Abstract
Vinyltri(phenylethynyl)silane ((ph–C≡C)3–Si–C=CH2; VTPES) and phenyltri(phenylethynyl)silane ((ph–C≡C)3–Si–ph; PTPES) were synthesized by Grignard reaction. Their molecular structures were characterized by means of 1H NMR, 13C NMR, 29Si NMR, and FT-IR spectroscopy. Their nonisothermal thermal curing processes were characterized by DSC, and the corresponding kinetic data, for example activation energy (E), pre-exponential factor (A), and the order of the reaction (n), were obtained by the Kissinger method. The results showed that the melting points of VTPES and PTPES were 84 and 116 °C, respectively. Their curing reaction rates were consistent with first-order kinetic equations. VTPES monomer had a lower activation energy and curing temperature as a result of coordination between reactive groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 1968, Luneva first reported the synthesis of diethynyldiphenylsilane in a nitrogen atmosphere [1]. This kind of silicon-containing arylacetylenic monomer can be cured thermally without formation of volatiles. The polymers’ high-temperature properties and high char yield resulted in their rapid development, because of their potential applications in biomaterials, optoelectronics, and ceramics precursors, etc. [2–8].
Synthesis of different alkynylsilane monomers has been reported in recent years [9, 10]. The main synthetic routes were dehydrogenation coupling reactions between hydrosilanes and alkynic compounds using metal compounds as catalysts. H2PtCl6/LiI, I2, IrH2(SiEt3)(COD)(AsPh3), [IrH(H2O](bp)L2)]SbF6, and RhClL3 (L = PPh, bp = 7,8-benzoquinolinato) have been used as catalysts [11]. In these cases, dehydrogenating cross-coupling reactions were found to accompany the significant hydrosilylation reactions to produce the corresponding alkenylsilanes, and dehydrocoupling in low yield was also found to accompany hydrosilation and hydrogen-transfer reactions [12, 13]. Calas and Bourgeois obtained alkynylsilane (n-Bu–C≡C–SiEt3) from Et3SiH and 1-hexyne using Na or NaH as a catalyst [11]. Liu and Harrod reported that the CuCl/amine catalyst led to highly selective dehydrogenative cross-coupling reactions of hydrosilanes with alkynes [14]. Itoh et al. also found that solid bases (MgO) and metal hydrides (LiAlH4, NaAlH4, LiBH4, and LiAlH(Ot–Bu)3) catalyze the same selective dehydrogenating cross-coupling reactions [15]. However, these synthetic methods were carried out under moisture-free and oxygen-free conditions, the catalysts and raw materials (e.g. LiAlH4 and phenylsilane) used were dangerous, and the products were rather expensive. Further, the hydrosilanes were mainly synthesized from chlorosilanes and lithium aluminum hydride in anhydrous diethyl ether or dioxane [16–18]. As a result, those methods were mainly limited to laboratory preparations, and the synthesized monomers were only characterized in the structures. However, the Grignard reaction is a common reaction method and the reaction conditions are mild [19]. Methyldi(phenylethynyl)silane and [–SiH(R)–C≡C–C6H4–C≡C–] (R = Ph, CH3, H) were synthesized by use of the Grignard reaction and yields were better than 75% [20, 21], but the synthesis of vinyltri(phenylethynyl)silane and phenyltri(phenylethynyl)silane by use of Grignard reactions has not been reported.
The main objectives in this work were to prepare vinyltri(phenylethynyl)silane and phenyltri(phenylethynyl)silane monomers by the Grignard reaction, using the relatively inexpensive vinyltrichlorosilane or phenyltrichlorosilane as raw materials, and to characterize the monomers’ structures by use of FT-IR and NMR, and the non-isothermal curing process by use of DSC.
Experimental
Materials and methods
Phenylacetylene was purchased from Hanwang Reagent Company, China. Vinyltrichlorosilane and phenyltrichlorosilane were purchased from Huarong Chemical Materials Company, China. Solvents were bought from Shanghai Reagent Company, and all chemicals and solvents were distilled or dried if necessary according to standard procedures.
Preparation of vinyltriphenylethynylsilane (VTPES) (monomer a)
An organic magnesium Grignard reagent was prepared as follows: Magnesium turnings (6.72 g, 0.28 mol) were introduced into a 500-mL four-necked flask and the atmosphere of the flask was replaced with dry nitrogen gas. THF (80 mL) which had been dried over sodium was subjected to simple distillation and introduced into the flask. A small piece of iodine was then added and the mixture was stirred to activate the magnesium. To the activated magnesium was added dropwise a solution of 20 mL (0.268 mol) ethyl bromide in THF (100 mL) at room temperature over approximately 1 h. The resulting mixture was reacted while being heated under reflux for 3 h to produce ethylmagnesium bromide. The reaction system was then added dropwise to a solution of 29 mL (0.264 mol) phenylacetylene in THF (100 mL) in a ice-bath over 2 h, with stirring, and the reaction was continued for an additional 3 h while being heated under reflux to produce the intended phenylethynylene Grignard reagent (0.264 mol).
Monomer a was then prepared by the following procedure. The reaction was performed subsequent to the foregoing preparation of the phenylethynylene reagent. A solution of 11 mL (0.086 mol) vinyltrichlorosilane in THF (100 mL) was added dropwise to the flask in a ice-bath over 1 h, with stirring, and the reaction system was further reacted for 3 h while being heated under reflux, following by the color variation from green to yellow. The reaction system was then post-treated as follows. An aqueous solution of hydrochloric acid (1 mol/L, 300 mL) was placed in another 500-mL flask and the reaction solution prepared in the 500-mL flask was transferred to a dropping funnel. The aqueous hydrochloric acid solution was gently stirred while the reaction solution was slowly added dropwise by use of the dropping funnel (over 30 min) in a ice-bath. Benzene (50 mL) was added to the reaction solution and the resulting oil phase was separated by use of a separatory funnel, washed with distilled water until no acid was present, then dried by adding anhydrous calcium chloride while standing overnight. The solution was filtered through a ceramic filter to remove the dehydrating agent. The solvent was removed from the solution by distillation under vacuum, eventually giving a viscous, oily, crude product. The crude product was repeatedly recrystallized in methanol to cause precipitation. The resulting precipitates were isolated by filtration and dried to give pin-like crystals (approx. 23 g, 74.7% yield) of the intended monomer a (Scheme 1).
Preparation of phenyltriphenylethynylsilane (PTPES) (monomer b)
The same procedures used for preparation of monomer a were repeated, except that phenyltrichlorosilane was substituted for vinyltrichlorosilane to give columnar crystals of monomer b (70% yield).
Characterization
FT-IR spectroscopic characterization was carried out with a Nicolet 8700 FT-IR spectrometer and use of potassium bromide (KBr) pellets. 1H NMR, 13C NMR, and 29Si NMR spectra were recorded on an Avance AV-400 superconducting Fourier digital NMR spectrometer (400 MHz for 1H NMR, 100.61 MHz for 13C NMR and 79.49 MHz for 29Si NMR). The chemical shifts were recorded relative to tetramethylsilane (δ, 0.0 ppm) for 1H NMR and 29Si NMR, and CDCl3 (δ, 77.7 ppm) for 13C NMR. The differential scanning calorimetry (DSC) study was performed with a Mettler-Toledo-DSC 821e/400 under nitrogen atmosphere.
Results and discussion
Structural characterization of the monomers
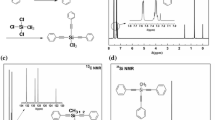
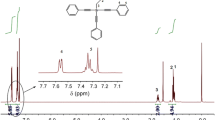
Typical proton NMR spectra of the two monomers are shown in Fig. 1. The aromatic hydrogen resonated at 7.35–7.59 ppm and silicon vinyl protons resonated at 6.33 ppm. In a precise study of NMR peak integration, vinyl protons (m, 3H, CH=CH2), aromatic hydrogen bonded to ethynyl (m, 6H, PhH–C≡C), and aromatic hydrogen (m, 9H, PhH) were in the ratio 3:6:9 for VTPES (Fig. 1a). For PTPES, aromatic hydrogen bonded to silicon (m, 2H, PhH–Si), aromatic hydrogen (m, 3H, PhH–Si), aromatic hydrogen bonded to ethynyl (m, 6H, PhH–C≡C), and aromatic hydrogen (m, 9H, PhH–C≡C) were in the ratio 2:3:6:9 (Fig. 1b). These results were in agreement with the structures of the silicon-containing arylacetylenic monomers [20–23].
The carbon spectra of the two monomers, with band assignments are shown in Fig. 2. The acetylenic carbons appeared as a pair of resonances at 86 and 107 ppm as they were bonded to the silicon and phenyl units of the two monomers. The phenyl carbon bonded to C≡C and the side-chain phenyl carbon bonded to Si were observed at 128–134 ppm. For VTPES (Fig. 2a), 137 ppm corresponded to carbon attached to C=C. The aromatic carbons bonded to C≡C and vinyl carbons bonded to Si were similar and difficult to separate between 128 and 134 ppm, because of similar chemical environments. Accurate chemical shift information will be investigated in the future.
29Si NMR spectra of two monomers are shown in Fig. 3. The two signal peaks assigned to the silicon were observed at −71.34 ppm (VTPES) (Fig. 3a) and −69.15 ppm (PTPES) (Fig. 3b) because of the different chemical environments [18]. On the basis of the 1H NMR, 13C NMR and 29Si NMR spectra we can see that the chemical shift of phenylethynyl bonded to Si has not been affected dramatically by vinyl and phenyl, probably because of small steric and inductive effects in the silicon-containing monomers [22].
Non-isothermal curing kinetics of the two monomers
Cure reactivity of the two monomers
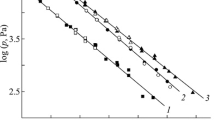
The heat curing behavior of VTPES and PTPES was examined by DSC (Fig. 4). The monomers gave distinct endothermic peaks at 84 and 116 °C corresponding to their melting points. The curing temperatures of the two monomers were also shown in Table 1. It was noticed that with increasing heating rate, the curing time decreased, the peak became sharp, and the thermal curing temperature moved to the high-temperature region (Fig. 5). This is because with increasing heating rate, the thermal effect per unit time increased, and a larger temperature difference was generated. As a result, the endothermic peak was shifted to higher temperature region. At the same time, we could see that the peak temperature of polymerization (T p) of VTPES was 316 °C, which was 47 °C lower than that of PTPES (Table 1), and the reactive activation energy of VTPES (114.25 kJ/mol) was also 40 kJ/mol lower than that of PTPES. It is well known that thermal polymerization of phenylethylene monomers is a radical mechanism [24, 25]. Thus, it was theoretically possible to deduce that radicals formed by VTPES would be stabilized to a greater extent and more active than those formed by PTPES, and the coordination role reduced the reactive energy barrier at the unitary level between double bonds and acetylenic bonds.
Non-isothermal curing kinetics analysis of the two monomers by DSC
The generation of accurate time–temperature−degree of conversion curves is of great practical importance for establishing optimum cure schedules. Mechanistically, evaluation of the activation energy of the cure reaction could give valuable information about barriers to reaction and about mechanisms. The dynamic DSC method was used in this study to obtain kinetic data on the basis of the variation in the peak exotherm temperature (T p) with heating rate (φ). A simple relationship between activation energy, heating rate, and peak exotherm temperature was based on the work of Kissinger, which had been shown to be effective in calculation of cure kinetics data [26]. The general rate equation is:
Where α is the degree of cure, t the reaction time, k the rate constant defined by the Arrhenius relationship, n the order of the cure reaction, A the frequency factor, E the activation energy, R the universal gas constant, and T the temperature in Kelvin. Combining Eqs. (1) and (2):
A was found from the relationship:
and n was determined by use of the Crane equation:
A plot of Ln φ versus 1/T p from several DSC curves would give a straight line with slope (−E/nR), when E/nR ≫ 2T p.
In our study, the cure reactions of the above-mentioned monomers were investigated by use of the non-isothermal DSC technique at different heating rates of 5, 15, 20, and 25 °/min under a nitrogen atmosphere with a flow rate of 100 mL/min (Fig. 5). Table 1 shows the relationship between ln(β/T 2p ) and (1/T p) or lnβ and (1/T p), obtained by use of the Kissinger method. The numerical values were fitted to a linear equation and the correlation coefficients were almost unity. The corresponding activation energy, frequency factor, and reaction order were also obtained. The results of calculation showed that the curing reactions of VTPES and PTPES approached first-order kinetics, with reaction orders of 0.92 and 0.93, respectively. Similar studies have been conducted on methyldi(phenylethynyl)silane and multisubstituted acetylenic monomers; a first-order cure reaction was reported for both, suggesting that the order n might not be a function of the substituted groups on the resin and was probably only correlated with acetylenic bonds [27, 28]. However, the exact cure mechanism to account for the observed value of n = 1 in these thermal polymerizations was unclear. The theoretically predicted value for cyclotrimerization was 189 ± 10 kJ/mol based on bond energy calculations for the thermal polymerization of the acetylenic group [29], and the experimentally determined heats of polymerization for the phenyl and vinyl-substituted acetylenic monomers were 338 and 288 kJ/mol, respectively, which were higher than the predicted enthalpy of cyclotrimerization, suggesting that trimerization of the acetylenic groups might be the preferred reaction pathway, although trimerization of these secondary acetylenic groups to form hexasubstituted benzene rings seemed to be a sterically unfavorable process. Surprisingly, the frequency factor for PTPES was two orders of magnitude larger than that for VTPES. The differences between the activation energies and frequency factors were probably because of the existence of relatively stable radicals and lower steric factors [30], which caused the VTPES to react more easily. The analyzed results were in accordance with cure reactivity of the two monomers.
At the same time, a precursor monomer suitable for preparing heat-resistant polymer must have an adequately large processing window, i.e. a temperature range above the melting point of the monomer and below the onset of appreciable cure where the monomer can be held in a state of liquid flow. The ideal processing window should be as broad as possible. Therefore practically, a processing window of at least 5–10 °C is necessary to ensure adequate flow behavior within processing temperature fluctuations. From Table 1, we can see that both monomers had a wide processing window above 200 °C, and that for the PTPES monomer was approximately 20 °C higher than that for VTPES. These broad processing windows mean the monomers have adequate time to impregnate other materials for preparing high-temperature polymers. Thus, the two monomers should be easily processed and are excellent candidates for further study as high-temperature-resistant polymers.
Non-isothermal curing analysis of the two monomers by FT-IR
In order to better understand the curing process of two monomers, FT-IR spectra were used to monitor the precise extent of the cure process and the chemistry of various reactions taking place. Spectra were taken every 5 min by means of four scans with a resolution of 4 cm−1. Figure 6 shows the FT-IR spectra obtained as the two monomers were cured at different temperatures. The strong peaks at approximately 2160 cm−1 indicate the presence of acetylenic functionality; the absence of primary acetylenes is also apparent from the lack of absorption at 3295 cm−1 (C≡C–H). Other functionality was as follows: 1486–1629 cm−1 (aromatic, C=C), 1009–1220 cm−1 (CH2–H), 756 cm−1 and 838 cm−1 (Si–C) from curve 1. The peak at 2160 cm−1 broadened with increasing curing temperature and vanished when the curing temperature reached 450 °C. This decrease in peak intensity was caused by conformational distribution of the products by a crosslinking reaction and was accompanied by an increase in intensity at 3056 cm−1 assigned to absorption of C–H bonds in the benzene ring with curing. No new absorption peaks were observed in spectra except for the increased intensity of the absorption peak of the benzene ring, which indicated the occurrence of a trimerization reaction [31]. The results were in agreement with the DSC analysis, and a small red shift in the position of the triple bonds peak occurred from 2160 to 2139 cm−1, which further confirmed occurrence of the trimerization reaction. It is worth mentioning that the peak of VTPES at 1392 cm−1 attributed to absorption of vinyl C–H bonds bonded to silicon showed changes and vanished eventually, accompanied by an increase in the intensity of the 2928 cm−1 peak assigned to absorption of methylene C–H bonds when the curing temperature was beyond 360 °C. These results suggest that, in the presence of oxygen, benzene rings are formed from two C≡C bonds, and the C=C bonds changed into C–C bonds with curing [25]. Thus, the introduction of unsaturated groups contributed to the lower thermal curing temperature of the phenylacetylsilanes and improved process conditions.
Conclusions
Vinyltriphenylethynylsilane and phenyltriphenylethynylsilane were synthesized as functional monomers. Spectral analysis confirmed the structures of the products. The curing reactions were consistent with a first-order reaction according to the Kissinger method. The two monomers had a broad processing window for preparation of highly resistant materials by cyclotrimerization. The introduction of unsaturated groups contributed to improving the process conditions for the phenylethynylsilane monomers.
References
L.K. Luneva, A.M. Sladkov, V.V. Korshak, Synthesis of organosilicon and organogermanium polymers containing diacetylenic groupings in the chain. Izv. Akad. Nauk. SSSR. 1, 160–163 (1968)
T.J. Barton, I.M. Sina, Y. Pang, Thermal and catalytic polymerization of diethynyldiphenylsilane. Macromolecules 24(6), 1257–1260 (1991)
Y. Pang, I.M. Sina, T.J. Barton, Catalytic synthesis of silylene-vinylene preceramic polymers from ethynylsilanes. Macromolecules 26(21), 5671–5675 (1993)
M. Itoh, K. Inoue, K. Iwata, A heat-resistant silicon-based polymer. Adv. Mater. 9(15), 1187–1190 (1997)
M. Itoh, K. Inoue, N. Hirayama, Fiber reinforced plastics using a new heat-resistant silicon based polymer. J. Mater. Sci. 37, 3795–3801 (2002)
W.Y. Wong, C.K. Wong, G.L. Lu, Triplet emission in platinum-containing poly(alkynylsilanes). Macromolecules 36(4), 983–990 (2003)
J. Zhang, J. Huang, W. Zhou, Fiber reinforced silicon-containing arylacetylene resin composites. Express. Polym. Lett. 12, 831–836 (2007)
J.C. Sanchez, W.C. Trogler, Hydrosilylation of diynes as a route to functional polymers delocalized through silicon. Macromol. Chem. Phys. 209, 1527–1540 (2008)
M.E. Freeburger, L. Spialter, Vinyl-allyl-, and phenylethynylsilanes. Effect of silicon on their reactions as dienophiles and the synthesis of phenylated phenyl- and benzylsilanes. J. Organomet. Chem. 35(3), 652–658 (1970)
H.R. Lang, S. Blau, G. Rheinwald et al., Synthese, koordinationsverhalten und thermolyse alkinylfunkionalisierter übergangsmetall- komplexe; kristallstruktu r(Mo2(η5–C5H5)2(CO)4((μ4-η2:2:2:2-Me3SiC≡C–C≡CSiMe3)-Co2(CO)6)). J. Organomet. Chem. 494(1–2), 65–73 (1995)
J. Ishikawa, K. Inoue, M. Itoh, Dehydrogenative cross-coupling reactions between phenylsilane and ethynylbenzene in the presence of metal hydrides. J. Organomet. Chem. 552(1–2), 303–311 (1998)
L.A. Oro, M.J. Fernandez, M.A. Esteruelas, Hydrosilylation of alkenes by iridium complexes. J. Mol. Catal. 37, 151–156 (1986)
E. Lukevics, O. Pudova, R. Sturkovich, Hydrosilylation of ethynylsilanes with dimethyl(2-thienyl)silane. J. Organomet. Chem. 346, 297–303 (1988)
H.Q. Liu, J.F. Harrod, Copper(I) chloride-catalyzed cross- dehydrocoupling reactions between silanes and ethynyl compounds: a new method for the copolymerization of silanes and alkynes. Can. J. Chem. 68(7), 1100–1104 (1990)
M. Itoh, M. Mitsuzuka, T. Utsumi et al., Dehydrogenative coupling reactions between hydrosilanes and monosubstituted alkynes catalyzed by solid bases. J. Organomet. Chem. 476(2), C30–C31 (1994)
R.H. Krieble, C.A. Burkhard, Cyclic dimethylpolymethylenedioxysilanes. J. Am. Chem. Soc. 69, 2689–2692 (1947)
S. Tannenbaum, S. Kaye, G.F. Lewenz, Synthesis and properties of some alkylsilanes. J. Am. Chem. Soc. 75, 3753–3757 (1953)
A.C. Bond, L.O. Brockway, The molecular structures of mono-, di- and trimethylsilane. J. Am. Chem. Soc. 76, 3312–3316 (1954)
J.F. Garst, F. Ungvary, in Grignard Reagents, ed. by R.S. Richey (John Wiley & Sons, New York, 2000), p. 185
M. Itoh, K. Inoue, K. Iwata et al., New highly heat-resistant polymers containing silicon: poly(silyleneethynylenephenyleneethynylene). Macromolecules 30(4), 694–701 (1997)
Q. Chen, Y. Li, Z.L. Dai et al., Synthesis and characterization of methyl- di(phenylethynyl)silane and its network polymer. Acta. Chim. Sin. 63(3), 254–258 (2005)
A.P. Melissaris, M.H. Litt, New high-Tg, heat-resistant, cross-linked polymers 1 Synthesis and characterization of di-p-ethynyl-substituted benzyl phenyl ether monomers. Macromolecules 27(4), 883–887 (1994)
J. Ohshita, T. Iida, M. Ikeda et al., Synthesis of poly{[bis(diethynylphenyl)- silylene]phenylene}s with highly heat-resistant properties and an application to conducting materials. J. Organomet. Chem. 689, 1540–1545 (2004)
S.A. Amdur, T.Y. Cheng, C.J. Wong, Free-radical polymerization of phenylacetylene. J. Polym. Sci: Polym Chem Edn. 16, 407–414 (1978)
P.M. Hergenrother, J.G. Smith, Chemistry and properties of imide oligomers end-capped with phenylethynylphthalic anhydrides. Polymer 35(22), 4857–4864 (1994)
D.E. Kissinger, Reaction kinetic in differential thermal analysis. Anal. Chem. 29(11), 1702–1706 (1957)
Z.L. Dai, Q. Chen, L.Z. Ni et al., Curing kinetics and structural changes of a of di[(N-m-acetenylphenyl)phthalimide]ether/[(methyl)diphenylacetylene]silane copolymer. J. Appl. Polym. Sci. 100, 2126–2130 (2006)
S.B. Sastri, J.P. Armistead, T.M. Keller, Cure kinetics of a multisubstituted acetylenic monomer. Polymer 36(7), 1449–1454 (1995)
J.J. Ratio, P.J. Dynes, C.L. Hammeresh, The synthesis and thermal polymerization of 4,4′-diethynylphenyl ether. J. Polym. Sci: Polym. Chem. Eds. 18, 1035–1046 (1980)
S.B. Sastri, T.M. Keller, M. Kenneth, Studies on cure chemistry of new acetylenic resins. Macromolecules 26(23), 6171–6174 (1993)
M.D. Sefcik, E.O. Stejskal, R.A. Mckay, Investigation of the structure of acetylene-terminated polyimide resins using magic-angle carbon-13 nuclear magnetic resonance. Macromolecules 12(3), 423–425 (1979)
Acknowledgments
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (NSFC) (Grant No. 50773017, 50973024).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, D., Shi, T. & Li, Z. Synthesis, characterization, and non-isothermal curing kinetics of two silicon-containing arylacetylenic monomers. Res Chem Intermed 37, 831–845 (2011). https://doi.org/10.1007/s11164-011-0291-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-011-0291-1