Abstract
The inhibition of mild steel corrosion in aerated acid mixture of 0.5 N H2SO4 and 0.5 N HCl solution was investigated using potentiodynamic polarization studies, linear polarization studies, electrochemical impedance spectroscopy, adsorption, and surface morphological studies. The effect of inhibitor concentration on corrosion rate, degree of surface coverage, adsorption kinetics, and surface morphology is investigated. The inhibition efficiency increased markedly with increase in additive concentration. The presence of PEG and PVP decreases the double-layer capacitance and increases the charge-transfer resistance. The inhibitor molecules first adsorb on the metal surface following a Langmuir adsorption isotherm. Both PEG and PVP offer good inhibition properties for mild steel and act as mixed-type inhibitors. Surface analysis by scanning electron microscopy (SEM) and atomic force microscopy (AFM) shows that PVP offers better protection than PEG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acid solutions are generally used for removal of rust and scale in industrial processes, and the deterioration of the metal due to these processes is very significant. Inhibitors are used in these processes to control metal dissolution. Hydrochloric acid is widely used in pickling of steel and different steel-based alloys. The most popular inhibitors are organic compounds containing N, S, and O atoms [1, 2]. Organic compounds containing functional electronegative groups and π electrons in triple or conjugated double bonds are also good inhibitors. The study of corrosion processes and their inhibition by organic compounds is an active area of contemporary research. Protection of steel against corrosion using polymeric coatings has become another popular method and the subject of considerable research in recent years [3, 4]. The advantages of polymeric coatings compared with chemical inhibitors are their low cost, extreme stability in acidic media, presence of multiple adsorption sites, ability to form complexes with metal ions, and extended surface coverage on metal surfaces. Several studies related to use of conducting polymer coatings on metals and alloys for corrosion protection have been reported in recent years [5–15]. In addition to these polymers, poly(N-ethylaniline), polythiophene, and polyindole were also investigated by several researchers [16, 17]. Electropolymerization of conducting polymers on metals and alloys is conducted within the solubility potentials of the electrode. The electrode should be kept in the passive region to be protected from corrosion. Passivity properties of both metals and alloys depend on their potential, temperature, and medium pH. Knowledge of these parameters is highly important for determining the conditions of studies of conducting polymer coatings. Even though monolayer polymeric coatings offer good corrosion protection for metals and alloys, bilayer polymeric coatings were found to be more effective in preventing corrosion [18, 19]. It is well known that polymers which contain conjugated π bonds provide the highest conductivity.
Experimental
Inhibitors
Two inhibitors, polyethylene glycol (PEG) [Mn = 10,000 g/mol, Sigma Aldrich] and polyvinyl pyrrolidone (PVP) [Mn = 44,000 g/mol, Sigma Aldrich], were used for the present study. The structure of the inhibitors is given in Fig. 1.
Medium
The medium for the study was prepared from reagent-grade H2SO4, HCl (E. Merck), and double-distilled water. All tests were performed in aerated medium under normal atmospheric pressure and room temperature (300 K).
Materials
The mild steel samples used were of the following chemical composition (wt.%): C (0.20 %), Mn (1 %), P (0.03 %), S (0.02 %), and Fe (98.75 %). Mild steel specimens of required dimensions were first rubbed with different grades of emery papers to remove rust particles and then subjected to the action of a buffing machine with a cotton wheel and fiber wheel having buffing soap to ensure mirror bright finish. The mild steel specimens used for electrochemical studies were cut into 4.8 × 1.9 cm2 coupons, but only 1 cm2 area was exposed during each measurement.
Electrochemical measurements
Electrochemical tests were carried out in a conventional three-electrode cell with metal specimen as the working electrode, platinum sheet (1 cm2 surface area) as auxiliary electrode, and saturated calomel electrode as reference electrode. The working electrode was first immersed in the test solution, and after establishing a steady-state open-circuit potential (OCP), electrochemical measurements were carried out with a Gill AC computer-controlled electrochemical workstation (model no. 1475; ACM, UK). Electrochemical impedance spectroscopy (EIS) measurements were carried out with amplitude of 10 mV (root mean square, RMS) alternating-current (AC) sine wave in the frequency range from 10 kHz to 0.1 Hz. Potentiodynamic polarization curves were obtained in the potential range from −250 mV to +250 mV at sweep rate of 1 mV/s.
Adsorption studies
It is generally assumed that the adsorption of inhibitors onto a metal surface is the primary step in the inhibition mechanism. To determine the adsorption mode, various isotherms were constructed from the results obtained from polarization studies, and the one that gave a straight-line graph in a plot of surface coverage against concentration was considered the Langmuir isotherm.
Scanning electron microscopy (SEM)
The surface morphology of the sample under study in the absence and presence of inhibitors was investigating using a Digital Microscope Imaging scanning electron microscope (model SU6600, serial no. HI-2102-0003) at accelerating voltage of 20.0 kV. Samples were attached on the top of an aluminum stopper by means of carbon conductive adhesive tape. All micrographs of the specimen were taken at 500× magnification.
Atomic force microscopy (AFM)
Mild steel strips of 4.8 × 1.9 cm2 size were prepared as described in the above section. After immersion in 0.5 N HCl and 0.5 N H2SO4 acid mixtures with and without 200 ppm of inhibitor at 300 K for 120 h, the specimens were cleaned with distilled water, dried, and then used for AFM studies.
Results and discussion
Potentiodynamic polarization studies
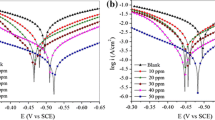
Polarization studies were carried out to generate knowledge concerning the kinetics of the anodic and cathodic reactions. The potentiodynamic polarization curves for mild steel in acid solution in the absence and presence of various concentrations of the inhibitors are shown in Fig. 2. The values of electrochemical kinetic parameters such as the corrosion potential (E corr), corrosion current density (I corr), and Tafel slopes (β a and β c) obtained from these graphs by extrapolation method are listed in Table 1. The corrosion inhibition efficiency was calculated using the relation
where \( I_{{{\text{corr}}^{*} }} \) and I corr are the uninhibited and inhibited corrosion current density, respectively, obtained by extrapolation of Tafel lines in the corrosion potential. It is evident from Table 1 that the value of I corr decreased from 2.3175 μA cm−2 for the blank to 0.1156 μA cm−2 in presence of 10 ppm of inhibitor, and it further reduces to 0.0150 μA cm−2 as the concentration of PVP was gradually increased to 200 ppm; also, the value of I corr decreased from 2.3175 μA cm−2 for the blank to 0.1385 μA cm−2 in presence of 10 ppm of inhibitor, and it further reduces to 0.0291 μA cm−2 as the concentration of PEG was increased to 200 ppm. The inhibitor molecule first adsorbs on the mild steel surface and blocks available reaction sites [20]. The surface coverage increases with the inhibitor concentration. The formation of a surface inhibitor film on the metal surface provides considerable protection of the metal against corrosion [21, 22]. This film reduces the active surface area exposed to the corrosive medium and delays the hydrogen evolution and iron dissolution. To determine the polarization resistance, R p, the potential of the working electrode was ramped ±10 mV in the vicinity of the corrosion potential at scan rate of 60 mV/s. The polarization resistance was determined from the slope of potential versus current lines:
where A is the surface area of the electrode.
Electrochemical impedance spectroscopy
All impedance measurements were performed under potentiostatic conditions after 1 h immersion in the corrosion medium. Nyquist plots of uninhibited and inhibited solutions containing different concentrations of polymer materials were obtained over a frequency range of 0.1–10,000 Hz, and the Nyquist plots and corresponding Bode plots for various concentrations of PVP and PEG are given in Figs. 3a, b and 4a, b. The Nyquist and Bode diagrams have similar shape with different concentrations of the inhibitor molecules. It is therefore clear that the mechanism of corrosion remains unaffected during the addition of inhibitor molecules. The simplest fit is represented by a Randles equivalent circuit (Fig. 5), which is a parallel combination of the charge-transfer resistance (R ct) and a constant-phase element (CPE), both in series with the solution resistance (R s). The impedance spectra exhibit one single depressed semicircle, the diameter of which increases with increasing inhibitor concentration. The single semicircle indicates that charge transfer takes place at the electrode–electrolyte interface, and the corrosion reaction of steel is controlled by the charge-transfer process [23, 24]. Also, these impedance diagrams are not perfect semicircles, which is related to frequency dispersion due to of the roughness and inhomogeneity of the electrode surface [25]. Furthermore, the impedance response of the corrosion rates in uninhibited solution changed significantly after addition of inhibitor to the corrosive solution; as a result, the real-axis intercept at high and low frequencies in the presence of inhibitor is larger in the absence of inhibitor (blank solution) and increases as the inhibitor concentration increasing. The capacitive loop corresponds to the charge-transfer reaction, which depends on either direct electron transfer at the metal surface or electron conduction through the film surface. The impedance (Nyquist) diagrams contain depressed semicircles with center under the real axis. Such behavior is characteristic of solid electrodes, being attributed to different physical phenomena such as roughness, inhomogeneities of the solid surfaces, impurities, grain boundaries, and the distribution of surface-active sites. In this case, a constant-phase element (CPE) is introduced into the circuit instead of a pure double-layer capacitor to give a more accurate fit [26, 27]. The impedance function of a CPE has the following equation:
where A is the CPE constant (in Ω−1 Sn cm−2), W is the sine wave modulation angular frequency (rad−1), i2 = −1 is the imaginary number, and α is an empirical exponent (0 ≤ n ≤ 1), which measures the deviation from ideal capacitive behavior [28, 29]. The opposite trend in the values of R ct and C dl (Table 2) over the whole concentration range can be supposed to indicate that a protective layer covers the whole surface of the electrode. The impedance values corresponding to PVP and PEG are given in Table 2. The charge-transfer resistance increases from 12.38 for inhibitor-free solution to 499.40 and 365.90 μF cm−2 upon addition of 200 ppm PVP and PEG, resulting in 97.52 and 96.62 % inhibition efficiency. The increase in R ct value is attributed to the formation of an insulating protective film at the metal–solution interface [30]. The double-layer capacitance decreases from 272.71 to 88.14 Ω cm2 in the presence of 200 ppm PVP and decreases from 272.71 to 85.21 Ω cm2 in the presence of 200 ppm PEG. The initial decrease in C dl value from blank solution to electrolyte containing inhibitor is due to a decrease in the local dielectric constant, while further decrease in C dl with increasing concentrations of inhibitor is due to increase in the thickness of the electrical double layer [31–33]. The time constant of the charge-transfer process (τ d) is defined by
The adsorption of inhibitor molecules on the metal surface decreases the double-layer capacitance (C dl) and increases the time constant (τ d) value (Table 2) significantly due to the decrease of the rate of charge and discharge processes at the metal–solution interface. This shows that there is agreement between the amount of charge that can be stored (i.e., the capacitance) and the discharge velocity at the interface [34]. The percentage inhibition efficiency was evaluated from the R ct values using the relation
where \( R_{{{\text{ct}}^{*} }} \) and R ct are the values of the charge-transfer resistance observed in the presence and absence of inhibitor molecules. In this case, the Mild steel (MS) corrosion takes place only at the free surface of the metal and/or within the pores due to the diffusion of dissolved oxygen, chlorine, or sulfate.
Adsorption studies
The extent of corrosion inhibition depends on the surface conditions and the mode of adsorption of the inhibitors [35]. The adsorption of an organic adsorbate at a metal–solution interface can be presented as a substitution adsorption process between the organic molecules in aqueous solution, (Orgaq), and the water molecules on the metallic surface, (H2Oads)
where X, the size ratio, is the number of water molecules displaced by one molecule of organic inhibitor, as represented in Fig. 6, under the assumptions that the corrosion of the covered parts of the surface is equal to zero and that corrosion takes place only on the uncovered parts of the surface (i.e., the inhibitor efficiency is mainly due to the blocking effect of the adsorbed species). The degree of surface coverage θ was estimated from the chemical and electrochemical techniques employed in this study as follows: θ = IE(%)/100 (assuming a direct relationship between surface coverage and inhibition efficiency). The adsorption on the corroding surfaces never reaches the real equilibrium and tends to reach an adsorption steady state. However, when the corrosion rate is sufficiently small, the adsorption steady state has a tendency to become a quasi-equilibrium state. In this case, it is reasonable to consider the quasi-equilibrium adsorption thermodynamically using the appropriate equilibrium adsorption isotherms. Basic information on the interaction between the inhibitor and the mild steel surface can be provided by the adsorption isotherm. In order to obtain the isotherm, the linear relation between the values of θ and the inhibitor concentration (C inhi) must be found. Attempts were made to fit the θ values to various isotherms, including those of Langmuir, Temkin, Flory–Huggins, Dahar–Flory–Huggins, and Bockris–Swinkel [36–38]. By far the best fit was obtained for the Temkin isotherm (log C versus log θ) and Langmuir isotherm (C versus C/θ), as shown in Fig. 7a, b.
Scanning electron microscopy
Surface examination using SEM was carried out to understand the effect of inhibitor molecules on the surface morphology of mild steel [39, 40]. Figure 8a shows an SEM image of a polished mild steel surface. Figure 8b shows an SEM image of the surface of mild steel after immersion in acid mixture without inhibitor molecules for 24 h. This micrograph shows the effect of acid in terms of surface damage. Figure 8c shows an SEM image of the surface of mild steel immersed in acid solution containing 200 ppm PVP, and Fig. 8d for the same time interval of PEG. The faceting observed in Fig. 8c, d is not present, and the surface was free from pits and appeared smooth. So, it can be concluded that corrosion is much less in the presence of inhibitors, and the appearance of the more polished surface obtained in the case of PVP compared with PEG proves its higher inhibition efficiency.
Atomic force microscopy (AFM)
To determine whether the inhibition is due to film formation on the metal surface through adsorption, atomic force micrographs were taken [41]. The three-dimensional AFM images are shown in Fig. 9a–d. As can be seen from Fig. 9c, d, there was much less damage on the surface of mild steel in the presence of PEG and PVP. The average roughness of polished mild steel (Fig. 9a) and mild steel in acid mixture without inhibitor (Fig. 9b) were calculated to be 15 nm and 252 nm, respectively. The mild steel surface in the free acid solution was cracked due to acid attack on the surface (Fig. 9b). However, in the presence of 200 ppm of inhibitors, the average roughness was reduced to 53 and 44 nm for PEG and PVP, respectively.
Mechanism of adsorption
Anodic dissolution of iron in acidic solutions has been reported to proceed as
The accompanied cathodic hydrogen evolution reaction follows the steps
As a consequence of this reaction plus the high solubility of the corrosion products, the metal loses weight in the solution. Corrosion inhibition is thought to be initiated by the inhibitor species, leading to specific adsorption of the inhibitor onto the metal surface (calculation shows that one molecule of PVP replaces 0.9858 molecules of water while PEG replaces 0.9642 molecules of water; Fig. 6). Examination of the above graph shows that the inhibition efficiency increases with increasing inhibitor concentration. This suggests that more inhibited molecules were adsorbed onto the metal surface at higher concentration, leading to greater surface coverage. Adsorption takes place through heteroatoms such as N, S, O, and P, double or triple bonds, or aromatic rings. The inhibition efficiency should increase in the order: P > S > N > O. Examination of the chemical structure of PEG and PVP reveals that PEG contains only oxygen heteroatom whereas PVP contains nitrogen in addition to oxygen atom. Also, the presence of rings in PVP leads to increased electron density on nitrogen atom, and PVP acts as a better inhibitor than PEG.
Conclusions
-
1.
The inhibition efficiency of both polymer PEG and PVP increases with increasing concentration.
-
2.
PVP shows better inhibition performance in acid medium compared with PEG.
-
3.
Both PEG and PVP act as mixed-type inhibitors.
-
4.
The adsorption of both polymers onto a mild steel surface obeys a Langmuir adsorption isotherm.
-
5.
Calculation shows that one molecule of PVP replaces 0.9858 molecules of water whereas PEG replaces 0.9642 molecules of water at 298 K.
-
6.
Surface analysis using SEM and AFM shows that PVP offers better protection than PEG.
References
O. Yuce, G. Kardas, Corros. Sci. 58, 86 (2012)
S. zhang, Z. Tao, W. Li, B. Hou, Appl. Surf. Sci. 255, 6757 (2009)
S. Rajendran, S.P. Sridevi, N. Antony, A. John, M. Sundearvadivelu, Anti. Corros. Methods Mater. 52, 102 (2005)
J. Jianguo, W. Lin, V. Otieno-Alego, D.P. Schweinsberg, Corros. Sci. 37, 975 (1995)
A. Yurt, B. Duran, Mater. Chem. Phys. 105, 114 (2007)
S.A. Umoren, I.B. Obot, Surf. Rev. Lett. 15, 277 (2008)
S.A. Umoren, O. Ogbobe, E.E. Ebenso, P.C. Okafor, Pigm. Resin Technol. 35, 346 (2006)
H. Ashassi-Sorkhabi, N. Ghalebsaz-Jeddi, Mater. Chem. Phys. 92, 480 (2005)
A.K. Dubey, G. Singh, Port. Electrochim. Acta. 25, 221 (2007)
S. Rajendran, B.V. Apparao, N. Palaniswamy, Electrochim. Acta 44, 533 (1998)
C. Jeyaprabha, S. Sathiyanarayanan, G Venkatachari, Appl. Surf. Sci. 253, 432 (2006)
S.A. Umoren, E.E. Ebenso, Mater. Chem. Phys. 106, 387 (2007)
S.A. Umoren, O. Ogbobe, I.O. Igwe, E.E. Ebenso, Corros. Sci. 50, 1998 (2008)
S.A. Umoren, U.M. Eduok, E.E. Oguzie, Port. Electrochim. Acta. 26, 533 (2008)
C. Jeyaprabha, S. Sathiyanarayanan, G. Venkatachari, J. Electroanal. Chem. 583, 232 (2005)
L. Liangcai, W. Ming, S. Huoming, L. Haiying, Q. Qingdong, D. Yuanlong, Polym. Adv. Technol. 12, 720 (2001)
J.E. Pereira da Silva, S.I. Cordoba de Torresi, R.M. Toressi, Corros. Sci. 47, 811 (2005)
S.A. Umoren, E.E. Ebenso, Ind. J. Chem. Technol. 15, 355 (2008)
A.D. Jones, Principles of Corrosion Control and Prevention, 2nd edn. (Printice-Hall, Saddle River, NJ, 1996), p. 32
S. John, B. Joseph, K.K. Aravindakshan, A. Joseph, Mater. Chem. Phys. 122, 374 (2010)
S. John, B. Joseph, K.V. Balakrishnan, K.K. Aravindakshan, A. Joseph, Mater. Chem. Phys. 123, 218 (2010)
H.H. Hassan, E. Abdelghani, M.A. Amin, Electrochim. Acta 52, 6359 (2007)
S.M.A. Hosseini, M. Salari, E. Jamalizadeh, S. Khezripoor, M. Seifi, Mater. Chem. Phys. 119, 100 (2010)
S.K. Singh, A.K. Mukerjee, J. Mater. Sci. Technol. 26, 264 (2010)
I. Ahamad, R. Prasad, M.A. Quraishi, Corros. Sci. 52, 933 (2010)
L. Larabi, M.Traisnel. Harek, A. Mansri, J. Appl. Electrochem. 34, 833 (2004)
M. Lebrini, M. Lagrene, H. Vezin, M. Traisnel, F. Bentiss, Corros. Sci. 49, 2254 (2007)
A.R.S. Priya, V.S. Muralidharam, A. Subramania, Corrosion 64, 541 (2008)
P. Bommersbach, C. Alemany-Dumont, J.P. Millet, B. Normand, Electrochim. Acta 51, 4011 (2006)
Q. Qu, Z.Z. Hao, L. Li, W. Bai, Y.J. Liu, Z.T. Ding, Corros. Sci. 51, 569 (2009)
M. Lagrene′e, B. Mernari, M. Bouanis, M. Traisnel, F. Bentiss, Corros. Sci. 44, 573 (2002)
L. Narvaez, E. Cano, D.M. Bastidas, J. Appl. Electrochem. 35, 499 (2005)
M. Bouklah, B. Hammouti, M. Lagrene, F. Bentiss, Corros. Sci. 48, 2831 (2006)
S. John, A. Joseph, Res. Chem. Intermed. (2011). doi:10.1007/s11164-0468-7
Z. Tao, S. Zhang, W. Li, B. Hou, Ind. Eng. Chem. Res. 50, 6082 (2011)
M.A. Migahed, A.A. Farag, S.M. Elsaed, R. Kamal, M. Mostfa, H. Abd El-Bary, Mater. Chem. Phys. 125, 125 (2011)
K.K. Al-Neami, A.K. Mohamed, I.M. Kenawy, A.S. Fouda, Monatsh Chem. 126, 369 (1995)
T. Szauer, A. Brandt, Electrochim. Acta 26, 1219 (1981)
F. El-Taib, A.S. Fouda, Radwan, Mater. Chem. Phys. 125, 26 (2011)
P. Zhao, Q. Liang, Y. Li, Appl. Surf. Sci. 252, 1596 (2005)
S. John, A. Joseph, Mater. Chem. Phys. 133, 1083 (2012)
Acknowledgments
One of the authors (S.J.) is grateful to CSIR New Delhi for providing a senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
John, S., Kuruvilla, M. & Joseph, A. Surface morphological and impedance spectroscopic studies on the interaction of polyethylene glycol (PEG) and polyvinyl pyrrolidone (PVP) with mild steel in acid solutions. Res Chem Intermed 39, 1169–1182 (2013). https://doi.org/10.1007/s11164-012-0675-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0675-x