Abstract
Europium and nitrogen co-doped TiO2 was successfully synthesized by the precipitation–peptization method. The structure and properties of the catalysts were characterized by X-ray diffraction, transmission electron microscopy, X-ray photoelectron spectroscopy, and UV–vis diffuse reflectance spectra. The photocatalytic efficiency was evaluated by monitoring the photocatalytic degradation of salicylic acid under visible light irradiation. It was verified that TiO2 co-doped with nitrogen and 1% europium showed the highest photocatalytic activity. The adsorption isotherms were obtained by measuring the salicylic acid concentration before and after the dark adsorption at different original solution concentrations. The results illustrated that the doping of Eu was beneficial to the adsorption of salicylic acid. The probable degradation mechanism of salicylic acid was examined by the addition of NaF, Na2S2O3, and K2S2O8 as the probe molecules. It was verified that salicylic acid was first adsorbed on the surface of the catalysts, followed by the degradation by the photogenerated holes (h +vb ).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Photocatalysis using TiO2 as a catalyst has been widely reported as a promising technology for removal of various organic and inorganic pollutants from contaminated water and air because of its stability, low cost, and non-toxicity [1–4]. However, the widespread technological use of TiO2 is impaired by its wide band gap (ca. 3.2 eV for crystalline anatase phase), the fast charge-carrier recombination, and the low interfacial charge-transfer rates [5]. Due to the above disadvantages, various approaches have been applied to enhance the photocatalytic activity under visible light illumination. Doping with nonmetals (such as N [6–9], S [10], C [11], and B [12, 13]) has been widely reported. Among them, N doping is the hottest topic. Asahi suggested that the N2p level could mix with O2p, which resulted in the narrowing of the band gap and enhancing of the photoactivity in the visible region [6]. Other researchers’ work supported that the formation of localized midgap states above the top of the valence band by nitrogen doping was responsible for the visible photoactivity [14]. Transition metals (such as Fe [15, 16], V [17], W [18], Ag [19], Au [20], and Pt [21, 22]) are another kind of efficient dopant which can facilitate the charge separation and hence reduce the recombination of electrons and holes efficiently. Lanthanide ions, owing to their special 4f electron configuration, can form complexes with various Lewis bases including acids, amines, aldehydes, alcohols, and thiols. It is beneficial to the interfacial charge transfer to the contaminant [23]. So co-doping with rare earths and nonmetal was an efficient way to enhance the activity in the visible region. Shen et al. [24] synthesized titania co-doped with nitrogen and cerium by the sol–gel route. The as-prepared photocatalyst could absorb the visible light and showed high photoactivity in the visible region because of the band gap narrowing. Cong et al. [25] synthesized TiO2 nanoparticles co-doped with nitrogen and lanthanum in a homogeneous precipitation–hydrothermal process. Some other researchers [26] have also reported rare earth and nitrogen co-doped TiO2. All of them used the organic solvent or organic titanium precursor which is expensive or poisonous to our environment and health. Furthermore, sometimes annealing is indispensable to remove organic materials and reduce the effect of carbon on photoactivity. Calcination will also lead to the growth of the nanoparticle size, while doped nitrogen species may be removed under high temperature condition [27].
In this study, we prepared europium and nitrogen co-doped titanium dioxide (Eu/N–TiO2) by the precipitation–peptization method, using inorganic titanium (titanium sulfate) as the titanium precursor. During the total synthesis process, no organic material was used. The dopant was dispersed homogeneously during the peptization process. In order to investigate the mechanism of photocatalytic reaction, adsorption isotherms were obtained which indicated that europium had a great effect on the adsorption ability. In principle, some valuable additional information on the mechanism of photocatalytic degradation of salicylic acid can be obtained by adding different probe molecules into the reaction systems. The addition of NaF illustrated that adsorption was a key step in the heterogeneous reaction. The important role of holes in the degradation of salicylic acid was confirmed by using K2S2O8 as an electron acceptor and Na2S2O3 as an efficient scavenger for holes.
Experimental
Materials
Titanium sulfate [Ti(SO4)2], ammonia (NH3·H2O), and nitric acid (HNO3) were obtained from Shanghai Reagent. Salicylic acid (purity 99.5%) was purchased from Shanghai Lingfeng. Sodium fluoride (NaF), sodium hyposulfite (Na2S2O3), and potassium peroxydisulfate (K2S2O8) were purchased from Huadong Medicine, China. All chemicals were of analytical grade or higher and were used as received. Double-distilled water was used for all synthesis and treatment processes.
Samples preparation
Nine grams of Ti(SO4)2 was dissolved in 36 mL water under vigorous stirring. After that, an aqueous ammonia (30 wt%) solution was added dropwise until pH = 8.0. After aging in the mother liquid for 12 h, the precipitate obtained was suction-filtered and washed with double-distilled water until the removal of SO4 2− (as was confirmed by the BaCl2 test). The obtained precipitates were dispersed homogeneously in 60 mL water with different amounts of Eu(NO3)3·6H2O, adjusting the pH of solution with HNO3 up to a certain value. The precipitates were peptized at 60 °C under continual magnetic stirring, and a transparent sol was formed after about 2 h. The sol was poured onto a plate and dried with water vapor. Eu, N co-doped TiO2 was obtained after the gel annealed at 300 °C for 2 h. The pure TiO2 was obtained by the addition of a 12-M NaOH solution instead of ammonia solution during the precipitation process, and HCl was used to adjust the pH value during the peptization process.
Characterizations
X-ray diffraction (XRD) patterns of all samples were collected in the range 10–80° with a stepwidth of 0.02° s−1 using a Rigaku D/MAX 2550 VB/PC apparatus (Cu Kα radiation, λ = 1.5406 Å), operated at 40 kV and 100 mA. The surface morphologies and particle sizes were observed by transmission electron microscopy (TEM, JEM-2011), using an accelerating voltage of 200 kV. The samples were prepared by grinding and subsequently dispersing the powder in acetone and applying a drop of very dilute suspension on carbon-coated grids. The UV–vis absorbance spectra were obtained for the dry-pressed disk samples using a Scan UV–vis spectrophotometer (Varian, Cary 500) equipped with an integrating sphere assembly, using BaSO4 as the reflectance sample. The spectra were recorded at room temperature in air within the range 200–800 nm. To investigate the chemical states of the photocatalyst, X-ray photoelectron spectroscopy (XPS) was recorded with Perkin Elmer PHI 5000C ESCA System with Al Kα radiation operated at 250 W. The shift of the binding energy due to relative surface charging was corrected using the C1 s as an internal standard at 284.6 eV.
Evaluation of photocatalytic activity
The photocatalytic activity was evaluated by measuring the decomposition of 50 mg/L salicylic acid solution. A 1,000-W tungsten halogen lamp was used as the light source, surrounded with a water circulation facility at the outer wall through a quartz jacket. The short wavelength components (λ <420 nm) of the light were cut off using UV cut-off filters. The distance between the lamp and the center of the quartz tube was 10 cm. For a typical photocatalytic experiment, 50 mg of catalyst powder was added to 50 mL of the above salicylic acid solution in the quartz tube. The initial pH value of the solution was kept at 3.5. Prior to irradiation, the suspensions were magnetically stirred in the dark for 30 min to ensure the establishment of an adsorption/desorption equilibrium. The above suspensions were kept under air-equilibrated conditions before and during the irradiation. After specific intervals, about 4 mL liquid was taken and centrifuged. The supernatant liquid was analyzed by recording the absorbance at 297 nm of salicylic acid solution using a UV–vis spectrophotometer (Varian, Cary 100). According to the standard curve of concentration and absorption, the value of ΔC/C 0 was calculated which indicated the decomposition efficiency.
In the experiment requiring scavengers, different amounts of probe molecules (NaF, K2S2O8, and Na2S2O3) were added to the salicylic acid solution before the introduction of the 1.0% Eu/N–TiO2 as the catalyst. The rest of the procedure was the same as the above photoactivity evaluation.
Adsorption isotherm measurement
The adsorption behavior of various samples to salicylic acid was carried out in dark. An amount of 30 mg catalyst was added into 30 mL salicylic acid solution with different initial concentration. After 30 min stirring, the concentration of salicylic acid was found to be constant. The adsorbed amount of salicylic acid on sample with different initial solutions was obtained by comparing the original concentration and the equilibrium concentration.
Results and discussion
Crystal structure
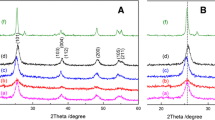
Figure 1 shows the XRD patterns in the 2θ region from 10 to 80° for pure TiO2 and Eu, N co-doped TiO2 calcined at 300 °C. The patterns show that all samples contain a little brookite structure but are dominated by anatase. The diffraction peaks due to the TiO2 anatase in the samples become broader and weaker with increasing amounts of Eu. This indicates that, with the increase of Eu dosage, the crystallinity of the TiO2 anatase phase deteriorates and the crystallite size of the TiO2 anatase becomes smaller.
From the (101) diffraction peak of anatase TiO2, the average crystallite sizes of TiO2 anatase can be calculated from the Debye–Scherrer equation. The results are summarized in Table 1. It can be seen that the average crystallite size decreased from 7.5 nm (pure TiO2) to 5.4 nm (2% Eu/N–TiO2). TiO2 has a tetragonal structure, whose unit cell parameters can be calculated according to Eqs. 1 and 2:
In this study, the diffraction peaks from (101) to (200) planes of TiO2 anatase were employed to calculate the unit cell parameters of TiO2 anatase. Table 1 shows the unit cell parameters of TiO2 anatase calculated from the XRD data. The unit cell parameters of TiO2 anatase do not change much from the pure TiO2 to Eu/N–TiO2. This means Eu3+ did not enter the TiO2 matrix. Generally, the ionic radius of Eu3+ (0.95 Å) is larger than that of Ti4+ (0.68 Å), and Eu3+ can hardly enter the lattice of TiO2 [28], but the highly dispersed rare earths inhibit grain growth by restricting the coalescence of some smaller neighboring grains.
TEM analysis
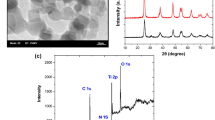
The TEM and HRTEM images of 1.0% Eu/N–TiO2 calcined at 300 °C are shown in Fig. 2. During the calcination, europium nitrate was converted into Eu2O3 and deposited onto the surface of N–TiO2. Figure 2a shows that the prepared sample has a small crystallite size of around 6 nm in diameter, which is in accordance with the XRD result. Figure 2b shows part of one nanocrystal having several lattice planes. The d-spacing of 0.352 nm corresponding to the distance between the (101) planes is the characteristic lattice fringes of the anatase TiO2 crystal. Figure 2c presents the selected area electron diffraction and lattice fringes. This result further proves that the crystallite is dominated by the anatase phase and with high uniform morphology.
UV–vis absorbance spectra
Figure 3a presents the UV–vis absorption spectra of the prepared samples. With the doping of nitrogen, there is a red shift toward the visible region. Figure 3b shows the (αhv)1/2 versus energy of light for direct band gap transition of pure TiO2 and modified TiO2. The band gap of pure TiO2 is 3.1 eV, and the band gap of N–TiO2 and 1.0% Eu/N–TiO2 is 2.8 and 2.75 eV, respectively.
XPS analysis
XPS is known as a particle surface analysis technique. In XPS, excitation by X-rays allows core atomic levels to be probed, and the resultant chemical shift provides an indication of the oxidation state of the material on the surface. Carbon is always present in XPS as a result of hydrocarbon deposits in air and/or vacuum, which is commonly used as an internal energy calibrated at 284.6 eV. The XPS spectra of Ti2p, O1s, N1s, and Eu4d of 1.0% Eu/N–TiO2 are presented in Fig. 4. The spectrum of Ti in Fig. 4A shows two well-resolved peaks. One of them is centered at 458.2 eV, while the other is centered at 463.9 eV. The peaks are due to the Ti4+ ions in titania assigned to Ti(2p3/2) and Ti(2p1/2) states, respectively. But for pure anatase TiO2, peaks for Ti(2p3/2) and Ti(2p1/2) states are at 458.6 and 464.3 eV, respectively [29]. The position of Ti2p was shifted to lower binding energy compared with pure anatase TiO2. This result indicated that the doping of nitrogen has an effect on the surrounding electron. Partial electrons transferred from the N to the Ti and the O, due to the lower electronegativity of nitrogen compared with oxygen. Therefore, the electron density of Ti increased and the binding energy decreased. This result is in agreement with other researchers [8, 30].
The shape of the O1s XPS spectrum in Fig. 4B indicates that it is composed of two peaks. One is centered at 529.9 eV, which is due to the contribution of oxygen atoms from metal oxide, e.g., from titanium dioxide and europium dioxide. The presence of additional broad photoelectron peak at the higher binding energy range was centered at 531.7 eV. This peak could be attributed to the hydroxyl group. It is reported that the hydroxyl group measured by XPS is ascribed to the water chemisorbed on the surface of titanium dioxide [31, 32]. This indicates that the doping of Eu enhances hydrophilic properties of the sample.
As for the N1s XPS spectrum, previous studies have shown the typical binding energy of nitrogen in TiN and NaNO3 at 397.2 and 408 eV [33], respectively. In this work, the N1s XPS spectrum can be fitted to three kinds of chemical states using multi-peak Gaussian approximation (Fig. 4C). The peak at 396.9 eV can be assigned to the formation of N–Ti–N [6, 34, 35]. In addition, two small peaks are observed at 398.7 and 400.3 eV. As is well known, the element adjacent to nitrogen directly influences its binding energy. The stronger the electronegativity of the adjacent element, the higher the binding energy of nitrogen. We attribute the peak at 398.7 eV to the N atom in the environment of O–Ti–N. The peak at 400.3 eV is the N atom in the environment of Ti–O–N. The analysis of the N1s XPS confirmed that nitrogen atoms were successfully doped into TiO2, and some substituted the sites of oxygen atoms.
For the spectrum of Eu4d in Fig. 4D, the prominent peak around 135.6 eV can be assigned to the Eu3+4d5/2 configuration. The other spin–orbit component, the 4d3/2 appears at 141.4 eV. The two-component separation is 5.8 eV, which is in good agreement with other measurements [36]. The signal of Eu4d suggests the presence of Eu2O3 in sample.
Photocatalytic activity
The photocatalytic activity of pure TiO2 and N–TiO2 doped with different amount of europium are shown in Fig. 5. It can be seen that the europium-doped N–TiO2 has better activity than the pure TiO2. A better and more quantitative method of presenting the activities of the samples is the use of the rate constant k, while k is calculated according to the following equation:
where C 0 and C t represent initial equilibrium concentration and the reaction concentration of salicylic acid, respectively. In photocatalysis reaction, k is almost independent of the temperature and concentration used because the photoactivtion process depends only on the radiant flux and on the visible light spectrum of the lamp. The reaction kinetics of different samples are shown in Fig. 6, and the rate constants are listed in Table 2. The rate constant k of pure TiO2 is 0.0005 min−1 and the degradation is 14%. For the pure TiO2, the catalyst turned pale yellow upon immersion in salicylic acid solutions which are photosensitive and lead to degrading by pure TiO2 [23]. With the doping of nitrogen, the rate constant k of N–TiO2 is 0.0026 min−1 with a degradation of 54%. The enhancement in the degradation of salicylic acid is attributed to absorption in the visible light region by the doped nitrogen species. With the increase of the europium dosage, the photocatalytic activity increased initially, but decreased when the doping content of europium was higher than 1%. Obviously, 1.0% Eu/N–TiO2 achieved the best photocatalytic performance with k of 0.0064 min−1 and the highest degradation of 88%. This might be due to the fact that there was an optimum doping content in the TiO2 particles for the most efficient separation of photoinduced electron–hole pairs.
Mechanism of the degradation of the salicylic acid
Adsorption behavior
In heterogeneous catalytic reaction, adsorption and desorption of reactants and intermediates have great influence on the efficiency of the overall degradation process. Organic compounds chemisorbed on TiO2 surface directly react with photogenerated holes, while reactant dissolved in aqueous environment are generally attacked by radicals such as ·OH, or O2 − on the surface or in the bulk of aqueous phase. Thus, adsorption experiments are considered as essential for a detailed study in this field. In this experiment, adsorption behavior was investigated by a set of adsorption experiments, as shown in Fig. 7. The adsorption isotherms of salicylic acid can be analyzed by Langmuir adsorption model:
where C e is the concentration of the salicylic acid in the solution at equilibrium (mmol/L), Q e is the adsorption amount in the solution at equilibrium (mmol/g). K a is the Langmuir adsorption equilibrium constant (L/mmol). Q max is the saturated adsorption amount (mmol/g). The plot of C e/Q e versus C e would give a straight line, and then Q max and K a can be derived from the slope and the intercept of the plot. The correlation coefficient R represents the conformity between the experimental results and the Langmuir adsorption isotherm model. According to the adsorption experimental data of salicylic acid on different catalysts, K a, Q max and the correlation coefficient are obtained and presented in Table 3.
The results indicate that the adsorption equilibrium constants (K a) of Eu, N co-doped TiO2 catalysts are much higher than that of the pure TiO2 catalyst, and Q max are also higher than that of the pure TiO2 catalyst. The factors that led to the enhanced adsorption capacity should involve the change of the physical or chemical properties of the catalysts owing to Eu ion doping. From Table 1, it can be seen that, with the increase of the dopant of Eu, the crystallite size decreased. The smaller crystal size and larger specific surface area of TiO2 catalysts would result in better physical adsorption of salicylic acid. Moreover, it was reported that rare earth ions can coordinate with salicylic acid for its special 4f electron configuration [27]. The adsorption equilibrium constant for the formation of Ti4+-salicylic acid is estimated to be K = 2.2 × 102 which is lower than that of Ln3+-salicylic acid K = 5.0 × 102 [23]. Therefore, the modified TiO2 has a stronger adsorption ability than the pure TiO2.
Effect of different probe molecules on the photocatalytic activity
In order to further illustrate the effect of adsorption on the photocatalytic activity, different amounts of NaF were added to the reaction solution. The degradation of salicylic acid with different amounts of NaF are shown in Fig. 8a. When there is no NaF, the degradation of salicylic acid by the 1.0% Eu/N–TiO2 under visible light irradiation is 88%. With the molar ratio of NaF to salicylic acid increased from 1:1 to 10:1, the degradation decreased from 61 to 13%. This can be explained by F− having a stronger adsorption ability on the surface of TiO2 catalysts than the salicylic acid. Thus, the surface of the catalyst was dominated by F−. The rate of degradation of salicylic acid is greatly inhibited by the presence of NaF. Thus, it can be concluded that the preferential adsorption on the surface of catalyst is a crucial step in this photocatalytic reaction.
The effect of the addition of K2S2O8 on the photocatalytic activity is shown in Fig. 8b. With the addition of K2S2O8, the photocatalytic activity noticeably increased. When the molar ratio of K2S2O8 to salicylic acid was 10:1, the degradation of salicylic acid was 91% after being irradiated for 3 h. As a kind of strong electron acceptor, the persulphate ions are known to capture the photogenerated electrons quickly and to generate hydroxyl radicals by the mechanisms shown in Eqs. 5–7:
The respective one-electron reduction potentials of different species are: E(O2/O2 −) = −155 mV, E(S2O8 2−/SO4 −) = 1,100 mV [37], From the thermodynamic point of view, the persulphate ion is a more efficient electron acceptor than molecular oxygen. So the addition of K2S2O8 reduces the recombination of electrons and holes, thus greatly enhancing the photocatalytic activity. The effect of K2S2O8 on the photoactivity has also been observed in a number of previous studies [38–40].
Figure 8c shows that the photocatalytic degradation of salicylic acid was strongly inhibited by the addition of Na2S2O3. The degradation of salicylic acid decreased from 88 to 68 and to 2% with the presence of molar ratios of salicylic acid to Na2S2O3 of 1:1 and 1:10. This is because Na2S2O3 is a strong reducing agent and can readily react with valance band holes (h +vb ) [41, 42]. It was oxidized to SO3 2− and subsequently to S2O6 2−. The process can be summarized as Eqs. 8 and 9.
From the above, it can be concluded that salicylic acid was adsorbed on the surface of the catalysts and oxidized under the effect of holes. The h +vb has an important role in the degradation of salicylic acid.
Conclusions
The present study demonstrated that titanium dioxide co-doped with europium and nitrogen with high visible light catalytic activity was successfully synthesized by inorganic chemicals. The doped nitrogen extended the absorption to the visible region, and the doped europium increased the adsorption ability to the salicylic acid. An amount of 1.0% Eu/N–TiO2 exhibited the best catalytic activity. Various probe molecules were added to the reaction system to explore the reaction mechanism. It can be deduced that the contaminants were first adsorbed on the surface of the catalyst, and were then decomposed quickly under the effect of the holes.
References
T.X. Liu, F.B. Li, X.Z. Li, J. Hazard. Mater. 152, 347 (2008)
J. Grzechulska, A.W. Morawski, Appl. Catal. B Environ. 36, 45 (2002)
C. Young, T.M. Lim, K. Chiang, J. Scott, R. Amal, Appl. Catal. B Environ. 78, 1 (2008)
M.R. Hoffmann, S.T. Martin, W. Choi, D.W. Bahnemann, Chem. Rev. 95, 69 (1995)
W. Balcerski, S.Y. Ryu, M.R. Hoffmann, J. Phys. Chem. C 111, 15357 (2007)
R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki, Y. Taga, Science 293, 269 (2001)
Y. Cong, J.L. Zhang, F. Chen, M. Anpo, J. Phys. Chem. C 111, 6976 (2007)
M. Sathish, B. Viswanathan, R. Viswanath, C. Gopinath, Chem. Mater. 17, 6349 (2005)
Y.M. Wu, M.Y. Xing, B.Z. Tian, J.L. Zhang, F. Chen, Chem. Eng. J. 162, 710 (2010)
T. Umebayashi, T. Yamaki, S. Yamamoto, A. Miyashita, S. Tanaka, T. Sumita, K. Asai, J. Appl. Phys. 93, 5156–5160 (2003)
Y.M. Wu, J.L. Zhang, L. Xiao, F. Chen, Appl. Surf. Sci. 256, 4260 (2010)
Y.M. Wu, M.Y. Xing, J.L. Zhang, F. Chen, Appl. Catal. B Environ. 97, 182 (2010)
A. Zaleska, E. Grabowska, J.W. Sobczak, M. Gazda, J. Hupka, Appl. Catal. B Environ. 89, 469 (2009)
C. Di Valentin, G. Pacchioni, A. Selloni, S. Livraghi, E. Giamello, J. Phys. Chem. B 109, 11414 (2005)
H.K. Shon, D.L. Cho, S.H. Na, J.B. Kim, H.-J. Park, J.H. Kim, J. Ind. Eng. Chem. 15, 476 (2009)
J.F. Zhu, W. Zheng, J.L. Zhang, M. Anpo, J. Mol. Catal. A Chem. 216, 35 (2004)
B.Z. Tian, C.Z. Li, F. Gu, H.B. Jiang, Y.J. Hu, J.L. Zhang, Chem. Eng. J. 151, 220 (2009)
S.A.K. Leghari, S. Sajjad, F. Chen, J.L. Zhang, Chem. Eng. J. 166, 906 (2010)
V. Vamathevan, R. Amal, D. Beydoun, G. Low, S. McEvoy, J. Photochem. Photobiol. A. Chem. 148, 233 (2002)
Y.M. Wu, J.L. Zhang, L. Xiao, F. Chen, Appl. Catal. B Environ. 88, 525 (2009)
M. Qamar, Desalination 254, 108 (2010)
L. Shivalingappa, J. Sheng, T. Fukami, Vacuum 48, 413 (1997)
K.T. Ranjit, I. Willner, S.H. Bossmann, A.M. Braun, J. Catal. 204, 305 (2001)
X.Z. Shen, Z.C. Liu, S.M. Xie, J. Guo, J. Hazard. Mater. 162, 1193 (2009)
Y. Cong, B.Z. Tian, J.L. Zhang, Appl. Catal. B Environ 101, 376 (2011)
D.G. Huang, S.J. Liao, W.B. Zhou, S.Q. Quan, L. Liu, Z.J. He, J.B. Wan, J. Phys. Chem. Solids 70, 853 (2009)
Y.F. Ma, J.L. Zhang, B.Z. Tian, F. Chen, L.Z. Wang, J. Hazard. Mater. 182, 386 (2010)
J. Lin, J.C. Yu, J. Photochem. Photobio. A Chem. 116, 63 (1998)
Y. Li, D.-S. Hwang, N.H. Lee, S.-J. Kim, Chem. Phys. Lett. 404, 25 (2005)
X. Chen, C. Burda, J. Phys. Chem. B 108, 15446 (2004)
P.M. Kumar, S. Badrinarayanan, M. Sastry, Thin Solid Films 358, 122 (2000)
H. Perron, J. Vandenborre, C. Domain, R. Drot, J. Roques, E. Simoni, J.J. Ehrhardt, H. Catalette, Surf. Sci. 601, 518 (2007)
X. Chen, Y.B. Lou, A. Samia, C. Burda, J. Gole, Adv. Funct. Mater. 15, 41 (2005)
H. Irie, Y. Watanabe, K. Hashimoto, J. Phys. Chem. B 107, 5483 (2003)
J.L. Gole, J.D. Stout, C. Burda, Y. Lou, X. Chen, J. Phys. Chem. B 108, 1230 (2003)
C.S. Fadley, D.A. Shirley, Phys. Rev. A 2, 1109 (1970)
P. Wardman, J. Phys. Chem. Ref. Data 18, 1637 (1989)
S. Qourzal, N. Barka, M. Tamimi, A. Assabbane, Y. Ait-Ichou, Appl. Catal. A 334, 386 (2008)
S. Yamazaki, S. Matsunaga, K. Hori, Water Res. 35, 1022 (2001)
A. Mills, M.A. Valenzuela, J. Photochem. Photobio. A Chem 165, 25 (2004)
S. Boumaza, A. Boudjemaa, A. Bouguelia, R. Bouarab, M. Trari, Appl. Energy 87, 2230 (2010)
S. Boumaza, R. Bouarab, M. Trari, A. Bouguelia, Energy Convers. Manage. 50, 62 (2009)
Acknowledgments
This work has been supported by the National Nature Science Foundation of China (20977030, 21173077), National Basic Research Program of China (2010CB732306), The Project of International Cooperation of the Ministry of Science and Technology of China (2011DFA50530), Science and Technology Commission of Shanghai Municipality (10520709900, 10JC1403900) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, Y., Zhang, J., Tian, B. et al. Synthesis of visible light-driven Eu, N co-doped TiO2 and the mechanism of the degradation of salicylic acid. Res Chem Intermed 38, 1947–1960 (2012). https://doi.org/10.1007/s11164-012-0516-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0516-y