Abstract
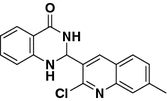
Montmorillonite K10 efficiently catalyzed a one pot–three component cyclocondensation of isatoic anhydride, NH4OAc and aromatic/heteroaromatic aldehydes under ambient conditions to produce the corresponding 2-substituted-2,3-dihydroquinazolin-4(1H)-ones in good yields. The 2-(2-chloroquinolin-3-yl)-2,3-dihydroquinazolin-4(1H)-ones 3a–d were screened for their antitumor activity against Ehrlich Ascites Carcinoma tumor cells
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of solid supported reagents has received considerable importance in recent years in organic synthesis due to their ease of handling, reaction rates, greater selectivity, simple work-up and recoverability of catalysts [1–3]. We have previously successfully utilized the montmorillonite K-10 clay for the reduction of 2-chloro-quinoline-3-carbaldehyde [2] and also for the syntheses of quinazolin-4(3H)-ones from anthranilic acid and amides under solvent-free condition [3]. The efficiency of montmorillonite K10 catalysis in organic synthesis has been demonstrated with their advantages of high atom efficiency, simplified isolation of product, and easy recovery and recyclables of the catalysts [4–6]. Montmorillonites have both Brönsted and Lewis acid sites and, when exchanged with cations having a high charge density, such as protons, they produce highly active catalysts for acid-catalyzed reactions [7]. The use of inexpensive clays as a solid source of protons in a number of industrially significant reactions are of greater interest [8].
Heterocyclic chemistry occupiesan important place in organic chemistry research worldwide [9–12] and forms the basis of many pharmaceutical, agrochemical and veterinary products. Especially, quinazolinone and its synthetic analogues have been found to exhibit a broad spectrum of biological activities, including antituberculos [13], anti-inflammatory [14], anticancer [15], antibacterial and antifungal [16].
Several methods have been reported for the synthesis of 2,3-dihydroquinazolinones [17–19]. However, they suffer from lengthy procedures and/or low yields and vigorous conditions [17–19]. Methods for the selective synthesis of 2,3-dihydroquinazolin-4(1H)-ones have not been explored before. Thus, developing versatile approaches to synthesize 2,3-dihydroquinazolin-4(1H)-ones still remains a highly desired goal in organic synthesis.
Recently, we successfully applied montmorillonite K10 in several reactions [2, 3]. These clays can be regarded simply as solid acids that act as heterogeneous catalysts, with all the advantages resulting from the easy removal of the catalyst from the product [2, 3]. As a result of our great interest in clay-catalyzed organic reactions, here we report an efficient one pot synthesis of new 2-(2-chloroquinolin-3-yl)-2,3-dihydroquinazolin-4(1H)-ones 3a–d by employing isatoic anhydride 1, ammonium acetate and 2-chloroquinoline-3-carbaldehydes 2a–d, and the quinazolinones 3a–d formed were screened and found to be potential antitumor agents.
Results and discussion
Initially, a mixture of isatoic anhydride 1, aromaticaldehyde 2, and ammonium acetate in EtOH was stirred under reflux condition in the presence of a catalytic amount of acidic alumina; the reaction produced the desired product in 45% of yield (Table 1, entry 5). After screening the catalysts, such as silica get, bentonite, montmorillonite KSF, basic alumina, acidic alumina, and montmorillonite K10, it was found that montmorillonite K10 was the best catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones in a shorter time and higher yield (Table 1, Entry 6) in comparision to the reaction carried out in the absence of the catalyst, (Table 2, entry 1). Montmorillonite K10 proved to be superior among all the catalysts screened in this transformation.

It should be noted that 10 mg of montmorillonite K10 was efficient to catalyze the reaction, and any increase in the amount of catalyst did not improve the yield significantly (Table 2, Entries 6–8). The reusability of the catalyst by recovering the montmorillonite K10 after the reaction was screened in new runs and it was found that the catalyst could be reused several times without any decrease in the product yield. The recyclability of the catalyst was investigated using a model reaction of ammonium acetate with isatoic anhydride 1 and 2-chloroquinoline-3-carbaldehyde 3a. The catalyst can be removed from the organic phase by removing the ethanol under vacuum then washing with fresh EtOH and drying at 80 °C for 1 h. Then, it was utilized in second run of the reaction process. It was noticed that the use of recycled catalyst in subsequent experiments gave almost similar yields. Thus, the catalyst is not leached.
The role of montmorillonite K10 in the formation of 2-substituted-2,3-dihydroquinazolin-4(1H)-ones 3 is illustrated in Fig. 1. The first step may involve the condensation of isatoic anhydride 1 with ammonium acetate, with the liberation of anthranilamide I which in turn led to intermediate II by the condensation of I and heteroaldehydes 2 promoted by montmorillonite K10. The amide group of intermediate II is then tautomerized in the presence of montmorillonite K10, and simultaneously the imine group of intermediate II gets activated by montmorillonite K10 resulting in intermediate IV by intramolecule nucleophilic attack of the amide nitrogen on imine carbon. Subsequently, 2,3-dihydroquinazolin-4(1H)-ones 3 could be formed by a 1,5-proton transfer. The isatoic anhydride 1, aromaticaldehydes 2 and ammonium acetate (used as the source of ammonia) in the presence of montmorillonite K10, EtOH under reflux condition led to the 2-substituted 2,3-dihydroquinazoline-4(1H)-ones 3a–h in good yields (Scheme 1). The optimization of the reaction is shown in Table 2 and the results are summarized in Table 3. In order to investigate the scope of this reaction, a variety of substituted aromatic aldehydes 3a–h was utilized in this reaction (Table 3, entries 1–8). The results suggest that, irrespective of heteroaryl or aromatic aldehydes, the reaction proceeds well in the optimized conditions (Table 3, entries 1–8). The 2,3-dihydroquinazolin-4(3H)-ones 3a–d were screened for their antitumor activity against Ehrlich Ascites Carcinoma tumor cells. The result suggests that all compounds have lowest IC50 values compared to the standard 5-fluoro uracil (132.12 μg/mL) and hence higher cytotoxicity effects on EAC tumor cell lines than the standard (Table 4). The cytotoxicity order of synthesized compounds is as follows: 3a > 3b > 3c > 3d > Standard.

In summary, a green chemical methodology has been proposed for the construction of biologically active 2-heterosubstituted-2,3-dihydroquinazolin-4(3H)-ones 3a–d. Present methodology offers very attractive features such as reduced reaction times, higher yields and economic viability of the catalyst. To the best of our knowledge, this is the first time an efficient method for 2,3-dihydroquinazolin-4(3H)-ones by using montmorillonite K10 has been reported. The catalyst can be recovered and reused with no change in the yield and catalytic activity.
Experimental section
Solvents and reagents were commercially sourced and used without further purification or preparation. Montmorillonite K-10 catalyst having the surface area of 250 m2/g was purchased from Sigma-Aldrich, Bangalore, India. Thin-layered chromatography (TLC) was performed on preparative plates of silica gel (S.D. fine). Visualization was made with an iodine charmber. Column chromatography was performed by using silica gel (60–120 mesh). Melting points were taken on Elchem microprocessor-based DT apparatus in open capillary tubes. IR spectra were obtained on a Nucon infrared spectrophotometer using KBr pellets. The NMR spectra were recorded on a Bruker-500 MHz spectrometer using TMS as internal standard (chemical shifts δ in ppm). Mass was recorded on Finnigan Mat 8230 mass spectrometer.
2-Subsituted-2,3-dihydroquinazolin-4(3H)-ones (3)
General procedure
Isatoic anhydride 1 (1 mmol), aromatic aldehyde 2 (1 mmol), ammonium acetate (1.2 mmol) and montmorillonite K10 (10 mg) were added to ethanol (5 mL). The mixture was stirred at 70 °C for 30 min as indicated in Table 2. The progress of the reaction was monitored by TLC. After completion, the reaction mixture was then allowed to cool to room temperature and water (20 mL) was added. The corresponding solid product was obtained through simple filtering, and recrystallized from ethanol. Spectral data of the newly synthesized unreported compounds 3a–d are given below
-
3a: Pale yellow solid: mp 262 °C; 1H NMR (500 MHz, DMSO-d6):δ = 8.64 (1H, s), 8.34 (1H, s, -NH-CO), 7.96–7.94 (1H, d, J 8), 7.71 (2H, t, J 6.5), 7.56 (1H, t, J 7.5), 7.30 (1H, t, J 7), 7.15 (1H, s, -CH, quinazolinone ring), 6.81–6.75 (m, 2H), 6.26 (1H, s, -NH), 2.68 (3H, s, -CH3); 13C NMR(125 MHz, DMSO-d6) δ 164.0 (-C=O), 148.2, 148.0, 146.4, 138.8, 135.7, 134.0, 131.9, 131.7, 127.9, 127.8, 127.3, 126.7, 118.3, 115.4, 115.2, 64.5 (-CH, quinazolinone ring), 17.8 (-CH3). HRMS: m/z calcd for C18H14ClN3O, 271.0825; found 271.0821 M+.

-
3b: Pale yellow solid: mp 258 °C; 1H NMR (500 MHz, DMSO-d6):δ = 8.63 (1H, s), 8.32 (1H, s, -NH-CO), 8.03–8.02 (1H, d, J 8), 7.53–7.51 (1H, d, J 8), 7.29 (2H, t, J 7.5), 7.21–7.19 (1H, d, J 8), 7.14 (1H, s, -CH, quinazolinone ring), 6.84–6.76 (m, 2H), 6.23 (1H, s, -NH), 2.57 (3H, s, -CH3); 13C NMR(125 MHz, DMSO-d6) δ 164.1 (-C=O), 149.0, 148.1, 147.6, 142.2, 138.3, 133.9, 131.1, 130.2, 127.9, 126.8, 125.3, 118.2, 117.5, 115.5, 115.3, 64.6 (-CH, quinazolinone ring), 21.9 (-CH3). HRMS: m/z calcd for C18H14ClN3O, 271.0825; found 271.0823 M+.
-
3c: White solid: mp 265 °C; 1H NMR (500 MHz, DMSO-d6):δ = 8.54 (1H, s), 8.34 (1H, s, -NH-CO), 7.89 (2H, t, J 4), 7.70 (2H, t, J 7.5), 7.31–7.28 (1H, m), 7.15 (1H, s, -CH, quinazolinone ring), 6.81–6.74 (m, 2H), 6.22 (1H, s, -NH), 2.53 (3H, s, -CH3); 13C NMR(125 MHz, DMSO-d6) δ 164.0 (-C=O), 148.1, 147.9, 145.8, 137.8, 137.7, 134.0, 132.1, 127.9, 127.6, 127.5, 127.2, 118.2, 115.3, 115.2, 64.5 (-CH, quinazolinone ring), 21.5 (-CH3). HRMS: m/z calcd for C18H14ClN3O, 271.0825; found 271.0824 M+.

-
3d: White solid: mp 274 °C; 1H NMR (400 MHz, DMSO-d6):δ = 8.59 (1H, s), 8.32 (1H, s, -NH-CO), 7.88–7.86 (1H, d, J 8.32), 7.71–7.69 (1H, d, J 7.76), 7.53-7.50 (1H, d, J 8.36), 7.29 (1H, t), 7.13 (1H, s -CH, quinazolinone ring), 6.79 (1H, t), 6.78–6.76 (1H, t), 6.24 (1H, s, -NH), 2.63 (3H, s, -CH3), 2.49 (3H, s, -CH3); 13C NMR(100 MHz, DMSO-d6) δ 163.4 (-C=O), 147.5, 145.8, 139.1, 138.2, 133.4, 132.6, 130.2, 127.4, 125.1, 117.3, 114.8, 114.7, 63.9 (-CH, quinazolinone ring), 20.2 (-CH3), 12.9 (-CH3).

In vitro cytotoxic activity against Ehrlich Ascites Cacinoma cell
General procedure
Cytotoxicity was assessed by incubating 1 × 106 EAC cells in 1 mL phosphate buffer saline with varying concentrations of the complexes at 37 °C for 3 h in CO2 atmosphere ensured using a McIntosh field jar. The viability of the cells was determined by the trypan blue exclusion method. EAC cells were obtained through the courtesy of Amala Cancer Research Center, Trissur, India. They were maintained by weekly intraperitoneal inoculation of 106 cells.
Newly synthesized compound 2,3-dihydroquinazolin-4(3H)-ones 3a–d were screen for their antitumor activity against Ehrlich Ascites Carcinoma tumor cells. The latter type of cells was used in this study because they are the only tumor cells that grow in mice available in Egypt. The target compounds have not been reported hitherto. A set of sterile test tubes was used, where 1 × 106 tumor cells/mL were suspended in phosphate–buffered saline. Then, 100, 200, 300, 400 and 500 μg/mL from the tested compound were added to the suspension and kept at 37 °C for 3 h. Trypan blue dye exclusion test was then carried out to calculate the percentage of nonviable cells. 5-Fluorouracil (132.12 μg/mL) was taken as a positive control. The percentages of the non-viable cells were calculated by the following equation: percentage of non-viable cells = (N/N t) × 100, where N is the number of non-viable cells counted, and N t is the total number of cells.
The test was repeated four times for each compound. The results are summarized in Table 4.
References
G. Gil, S.J. Brse, Comb. Chem. 11, 175 (2009)
S.M. Roopan, F.N. Khan, ARKIVOC 13, 161 (2009)
S.M. Roopan, T. Maiyalagan, F.N. Khan, Can. J. Chem. 86, 1019 (2008)
M. Kowalska, D.L. Cock, Chemosphere 36, 547 (1998)
V.P. Evangetou, M. Marsi, M.M. Vandiviere, Plant Sol. 213, 63 (1999)
O.Y. Kwon, K.W. Park, S.Y. Jeong, Bull. Korean Chem. Soc. 22, 678 (2001)
B.K.G. Theng, Dev. Sedimentol. 35, 197 (1982)
P. Komadel, J. Madejova, in Handbook of Clay Science: Development in Clay, vol. 1, ed. by F. Bergaya, B.K.G. Theng, G. Lagaly (Elsevier, Amsterdam, 2006), p. 263
C. Larksarp, H. Alper, J. Org. Chem. Rev. 65, 2773 (2000)
V. Krchnak, M.W. Holladay, Chem. Rev. 102, 61 (2002)
B.B. Tour, D.G. Hall, Chem. Rev. 109, 4439 (2009)
S.M. Roopan, F.N. Khan, B.K. Mandal, Tetrahedron Lett. 51, 2309 (2010)
S.S. Parmar, R. Kumar, J. Med. Chem. 11, 635 (1968)
K.-I. Ozaki, Y. Yamada, T. Oine, T. Ishizuka, Y. Iwasawa, J. Med. Chem. 28, 568 (1985)
A. Kamal, E.V. Bharathi, M.J. Ramaiah, D. Dastagiri, J.S. Reddy, A. Viswanath, F. Sultana, S.N.C.V.L. Pushpavalli, M. Pal-Bhadra, H.K. Srivastava, G.N. Sastry, A. Juvekar, S. Sen, S. Zingde, Bioorg. Med. Chem. 18, 526 (2010)
M.S. Mohamed, M.M. Kamel, E.M.M. Kassem, N. Abotaleb, S.I.A. El-moez, M.F. Ahmed, Eur. J. Med. Chem. 45, 3311 (2010)
J.A. Moore, G.J. Sutherland, R. Sowerby, E.G. Kelly, S. Palemo, W. Webster, J. Org. Chem. 887 (1969)
W. Su, B. Yang, Aust. J. Chem. 55, 695 (2002)
D. Shi, L. Rong, J. Wang, Q. Zhuang, X. Wang, H. Hu, Tetrahedron Lett. 44, 3199 (2003)
D.Q. Shi, L.C. Rong, J.X. Wang, X.S. Wang, S.J. Tu, H.W. Hu, Chem. J. Chin. Univ. 25, 2051 (2004)
T.A.K. Smith, H. Stephen, Tetrahedron 1, 38 (1957)
Acknowledgments
This work was supported by Department of Science & Technology Government of India (Grant No. SR/FTP/CS-99/2006). We also acknowledge SAIF, IIT Madras, Chennai for providing NMR and MS facility. The authors are grateful to the VIT University management for their generous support and facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roopan, S.M., Nawaz Khan, F., Jin, J.S. et al. An efficient one pot–three component cyclocondensation in the synthesis of 2-(2-chloroquinolin-3-yl)-2,3-dihydroquinazolin-4(1H)-ones: potential antitumor agents. Res Chem Intermed 37, 919–927 (2011). https://doi.org/10.1007/s11164-011-0301-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-011-0301-3