pushpanaden, M. Ahamed, Corros. Sci. 32, 193 (1991)
Acknowledgments
The authors extend their appreciation to the members of Laboratory of Electrochemical and Analytical Chemistry at Tlemcen University for their help in carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benali, O., Selles, C. & Salghi, R. Inhibition of acid corrosion of mild steel by Anacyclus pyrethrum L. extracts. Res Chem Intermed 40, 259–268 (2014). https://doi.org/10.1007/s11164-012-0960-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0960-8