Abstract
A hemicyanine dye with imidazole as the acceptor was designed and prepared as a chemosensor for fluoride anions. The experimental results demonstrated that it is a fluorometric probe for fluoride anion with good sensitivity and high selectivity. This probe showed obvious changes in UV–Vis absorption and fluorescence spectra in the presence of fluoride anions, which are attributed to the deprotonation of N–H on the imidazole moiety with fluoride anions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Owing to its relative size and electronegativity, fluoride plays a fundamental role in industrial and biological processes [1–4]. Fluoride is associated with dental caries, treatment for osteoporosis, and the refinement of uranium used in nuclear weapon manufacture [5–7]. However, excess fluoride can lead to fluorosis; for example, ahigh level of fluoride has been reported to be implicated in dental and skeletal fluorosis, as well as inhibition of neurotransmitter biosynthesis in fetuses [8–10]. In a word, the diversity of its function, both beneficial and otherwise, makes the careful control of fluoride of great importance. As a consequence, the methodologies for indicating fluoride, which are developed to provide critical information for fluoride, have attracted growing attention [11–13]. Among these techniques, fluorescent molecular sensing, which can translate molecular recognition into tangible fluorescence signals, has received much attention [14–16].
A general approximation to the development of anion chemosensors is the coupling of at least two units: the binding site (receptor) and the signaling subunit (fluorophore) [17, 18]. Up to now, a variety of anion binding motifs appended to the fluorophore core for effective anion binding have been employed [19]. For most of the reported anion fluorescent sensors, molecules containing H-bond donors toward anions are widely used as receptors for recognition and sensing purposes [20]. Among these molecules, the N–H fragment of a receptor can be a most promising motif, because the H-bond donor tendency changes when interact with the fluoride anion [21, 22]. Kong et al. [21] reported a probe synthesized under microwave irradiation. The probe shows red shift and absorbance intensity changes in spectra in the presence of fluoride anions. Qu Yi et al. [22] presented a red fluorescent chemosensor based on diketopyrrolopyrrole; the addition of fluoride results in vivid orange-to-red absorption color change and yellow-to-red emission color change. Both of them are based on the deprotonation of N–H with fluoride anions.
Here, we present an easy-to-prepare fluorescent probe, 2-(2-(1H-imidazol-4-yl) vinyl)-1,3,3-trimethyl-3H-indolium iodide, for fluoride anion sensing based on incorporating an imidazole heterocycle via a methine linker into a fluorogenic indolium core. It is reported and used as a fluorescent chemosensor for fluoride anions for the first time. The interaction of anions with H-bond-donating receptors (imidazole) will lead to a change of the electron distribution of the whole molecule, which can be amplified to a macroscopic level—the color and the spectra—thus providing colorimetric and fluorescent sensing of the recognition event.
Experimental
Chemicals and instruments
All chemicals and reagents were commercially available and used without further purification. All solvents were carefully dried and freshly distilled according to standard procedures. 1H NMR and 13C NMR spectra were recorded in DMSO-d6 on a Varian Inova 500 MHz NMR spectrometer using TMS as an internal standard. Mass spectra were obtained with Bruker Daltonics Autoflex tof/tof III. UV–Vis absorption spectra were recorded on a PerkinElmer Lambda 750 spectrophotometer. Fluorescence spectra were obtained on a Varian Eclipse fluorescence spectrophotometer at room temperature.
Synthesis of the probe 2
1,2,3,3-tetramethyl-3H-indolium iodide (1) was prepared according to the literature procedure [23] described in Scheme 1.
1,2,3,3-tetramethyl-3H-indolium iodide (1) (1.54 g, 5 mmol) and imidazole carbaldehyde (0.58 g, 6 mmol) were added to 20 mL ethanol. The mixture was stirred at 85 °C for 6 h under a nitrogen atmosphere. The reaction mixture was cooled, filtered, and washed with ethanol, to afford a yellow powder 0.94 g, yield 49.9 %.1H NMR (500 MHz, DMSO-d6): δ 1.74 (s, 6H), 3. 98 (s, 3H), 7.36 (d, 1H, J = 15.74 Hz), 7.53–7.60 (m, 2H), 7.83 (t, 2H, J = 7.27 Hz), 8.08 (m, 2H), 8.46 (d, 1H, J = 15.74 Hz). 13C NMR (500 MHz, DMSO-d6): δ 26.28, 34.25, 52.04, 108.44, 115.00, 123.29, 129.06, 129.33, 142.35, 143.42, 181.75. HRMS: calculated for [M–I−]: 252.1495, measured: 252.1500.
Results and discussion
The fluorescent probe was successfully synthesized as shown in Scheme 1, and was characterized by NMR spectroscopy and mass spectrometry. Its optical properties were investigated by UV–Vis and fluorescence spectroscopy. The probe was composed of a imidazole heterocycle and a fluorogenic indolium core, and the electron-rich imidazole was designed to be the receptor group.
Spectra titration of 2 with 10 eq of halide anions
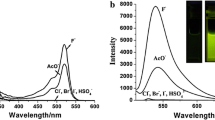
According to reports, the N–H fragment of imidazole can interact with the fluoride selectively. To confirm this assumption, we first investigated the selectivity of the probe. In the experiments, n-Bu4NX (TBAX) (X stands for F− Cl− Br− I−) as the halide anions source was gradually added to a dichloromethane (DCM) solution of the probe.
As can be seen from Fig. 1, when Cl− was added to the solution, the maximum absorption wavelengths (λmax) of the probe red shifted from 426 to 443 nm, while the absorbance intensity showed no obvious change. The results of Br− and I− were similar to that of Cl−, except for the intensity differences. Addition of TBAF exhibited remarkable spectral variations, revealing a new red-shifted absorption band at 491 nm, which is attributed to the deprotonation with fluoride anions, and the absorbance showed a sharp decrease, from 0.25 to 0.03.
Upon addition of halide anions, no significant change in the fluorescence spectra were observed; with the addition of Cl− or Br−, the other two halide anions showed different responses. The spectra of the probe with I− and F− exhibited a small blue shift and gradually diminished. While the reason may be different, the one with I− may have resulted from the heavy atom effect, while the F− resulted from deprotonation of the imidazole (Scheme 2).
With comprehensive consideration, the selectivity of designed probe 2 for fluoride anions over the other anions is remarkably high and efficient.
Fluorescence spectra titration of 2 with fluoride anion
After the determination of the selectivity, the sensitivity was also investigated. The result is presented in Fig. 2. Because the absorption spectra titration showed no apparent change, we mainly studied emission spectra. After the addition of different equivalences of fluoride anions, the fluorescence gradually diminished with the increase of fluoride. When the equivalence is 4 times that of the probe, it exhibited greatly decreased fluorescence intensity. The intensity of the probe went from 625 (with 0 F−) to 145 (4eq F−), and the maximum emission wavelengths (λem) blue shift from 525 to 500 nm. The change of the fluorescence spectra is attributed to the deprotonation with fluoride anions of the imidazole.
Conclusion
We have presented a hemicyanine dye as both a colorimetric and fluorescent selective chemosensor for fluoride anions in DCM, which operates by interaction of the H-bond contained in imidazole with the fluoride anions. The obvious absorption and fluorescence variation upon the addition of fluoride anions can be observed both by the naked eye and by optical responses. Other halide anions such as Cl−, Br− and I− were not found to induce any variation in either the absorption or fluorescence spectra.
References
Y. Li, L. Cao, H. Tian, J. Org. Chem. 71, 8279–8282 (2006)
R. Martínez-Manêz, F. Sancenón, Coord. Chem. Rev. 250, 3081–3093 (2006)
A. Susumu, G.D. Matthew, M.F. Thomas, T.G. Hibbert, T.D. James, G.I. Kociok-Köhn, Chem. Commun. 14, 1640–1641 (2004)
I.H.A. Badr, M.E. Meyerhoff, J. Am. Chem. Soc. 127, 5318–5319 (2005)
G. Zhou, Y. Cheng, L. Wang, X. Jing, F. Wang, Macromolecules 38, 2148–2153 (2005)
Y.P. Zhao, C.C. Zhao, L.Z. Wu, L.P. Zhang, C.H. Tong, Y.J. Pan, J. Org. Chem. 71, 2143–2146 (2006)
M. Takeuchi, T. Shioya, T.M. Swager, Angew. Chem. Int. Ed. 40, 3372–3376 (2001)
C. Suksai, T. Tuntulani, Chem. Soc. Rev. 32, 192–202 (2003)
T. Mizuno, W.H. Wei, L.R. Eller, J.L. Sessler, J. Am. Chem. Soc. 124, 1134–1135 (2002)
S.H. Mashraqui, R. Betkar, M. Chandiramani, D. Quinonero, A. Frontera, Tetrahedron Lett. 51, 596–599 (2010)
L. Panzella, A. Pezzella, M. Arzillo, P. Manini, A. Napolitano, Tetrahedron 65, 2032–2036 (2009)
X.F. Yang, H.P. Qi, L.P. Wang, Z. Su, G. Wang, Talanta 80, 92–97 (2009)
E. Galbraith, T. Fyles, M.F. Marken, M.G. Davidson, T.D. James, Inorg. Chem. 47, 6236–6244 (2008)
E.J. Cho, B.J. Ryu, Y.J. Lee, K.C. Nam, Org. Lett. 7, 2607–2609 (2005)
K.H. Lee, H.Y. Lee, D.H. Lee, J.I. Hong, Tetrahedron Lett. 42, 5447–5449 (2001)
C.H. Lin, S. Selvi, J.M. Fang, P.T. Chou, C.H. Lai, Y.M. Cheng, J. Org. Chem. 72, 3537–3542 (2007)
R.W. Sinkeldam, N.J. Greco, Y. Tor, Chem. Rev. 110, 2579–2619 (2010)
M. Sameiro, T. Gonçalves, Chem. Rev. 109, 190–212 (2009)
V. Amendola, D. Esteban-Goamez, L. Fabbrizzi, M. Licchelli, Acc. Chem. Res. 39, 343–353 (2006)
B. Liu, H. Tian, J. Mater. Chem. 15, 2681–2686 (2005)
F.P. Kong, Q.J. Liu, X. Wu, Z. Wang, Q. Chen, L.S. Chen, J. Fluoresc. 21, 1331–1335 (2011)
Y. Qu, J. Hua, H. Tian, Org. Lett. 12, 3320–3323 (2010)
L. David, J. Gallaher, J. Mitchell, Analyst 124, 1541–1546 (1999)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, L., Li, T. & Chen, L. A fluorescent chemosensor for fluoride anions based on a hemicyanine dye. Res Chem Intermed 39, 3525–3530 (2013). https://doi.org/10.1007/s11164-012-0859-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0859-4