Abstract
The effect of ultraviolet irradiation (UV), ozonation, and the combined UV/O3 systems on the decolorization and degradation of methyl orange, performed in a laboratory-scale reactor, was studied. Decoloration efficiency, UV–vis spectrum, chemical oxygen demand (COD), mass spectrum (MS), and total organic carbon (TOC) analyses were employed. Three oxidative processes including UV, ozonation (O3), and the combined UV/O3 system were assessed to select the most appropriate oxidative process in terms of methyl orange aqueous solution treatment and special emphasis was laid on the effect of reaction pH in the UV/O3 system. The results indicated that the pH value of methyl orange solution decreased with the treatment time, and it reached an acid value when oxidized for 150 min. The COD removal efficiencies of methyl orange were only 46.23 (UV), 44.54 (O3), and 71.17 % (UV/O3) in three processes, while the corresponding decolorization efficiencies were 94.8, 94.2, and 95.1 % after 150 min of treatment. The mineralization mechanism was suggested based on the analysis of the molecular structure of methyl orange, intermediate products, and final products by using MS. For the combined UV/O3 system, the basicity condition was good at the TOC removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The textile coloration industry produces large volumes of discharge effluent, and is considered as one of the major industrial polluters in China [1]. Amongst the 10,000 different dyes and pigments available, azo dyes constitute over 50 % of all textile dyes used in the industry [2]. As an important source of environmental contamination, dye-containing wastewater has aroused more attention because most of the dyes molecules have complicated structures and they are photostable, which renders them resistant toward conventional biochemical decomposition [3]. Through new environmental concerns and regulations, pressure is being placed on textile companies to reduce pollutants and reuse process water and chemicals [4].
Methyl orange is an anionic dye, widely used in the textile, printing, paper, food colorants, cosmetic, and pharmaceutical industries. The presence of the azo group (N=N) on its molecule and low biodegradability makes it an issue of concern for environmental science [5]. The strong electron-withdrawing character of the azo group stabilizes the molecule structure and it may produce secondary waste products which are also toxic against aquatic organisms and are suspected of causing adverse effects to human health. At the same time, it has carcinogenic and teratogenic effects on public health. Therefore, it should necessitate proper treatment before discharge into the environment.
At present, several physico-chemical methods, such as membrane filtration [6], flocculation [7], chemical oxidation [8], ozonation [9], ion-exchange [10], irradiation [11], and adsorption [12], have been proposed by researchers for the removal of dyes from aqueous solutions. But none of these treatments is satisfactory because of the effluent’s high degree of polarity and complex molecular structure. Furthermore, they are usually non-destructive, inefficient, and result in production of secondary waste products which need further treatment. A variety of AOPs (uv/ozone oxidation, photocatalysis, fenton oxidation) have been studied for textile wastewater treatments to meet this challenge. Promising results have been achieved by using these advanced oxidation processes: hydroxyl radicals, ozone, atomic oxygen, hydrogen peroxide, and perhydroxyl radicals which promote destruction of the target pollutant until mineralization. The hydroxyl radicals and ozone are very strong oxidizers and their reactions with organics are characterized by very high reaction rates [13]. The hydroxyl radical (∙OH), especially, is known to play an important role in degrading organics since its oxidation potential is higher than that of atomic oxygen and ozone [14]. The UV/O3 oxidation method is environmentally friendly since it does not involve the use of harmful chemical reagents. In addition, the method is easy to handle and the reactor is simple. There are still many problems yet to be solved. On the one hand, ozone is commonly used in AOPs and produces hydroxyl radical (∙OH) by electrical discharges. However, some dyes are not easily degraded by ozonation alone. On the other have, researches have been conducted to actively improve oxidative degradation performance by adding UV irradiation in an effort to make the treatment of high-concentration contaminations in wastewater more efficient. In fact, The efficacy of using ozone (O3), ultraviolet irradiation (UV), and the combined UV/O3 advanced oxidation process (AOP) to remove organic compounds in wastewater has been reported recently [15, 16]. However, many researchers have considered only the decoloration of the AOP while the mechanism research may have an important influence on the future research as well as the reaction condition which could give guidance to industrial use.
To our knowledge, there is no information reported in the literature to date concerning decolorization/degradation of methyl orange by O3, UV, and UV/O3. In this paper, a cylindrical reactor fixed UV light and pump was used to treat methyl orange water as the sample of azo dye effluent in the pattern of UV radiation, ozone oxidation, and UV/O3 combined. Salicylic acid was also treated in the reactor to detect the content of ∙OH produced by AOP reactor. Important effluent parameters, such as the structure, adsorption peak in UV–vis, and pH, were also investigated to explain the mechanism of the dye degradation. Finally, the decoloration rate, COD and TOC removal rate were monitored in an attempt to help analyze the effection of these methods.
Experimental
Reagents and materials
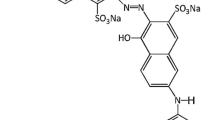
A commercial methyl orange was obtained from Tianjin Chemical Reagents and used in the present study without further purification. For the pH adjustment of the dye solution, hydrochloric acid (0.1 mol/L) and sodium hydroxide (0.2 mol/L) that purchased from Xi’an Chemical Reagents, China, were used. All chemicals were p.a. grade. Distilled water was used in this work. Salicylic acid was prepared as the capture agent of ∙OH free radical which produced by AOP apparatus. The initial concentration of salicylic acid was fixed at 0.05 g/L. The molecular structures of the methyl orange and salicylic acid are shown in Table 1.
Methods
The reactor system used in this study is represented in Fig. 1. This system consists of a raw water tank, a diving pump, a cylindrical photoreactor, a UV lamp, and an ozone generator. The tank was a stainless tank with 116 L working volume (0.46 m diameter × 0.7 m height). The cylindrical photoreactor was a stainless steel tube (0.07 m diameter × 1.55 m height) that held a high pressure UV lamp enclosed in stainless steel sleeve (0.015 m diameter × 1.2 m height), giving a net working volume of 0.02 m3. The average intensity of the lamp was 185 W. Ozone was generated from air by using an ozone generator with input rate of 0.25 m3/h. The recycling loop utilized a diving pump and latex soft tubing to recycle water from the base of the reactor to the top of the photoreactor back at a rate of 20 L/h.
Considering only ozone treatment, the light switch of UV lamp was turned off in the experimental apparatus above. When only UV treatment is considered, the operating conditions were similar to those described above for the reactor, with the airflow rate of 0.25 m3/h from gas bump.
Methods of analysis
The experiments were conducted in a batch process mode, whereby 6 L of methyl orange solution at room temperature (25 °C) was pumped into the cylindrical photoreactor and then subjected to a treatment for a specified duration. Three oxidation processes including ozone, UV, and UV/O3 systems were assessed to select the most appropriate oxidative process in terms of methyl orange solution treatment. Decolorization efficiency is defined as percent decrease of absorbance according to the following Eq. (1):
where A 0 is the absorbance at the maximum absorption wavelength (465 nm) of initial methyl orange solution, and A is the maximum absorption wavelength (465 nm) of the methyl orange solution after the oxidation treatment. The UV–vis spectrum of the methyl orange solution was recorded in the range from 200 to 800 nm using a UV 2300 analyst spectrophotometer (Shanghai Techcomp Instrument, China), showing that the maximum absorbance wavelength of methyl orange and salicylic acid was 465 and 296 nm, respectively. To determine the influence of pH value of the initial methyl orange solution on the decolorization efficiency, a pH of methyl orange solutions was adjusted to three different values (pH 9.00, 7.00, and 5.00) by adding sodium hydroxide or hydrochloric acid. The pH value of the solutions was measured by using a digital pH meter (Beckman F44). COD determination was conducted with the digestion system and COD determinator (Lianhua COD Determinator, China). The TOC values of degradation of methyl orange were detected by a JEOL JEM-3010 total organic carbon analyzer to determine the extent of mineralization. The reaction products of methyl orange were detected by mass spectrometry (MS). MS analysis were carried out on a matrix assisted laser desorption/ionization time of flight mass spectrometer (MALDI–TOF MS; Krato Analytical, Shimadzu Biotech, Manchester, UK) using a standard procedure involving 1 mL of the sample solution.
Results and discussion
Decolorization of methyl orange in different systems
Experiments were performed at the constant temperature of 25 °C with ultraviolet irradiation alone, ozonation alone, and the combined UV/O3 system, respectively, to investigate the different decolorization of three processes. The concentration C 0 of methyl orange was fixed at 200 mg/L with the initial pH of 7 and the gas flow rate was 0.25 m3/h (instead of ozone, air was bubbled into the reactor in the run of ultraviolet irradiation alone). Ozone concentration was 0.05 mg/L for ozonation alone and the combined UV/O3 system, whereas ultraviolet-light power was 185 W for ultraviolet irradiation alone and the combined UV/O3 system. As shown in Fig. 2, the decolorization rates of methyl orange at the 60th minute reached 79.02 % for ultraviolet irradiation, 68.8 % for ozonation, and 89.81 % for the combined UV/O3 system. Further, the decolorization of methyl orange was improved in the combined UV/O3 system and the decolorization rates were 94.8, 94.2, and 95.1 % after 150 min, respectively, in the process of ultralight alone, ozonation alone, and the combined UV/O3. This revealed that the decolorization of methyl orange in the three systems mainly occurred in the early stage of the reaction, and, in order to compare the effect of the decolorization rate on different conditions, we chose the decolorization rate of methyl orange before the 60th minute and estimated the values of the slope of the curve. Thus, the slope values of fitting curves for UV, O3, and UV/O3 were 1.32, 1.15, and 1.50, respectively. The results showed that the decolorization rate of methyl orange in combined UV/O3 was the highest, followed by ultraviolet irradiation alone, while ozonation alone was the lowest. This trend is similar with many reports in the literature [17–19]. The reason was that the rate of formation of hydrogen peroxide would be the highest and quickest transfer of ozone to more hydroxyl radicals which could enhance the degradation of the methyl orange. At the same time, UV light was an initiator which accelerated the formation of more hydroxyl radicals, compared to ozonation alone.
The pH value ranged from 5 to 9 was researched to investigate the UV/O3 oxidation reaction pathways of methyl orange. The results are presented in Fig. 3, where it is shown that a higher decolorization was presented in alkaline or acid condition than neutral condition, and the decolorization rates of methyl orange were 99.1, 95.1, and 99.3 %, respectively, at pH 5, 7, and 9 after 150 min. After 60 min, the decolorization rates in three different pH reached 90 %, which indicated that alkaline or acid condition was more favorable for the decolorization of methyl orange. As can be seen from Figs. 2 and 3, the decolorization rates of methyl orange increased with reaction time and the pH had an important influence on the results. It was good for color removal when the initial pH was basic or acid when the decolorization would reach 90 % after 150 min.
The UV–visible absorption spectra analysis
In order to investigate the changes in the molecule of methyl orange with the reaction time in the three methods, UV–vis spectra changes in the dye solution were observed and the corresponding spectra are shown in Fig. 4. This shows that the characteristic absorption peak of methyl orange in solution had two bands: the main band was in the visible region, with its maximum absorption at 465 nm, and the other band in the ultraviolet region located at 272 nm. The characteristic absorption peak is attributed to the dye chromophore structure. In the three systems, they were the same phenomena that the absorption intensity of methyl orange in solution became weaker along with the reaction time. It could be explained that the aromatic ring structure of methyl orange molecules were destroyed in the three systems. However, there were some differences in the reaction rate of methyl orange. In the UV/O3 process, the absorption peak quickly diminished in 60 min and the decolorization rate was the highest in the three processes during the same reaction time. The second one was the ultraviolet process and the single ozonation was the last one.
These results were in agreement with the above outcomes and further proved that the extended aromatic methyl orange absorbed at 465 nm and the aromatic ring absorbed in the range 200–280 nm. This trend suggested that the polyaromatic rings in methyl orange started to degrade, creating a mono-substituted aromatic, and a weak band appeared at 320 nm. With the reaction time increasing, both bands at 272 and 465 nm started to decrease and the peak at 320 nm also became weak. This indicated that the methyl orange had started to degrade into inorganic products.
Comparion of COD removal in UV, ozonation and the combined UV/O3 system
It is known that complete decolorization of methyl orange does not mean that it is completely mineralized. In order to discover the mineralization degree of methyl orange, COD was investigated at pH = 7 during the reaction. The results (Fig. 5) show that, after 150 min treatment, the COD removal effect was 46.23, 44.54, and 71.17 % in ultraviolet irradiation alone, ozonation alone, and the combined UV/O3 processes, respectively. It was thus found that methyl orange was not mineralized completely in these three processes. The synergy between ultraviolet and ozonation in the UV/O3 system could improve the COD removal effect. This result demonstrated that single ultraviolet irradiation and ozonation alone also produced hydroxyl radicals, but the production efficiency of the hydroxyl radicasl was less than that of the combined UV/O3 process. Furthermore, promoted by UV light, ozonation could generate more hydroxyl radicals and get a better degradation effect, since the hydroxyl radical is a very powerful oxidizing agent which could deal with organic compounds until mineralized.
Table 2 shows the decolorization efficiency after 150 min reaction and the COD removal efficiency of methyl orange after 150 min treatment. It shows the same trend between the decolorization efficiencies and the COD removal efficiencies in the three processes. However, the decolorization efficiency was much higher than the corresponding COD removal efficiency. The COD removal efficiencies of methyl orange were only 46.23, 44.54, and 71.17 % in the three processes, while the corresponding decolorization efficiencies were 94.8, 94.2, and 95.1 % after 150 min treatment. The reason is probably that the chromophore has been destoyed completely, but the organic pollutant has been oxidized to micromolecule pollutants, which have not been oxidized to CO2 and H2O [20–22].
Effect of initial pH on the degradation of methyl orange under the combined UV/O3 system
The amount of hydroxyl radicals produced by the combined UV/O3 system is greatly affected by the pH value, and as a result effective methyl orange decomposition is largely dependent on the pH of the solution. The variation of pH values of the solution during the treatment process is shown in Fig. 6 for all three initial pH values for methyl orange. The curves show that the pH values considerably decreased for all three initial pH. The initial pH values decreased with reaction time at higher than 5.0, while the final pH values in the solution remained almost constant at from 2.6 to 4.0.
The effect of initial pH values on the COD removal rate of methyl orange is shown in Fig. 7. This result indicated that methyl orange could be converted to organic acids such as formic acid and oxalic acid, thereby reducing the neutral and alkaline solutions pH. As demonstrated in Fig. 3, the decolorization rate of methyl orange sharply increased with increasing pH from 5 to 9, which was different from the changes of COD. This was largely attributed to hydroxyl radicals that formed from ozonation decomposition at high pH, whereas molecules of ozone remain dominant at low pH. Thus, optimal pH for the degradation could be required. To verify the intermediate process, further study would be needed to determine the degradation mechanism involving ozonation and hydroxyl radicals in the UV/O3 system.
Proposed reaction mechanism for degradation of methyl orange
The above results verified that ultraviolet irradiation and ozonation were combined to enhance the decolorization and mineralization of methyl orange in aqueous solution. The UV/O3 oxidation was more efficient than ozonation alone or UV irradiation for the quantity of hydroxyl radicals. The generated hydroxyl radicals acted as strong oxidizing agents that are responsible for attacking methyl orange to form intermediates before producing degradation products. To investigate the role of the hydroxyl radicals, the degradation process was carried out in the presence of a hydroxyl radical scavenger like salicylic acid. Salicylic acid is capable of deactivating the hydroxyl radicals and its derivatives. Salicylic acid reacted with the hydroxyl radicals and it would also be degraded at the same time. In order to investigate the formation of ∙OH radicals and the degradation productions of methyl orange, the MS was used to test the reactive oxygen species in the UV/O3 process. By the detection of MS, the reaction products were mainly formic acid, acetic acid, and N,N′-diethylformamide, which can be easily biodegraded into CO2 and H2O [23, 24].
The information of the degradation products of the methyl orange solution by UV/O3 system is shown in Fig. 8a, b revealing the several peaks which appeared in the aqueous solution of methyl orange with or without a scavenger in the same conditions. The results indicated that the addition of salicylic acid had weak effects on the characteristics of methyl orange. The 15 and 60 min degradation samples of methyl orange solution were analyzed using MS (Fig. 8c–d).
It can be inferred that the peak of m/z 304 corresponds to the remaining structure of the methyl orange molecule taking off a Na+, and the structure is shown in Fig. 8a. The structure corresponding to peak of m/z 136 was salicylic acid and is shown in Fig. 8b. Oxidative degradation of methyl orange occurs generally by the subsequent attacks of ∙OH radicals. It can be inferred that the peak of m/z 289 corresponds to the remaining structure of the methyl orange molecule taking off a Na+ and a –CH3. The molecule ion m/z 289 was a radical molecular ion and its structure is shown in Fig. 8c. Here, the –CH3 was taken off under the attack of the radical species in liquid phase [25, 26]. It can be proposed that oxidation of methyl orange proceeded by the attack of the ∙OH radicals, and the intermediate products were deduced according to the bond energies of the methyl orange molecule. The products such as benzene sulfonic acid, N,N′-dimethylaniline, and 4-hydroxy-N,N′-dimethylaniline could arise from the oxidation reaction. Furthermore, compared to the MS spectrograms of degradation solution in different reaction times (Fig. 8c–d), it can be inferred that the degradation process contains three stages: bond-breaking oxidation process, ring-opening process, and complete oxidation process. This was followed by the generation of inorganic final products, such as SO4 2− and NO3 −. The characteristic peak at m/z 304 disappears in Fig. 8d after complete decolorization of methyl orange (60 min), indicating the formation of other intermediate products. The degradation mechanism is suggested in Fig. 9. The nature of the degradation product was confirmed from total organic carbon analysis.
The total organic carbon concentration is very important because it is one of the best observations that determines the exact point in time when the methyl orange has been entirely degraded and its complete mineralization has been achieved. Figure 10 shows the TOC removal rate of methyl orange during UV/O3 reaction in different initial conditions. It can be easily found that TOC removal rate were gradually increased with UV/O3 reaction time no matter what the initial pH, with basic condition having a better removal rate than neutral condition. In other words, alkaline condition was good for the degradation of methyl orange.
Conclusion
This study showed ozonation in combination with 185 W ultraviolet irradiation had a synergistic effect on the decolorization of methyl orange, and the synergistic effect was more pronounced on the COD removal rate during the degradation process. The pH had an important influence on the degradation of methyl orange, and the alkaline or acid condition were better than neutral condition under the UV/O3 process. The pH was reducing with the time of reaction, and stayed in acid stability in the UV/O3 reaction which indicated that a large amount of methyl orange became acid matter at the end of the reaction and only a little methyl orange was totally converted to SO4 2− and NO3 −. It can be proposed that oxidation of methyl orange proceeded by the attack of the ∙OH radicals, and the intermediate products were deduced, according to the bond energies of the methyl orange molecule. The products such as benzene sulfonic acid, N,N′-dimethylaniline, and 4-hydroxy-N,N′-dimethylaniline could arise from the oxidation reaction. Future work will be focused on the mineralization mechanism of methyl orange in the UV/O3 process.
References
T. Kurbus, A.M. Marechal, D.B. Vončina, Dyes Pigm. 58, 245 (2003)
C. Tang, V. Chen, Water Res. 38, 2275 (2004)
B.P. Dojčinović, G.M. Roglić, B.M. Obradović, M. Kuraica, M. Kostić, J. Nešić, D. Manojlović, J. Hazard. Mater. 192, 763 (2011)
S. Haji, B. Benstaali, N. Al-Bastaki, Chem. Eng. J. 168, 134 (2011)
S. Hosseini, M.A. Khan, M.R. Malekbala, W. Cheah, T.Y. Choong, Chem. Eng. J. 171, 1124 (2011)
F. Du, A. Hawari, M. Baune, J. Thőming, J. Membr. Sci. 336, 71 (2009)
N.F. Jaafar, A.A. Jalil, S. Triwahyono, M.N. Muhid, N. Sapawe, M.A.H. Satar, H. Asaari, Chem. Eng. J. 191, 112 (2012)
Y. Kong, Z.L. Wang, Y. Wang, J. Yuan, Z.D. Chen, New Carbon Mater. 26, 459 (2011)
H. Zhang, L.J. Duan, D.B. Zhang, J. Hazard. Mater. 138, 53 (2006)
M.G. Kiseleva, L.V. Radchenko, P.N. Nesterenko, J. Chromatogr. A 920, 79 (2001)
T. Tasaki, T. Wada, K. Fujimoto, S. Kai, K. Ohe, T. Oshima, Y. Baba, M. Kukizaki, J. Hazard. Mater. 162, 1103 (2009)
Y.J. Yao, B. He, F.F. Xu, X.F. Chen, Chem. Eng. J. 170, 82 (2011)
A.N. Ökte, Ö. Yılmaz, Appl. Catal. A Gen. 354, 132 (2009)
G.K. Parshetti, A.A. Telke, D.C. Kalyani, S.P. Govindwar, J. Hazard. Mater. 176, 503 (2010)
C. Lee, J. Yoon, J. Photochem. Photobiol. A 165, 35 (2004)
Ş. Gül, Ö. Özcan-Yıldırım, Chem. Eng. J. 155, 684 (2009)
S. Al-Qaradawi, S.R. Salman, J. Photochem. Photobiol. A Chem. 148, 161 (2002)
H.S. El-Desoky, M.M. Ghoneim, N.M. Zidan, Desalination 264, 143 (2010)
C.P. Bai, X.F. Xiong, W.Q. Gong, D.X. Feng, M. Xian, Z.X. Ge, N. Xu, Desalination 278, 84 (2011)
Y. Akama, A. Tong, M. Ito, S. Tanaka, Talanta 48, 1133 (1999)
L.G. Devi, S.G. Kumar, K.M. Reddy, C. Munikrishnappa, J. Hazard. Mater. 164, 459 (2009)
X.F. Lü, J. Li, C. Wang, M.Y. Duan, Y. Luo, G.P. Yao, J.L. Wang, Appl. Surf. Sci. 257, 795 (2010)
S. Haji, B. Benstaali, N. Al-Bastaki, Chem. Eng. J. 168, 134 (2011)
M. Joshi, S.P. Kamble, N.K. Labhsetwar, D.V. Parwate, S.S. Rayalu, J. Photochem. Photobiol. A 204, 83 (2009)
F.M. Huang, L. Chen, H.L. Wang, T.Z. Feng, Z.C. Yan, J. Electrostat. 70, 43 (2012)
Y.H. He, F. Grieser, M. Ashokkumar, Ultrason. Sonochem. 18, 974 (2011)
Acknowledgments
This research was financially supported by the National Natural Science Foundation Project (No. 21177079) and the major State Basic Research Development Program of China (973 Program) (No. 2011CB612309) and Shaanxi University of Science and Technology Scientific Research Foundation for doctor (BJ11-19).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lü, Xf., Ma, Hr., Zhang, Q. et al. Degradation of methyl orange by UV, O3 and UV/O3 systems: analysis of the degradation effects and mineralization mechanism. Res Chem Intermed 39, 4189–4203 (2013). https://doi.org/10.1007/s11164-012-0935-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0935-9