Abstract
Thiamine deficiency complex (TDC) is a disorder resulting from the inability to acquire or retain thiamine (vitamin B1) and has been documented in organisms in aquatic ecosystems ranging from the Baltic Sea to the Laurentian Great Lakes. The biological mechanisms leading to TDC emergence may vary among systems, but in fishes, one common outcome is high mortality among early life stages. Here, we review the causes and consequences of thiamine deficiency in fishes and identify potential solutions. First, we examine the biochemical and physiological roles of thiamine in vertebrates and find that thiamine deficiency consistently results in impaired neurological function across diverse taxa. Next, we review natural producers of thiamine, which include bacteria, fungi, and plants, and suggest that thiamine is not currently limiting for most animal species inhabiting natural aquatic environments. A survey of historic occurrences of thiamine deficiency identifies consumption of a thiamine-degrading enzyme, thiaminase, as the primary explanation for low levels of thiamine in individuals and subsequent onset of TDC. Lastly, we review conservation and management strategies for TDC mitigation ranging from evolutionary rescue to managing for a diverse forage base. As recent evidence suggests occurrences of thiamine deficiency may be increasing in frequency, increased awareness and a better mechanistic understanding of the underlying causes associated with thiamine deficiency may help prevent further population declines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies have revealed an increasing number of wild fish populations that are deficient in thiamine (vitamin B1), an essential vitamin that is required for a range of metabolic functions (Bettendorff 2013). Low levels of thiamine result in a syndrome referred to as thiamine deficiency complex (TDC; Riley and Evans 2008) that can result in high early life stage mortality and lead to population declines (Brown et al. 2005b; Balk et al. 2009, 2016). Thiamine deficiency complex encompasses all symptoms resulting from thiamine deficiency at all life stages, including sublethal effects and direct mortality, and has emerged as a possible contributor to decreased survival and reduced reproductive success in a variety of fish taxa (Ketola et al. 2000; Brown et al. 2005b; Balk et al. 2016). This disorder was first documented in salmonine fishes in the Laurentian Great Lakes in 1968 (Marcquenski and Brown 1997) and the Baltic Sea in 1974 (Bylund and Lerche 1995), but the cause of the observed signs was not recognized as thiamine deficiency until decades later (Bylund and Lerche 1995; Fitzsimons 1995; Fisher et al. 1995). Here, we examine the causes and consequences of TDC by reviewing (1) the molecular and physiological functions of thiamine, (2) the natural and artificial sources of thiamine for fishes, (3) the possible causes and consequences of TDC, and (4) research needs and questions relevant to thiamine deficiency.
The geographic and taxonomic distribution of TDC occurrences has recently broadened, with low thiamine concentrations in tissues or eggs now being documented in previously unaffected populations. Thiamine deficiency has been most widely studied in salmonine fishes and has been identified in populations of lake trout (Salvelinus namaycush), Atlantic salmon (Salmo salar), Chinook salmon (Oncorhynchus tshawytscha), coho salmon (Oncorhynchus kisutch), steelhead trout (anadromous Oncorhynchus mykiss), and brown trout (Salmo trutta) from the Great Lakes and New York Finger Lakes (Fisher et al. 1995, 1996; Marcquenski and Brown 1997). Thiamine deficiency has also been widely reported in Baltic Sea populations of Atlantic salmon and brown trout (Bengtsson et al. 1999; Karlsson et al. 1999). Assessments of egg and tissue thiamine concentrations have been conducted in Alaskan Chinook salmon (Honeyfield et al. 2016) and American eels (Anguilla rostrata) from Lake Ontario (Fitzsimons et al. 2013), and the results suggest continued monitoring may be important in these populations. The broadening geographic distribution of TDC cases may indicate that thiamine availability is decreasing through decreases in thiamine production or increases in thiamine-degrading pathways in multiple aquatic systems. Alternatively, increased spatial distribution may reflect increased awareness of this issue, expanded research on thiamine, improved analytical methods for measurement of thiamine, and, consequently, increased sampling and detection.
Organisms susceptible to thiamine deficiency are thiamine auxotrophs (i.e., those that cannot synthesize thiamine on their own). In aquatic communities, thiamine is produced by prokaryotes, algae, and plants, and fishes are presumed to obtain most of their thiamine via grazing and predation on lower trophic level sources. Fishes may also obtain thiamine from symbiotic gut microbes (Ji et al. 1998), as has been documented in ruminant mammals (Breves et al. 1980, 1981) and insects (Sannino et al. 2018a, b), but the extent to which fishes exploit thiamine produced by resident microbiota has not been investigated.
Disruptions of the thiamine-producing community within an ecosystem could lead to decreased thiamine availability for consumers such as zooplankton, macroinvertebrates, and fishes. Changes in abiotic environmental factors including light availability, temperature, and salinity have been shown to affect the thiamine content of several phytoplankton species capable of synthesizing thiamine (Sylvander et al. 2013). Although diminished thiamine production at lower trophic levels could lead to thiamine deficiency in fishes, decreased production has not been implicated in documented cases of thiamine deficiency, and most research to date has focused on other causes.
In the Great Lakes, the most widely studied potential cause of TDC is an enzyme—thiaminase (specifically, thiaminase I, which is distinct from another thiamine-degrading enzyme referred to as thiaminase II or tenA; Jenkins et al. 2007)—that is present in some prey fishes. Thiaminase I can degrade thiamine in the gastrointestinal (GI) tract of fishes, and can ultimately decrease the amount of thiamine available to individuals consuming thiaminase I-containing prey (Honeyfield et al. 2005; Houde et al. 2015). Recent work demonstrates that thiaminase I in bacteria salvages precursors from environmental thiamine and thiamine analogs to synthesize thiamine (Sannino et al. 2018a). The surprising involvement of bacterial thiaminase I in thiamine synthesis, as well as degradation, is similar to the previously discovered role of tenA in thiamine salvage and synthesis (Jenkins et al. 2007). Thiaminase I activity is high in alewife (Alosa pseudoharengus; Tillitt et al. 2005), a ubiquitous clupeid that invaded the Great Lakes and forms the primary forage base for a number of vertebrates (including fishes and birds) (Stewart et al. 1981; Fox et al. 1990; Weseloh et al. 1995; Madenjian et al. 2002; Happel et al. 2017). Awareness of the consequences of thiaminase I consumption has led to detection and analysis of this enzyme in a wide variety of other taxa within aquatic food webs, including round goby (Neogobius melanostomus; Honeyfield et al. 2012) and dreissenid mussels (Dreissena spp.; Tillitt et al. 2009). Thiaminase I has also been a focus of TDC research in the Baltic Sea (Karlsson et al. 1999; Wistbacka et al. 2002), with some correlational data suggesting that other dietary factors (e.g., fat content, antioxidant availability) may exacerbate thiaminase I-induced TDC (Lundström et al. 1999b; Pettersson and Lignell 1999; Keinänen et al. 2012). Regardless of the proximate cause of TDC, the physiological effects of thiamine deficiency are consistent among fishes across systems and are related to the crucial role thiamine plays in bioenergetic pathways.
Functions of thiamine and its derivatives
Thiamine synthesis by living organisms produces thiamine monophosphate (TMP) (Bettendorff 2013). In animals, TMP must first be dephosphorylated to free thiamine before it can then modified by the addition of phosphate groups to serve a variety of physiological functions. Free thiamine and TMP have no known physiological function (Bettendorff et al. 2014), although free thiamine may act as an antioxidant (Lukienko et al. 2000). The addition of two phosphate groups to TMP yields thiamine triphosphate (TTP), which is constantly produced at a low rate in animal cells (Bettendorff et al. 2014). TTP has been suggested to be important for proper neuron function (Cooper and Pincus 1979; Bettendorff et al. 1993), but its precise role(s) in cellular pathways remain unclear (Bettendorff et al. 2014). By far, the most well-characterized thiamine derivative is thiamine diphosphate (TDP), which serves as a cofactor for 20 enzymes in humans (Widmann et al. 2010).
Enzymes requiring TDP as a cofactor are essential for metabolism and energy production, catalyzing reactions in the pentose phosphate pathway and tricarboxylic acid (TCA) cycle (Fig. 1). With additional roles in the production of neurotransmitters, antioxidants, and myelin, TDP-dependent enzymes are crucial to proper neurological function (Bettendorff 2013). In humans, diseases resulting from thiamine deficiency comprise a spectrum of clinical presentations, including a syndrome known as beriberi. Cases of beriberi are generally divided into two categories: “wet” beriberi primarily affects the cardiovascular system and is accompanied by edema whereas “dry” beriberi is characterized by peripheral nervous system impairment (Whitfield et al. 2018). Severe and prolonged thiamine deficiency can also lead to the development of Wernicke’s encephalopathy, which is a neurological disorder characterized by weakness and involuntary movement of eye muscles, ataxia, and abnormal stance and gait (Sechi and Serra 2007). Wernicke’s encephalopathy is often observed concurrently with Korsakoff’s psychosis, as indicated by amnesia, disorientation, and disturbances and distortions of memory (Harper et al. 1986). These two disorders are often collectively referred to as Wernicke–Korsakoff syndrome and are definitively diagnosed postmortem based on characteristic brain lesions (Sechi and Serra 2007). Similar lesions have been described in thiamine-deficient Atlantic salmon and lake trout, with severity of brain tissue necrosis increasing with disease progression (Lundström et al. 1999a; Lee et al. 2009). Furthermore, the neurological signs of Wernicke–Korsakoff syndrome in humans (e.g., abnormal stance and gait) are analogous to behavioral signs of TDC in juvenile and adult salmonine fishes (e.g., inability to remain upright, ataxia, corkscrew swimming patterns, lethargy; Fisher et al. 1995; Brown et al. 2005c; Fitzsimons et al. 2005a), suggesting that the pathology of thiamine deficiency may reflect conserved TDP-dependent enzyme functions across diverse vertebrate taxa.
Pathways requiring thiamine diphosphate (TDP) with TDP-dependent enzymes highlighted in yellow boxes. Arrows in pathways may represent multiple reactions and intermediates for the purpose of simplification. The central roles that thiamine plays in carbohydrate, branched-chain amino acid, and lipid metabolism are mediated by five thiamine-dependent enzyme complexes: pyruvate dehydrogenase (PDH), oxoglutarate dehydrogenase (OGDH), branched-chain α-ketoacid dehydrogenase (BCKDH), transketolase (TK), and 2-hydroxyacyl-CoA lyases 1 and 2 (HACL1 and HACL2) (Depeint et al. 2006; Casteels et al. 2007). These five complexes require the active form of thiamine, thiamine diphosphate (TDP; also referred to as thiamine pyrophosphate), as a coenzyme. Three of these complexes—PDH, OGDH, and BCKDH—catalyze oxidative decarboxylation of α-ketoacids to produce CO2 during the tricarboxylic acid (TCA) cycle, and are therefore central to mitochondrial energy production (Depeint et al. 2006). Specifically, PDH is also necessary for the production of the neurotransmitter acetocholine and of myelin, the insulating substance that coats neurons. OGDH is required for production of two other neurotransmitters, glutamate and γ-aminobutryic acid (GABA). TK catalyzes the first and last steps of the pentose phosphate pathway, linking the pathway to glycolysis (Bettendorff 2013). Among other products, the pentose phosphate pathway generates fatty acids, ribose for nucleic acid synthesis, and glutathione, which is a major endogenous antioxidant (Depeint et al. 2006). HACL1 and 2 catalyze α-oxidation of fatty acids in the peroxisome and endoplasmic reticulum, respectively (Casteels et al. 2007; Kitamura et al. 2017). By facilitating α-oxidation, HACLs are suspected to aid in maintaining homeostasis of a specific group of fatty acids important in the formation and maintenance of the myelin sheath (Kitamura et al. 2017)
Natural and artificial thiamine sources
Production and availability of thiamine
All fishes acquire thiamine through intestinal absorption. This thiamine is synthesized by bacteria and algae in the aquatic environment, consumed by planktivorous fishes, and later transferred via piscivory. Thiamine content in Great Lakes forage fishes varies seasonally and annually among species and locations, but does not seem to be limiting in any forage species under any particular environmental condition or set of conditions (Fitzsimons et al. 2005b; Tillitt et al. 2005). Thiamine may also be produced by resident microbiota in the GI tracts of fish. An experimental feeding study conducted in lake trout suggests that as much as 81% of thiamine present in the posterior portion of the intestine may be of non-dietary origin (i.e., synthesized by gut microbiota) (Ji et al. 1998). Gut microbes may therefore provide thiamine to their hosts, but the degree to which non-dietary (synthesized) sources of thiamine are absorbed by posterior intestinal tissues is unknown. If fishes do receive substantial amounts of thiamine from resident microbes, perturbations of gut microbial communities could influence thiamine availability for the host fish, but little is known on this subject.
Environmental (i.e., non-dietary) sources of thiamine may also be available to fishes. Degradation of any cellular material likely releases intact cellular thiamine, but longevity of free thiamine in natural waters is unknown. Assessment of thiamine concentrations in a marine system suggest that concentrations are generally low in the water column (30–280 pM) but can be much higher in sediment pore water (30–770 pM) (Monteverde et al. 2015). In shallow aquatic environments where mixing is prevalent, thiamine may readily diffuse out of the sediment. High benthic concentrations of thiamine may be particularly important for salmonines and many other fish species that incubate their eggs in and on freshwater sediments. Thiamine absorption across the chorion once the egg is water-hardened has not been observed or reported, but free embryos are capable of absorbing thiamine from water (see Thiamine therapies: hatchery treatments below). If benthic concentrations of aqueous thiamine are sufficient, 4–6 weeks residence of free embryos in spawning substrate after hatching may allow embryos to absorb enough thiamine to mitigate deficiency.
Thiamine therapies: hatchery treatments
Several effective methods of thiamine supplementation have been developed for multiple salmonine life stages. The method most commonly employed in hatcheries is the immersion of fertilized eggs in thiamine solution during water hardening (Wooster et al. 2000), the developmental stage where eggs absorb water and the egg membrane becomes rigid. Immersing Atlantic salmon eggs in a 1% thiamine hydrochloride (HCl) solution can yield a 14-fold increase in sac fry total thiamine concentration over untreated control fry (untreated: 0.625 nmol/g; treated: 8.706 nmol/g) (Wooster et al. 2000). The magnitude of increase in egg thiamine concentration is dosage-dependent; treating with higher concentrations of thiamine in an immersion bath results in a greater magnitude of increase in egg thiamine concentration (e.g., treatment with a 0.1% thiamine HCl solution results in a threefold increase in sac fry total thiamine; untreated: 0.625 nmol/g; treated: 1.845 nmol/g) (Wooster et al. 2000).
Treating individuals at the egg stage is more logistically tractable than at later developmental stages. In hatcheries that rear salmonines, fertilized eggs are often treated with an iodophor solution to prevent the spread of viral hemorrhagic septicemia (VHS) (Amend and Pietsch 1972). Hatchery biosafety protocols typically require that eggs be treated with iodophor solution first, followed by thiamine HCl (K. Kelsey, personal communication), and this series could lead to the degradation of thiamine by residual iodophor solution if the solution adheres to the eggs or if disinfected eggs are not thoroughly rinsed. Iodophor treatment also delays thiamine treatment and may limit the amount of thiamine that can be absorbed by eggs prior to chorion hardening. The possibility for iodophor–thiamine interactions has led to increased interest in thiamine treatments designed for fry and adult stages.
Thiamine-deficient fry may be treated with yolk sac thiamine injections or immersion in a 1% thiamine HCl bath for 1 h; both approaches have been shown to effectively eliminate TDC-related mortalities (Fisher et al. 1996; Wooster et al. 2000; Lee et al. 2009). These studies show that fry absorb aqueous thiamine, and gill tissues are plausible sites of thiamine absorption from the environment. However, whether gill epithelial cells transport thiamine from the environment across the cell membrane is unknown, and this route of uptake has yet to be experimentally demonstrated. One risk of treatment at the sac fry stage is the potential for sublethal effects resulting from short-term thiamine deficiency, but this mechanism has not been investigated in fishes (Mimouni-Bloch et al. 2014) (see Lethal and sublethal effects in fry below).
Although egg or fry immersion is an easy method of treating large numbers of individuals simultaneously, injection of prespawn females can achieve far greater increases in offspring thiamine concentrations (Ketola et al. 2000). Injection of prespawn coho salmon females with 50 mg thiamine HCl/kg body mass (hereafter, mg/kg) ~ 1 month prior to spawning can result in a 28-fold increase in egg total thiamine concentration relative to eggs from untreated females (untreated: 0.767 nmol/g; treated: 21.925 nmol/g) (Fitzsimons et al. 2005a). In systems with small numbers of returning females, or where females will be released to spawn in the natural environment, injection of prespawn females is a viable alternative to egg immersion.
As with egg and fry immersion, the magnitude of increase in egg thiamine concentration resulting from prespawn female injection is dosage-dependent (Wooster et al. 2000). Across separate studies, injection concentrations of 50 and 100 mg/kg have resulted in significant increases in egg thiamine concentrations for Atlantic salmon, coho salmon, and steelhead trout (Koski et al. 1999; Fitzsimons et al. 2005a; Futia et al. 2017). Injection of a much lower thiamine concentration (7 mg/kg) did not produce a significant effect on egg thiamine concentrations for Atlantic salmon females but did significantly decrease fry mortality from 98.6% in control females to 2.1% in thiamine-injected females (Ketola et al. 2000). This result demonstrates that large changes in egg thiamine concentration may not be required for increased fry survival in the short term, especially when egg thiamine concentrations are in the range of the dose–response curve where small increases in thiamine are expected to produce relatively large changes in survival. However, no study has continued to track offspring from treated and untreated females to detect potential sublethal effects of low egg thiamine at later life stages.
The amount of time between treatment and spawning may also impact the efficacy of treating prespawn females, although this effect has never been measured in a controlled manner. In two separate studies (both using a thiamine injection concentration of 50 mg/kg), treating steelhead trout females ~ 4 months prior to spawning resulted in a sixfold increase in egg total thiamine concentration (untreated: 3.4 nmol/g; treated: 20.4 nmol/g) (Futia et al. 2017), whereas treating coho salmon females ~ 1.3 months prior to spawning yielded a 28-fold increase in egg total thiamine concentration (untreated: 0.767 nmol/g; treated: 21.925 nmol/g) (Fitzsimons et al. 2005a). The 28-fold increase in egg thiamine concentration observed in the coho salmon study may be due to females allocating thiamine to eggs in greater proportions when spawning is imminent as opposed to when spawning is 4 months in the future, though some of these differences may also reflect interspecific differences in how excess thiamine is allocated.
Causes of TDC in natural populations
M74 in the Baltic Sea
Diverse causes of TDC, which may not be mutually exclusive, have been proposed. In 1974, anadromous Atlantic salmon sac fry in Baltic Sea hatcheries began exhibiting behavioral signs typical of thiamine deficiency, including ataxia and lethargy (Amcoff et al. 1998), and affected families experienced 100% mortality (Lundström et al. 1999b). Since the early 1990s, returning adult salmon have also displayed signs of thiamine deficiency, including lack of coordination and sideways swimming (Amcoff et al. 1998). This disorder—originally termed M74, for miljöbetingad (literally translated as “environmental”) and the year of its discovery (Bylund and Lerche 1995)—was eventually linked to low levels of thiamine at the egg and fry stages of affected individuals (Amcoff et al. 1998). Using data spanning 25 years, Karlsson et al. (1999) identified correlations suggesting that over-consumption of native sprat (Sprattus sprattus) had led to thiamine deficiency in Atlantic salmon. For the salmon whose diet consisted primarily of sprat, frequency of TDC was positively correlated with sprat biomass (Karlsson et al. 1999).
Sprat, along with Atlantic herring (Clupea harengus), comprise a large proportion of Atlantic salmon diets in the Baltic Sea (Hansson 2001) and tissues of both forage species exhibit comparable degrees of thiaminase I activity (Wistbacka et al. 2002; Wistbacka and Bylund 2008). Thiaminase I consumption is hypothesized to be the cause of M74, but other dietary factors may exacerbate thiamine deficiency. For example, young, small sprat have higher fat and lower thiamine contents than older, larger sprat. Thiamine:fat content increases as fish age, and young sprat contain roughly half as much thiamine per gram of fat as older sprat (range for sprat aged 1–5 years: 16–122 nmol thiamine/g fat; aged 6–13 years: 48–248 nmol/g) (Keinänen et al. 2012). The combination of high fat and low thiamine concentrations is hypothesized to be particularly detrimental for thiamine-deficient fishes for two main reasons. First, consumption of high amounts of easily oxidized fatty acids can lead to oxidative stress in Atlantic salmon, and this stress can be exacerbated when thiamine—which can function as an antioxidant (Lukienko et al. 2000)—occurs in low concentrations relative to lipids (Keinänen et al. 2012). Development of thiamine deficiency has also been associated with low concentrations of other antioxidants in salmonine tissues, including astaxanthin (Pettersson and Lignell 1999) and vitamin E (Palace et al. 1998), but a correlation between TDC and low levels of other antioxidants is not always present (Brown et al. 2005a). Low availability of other antioxidant vitamins may also increase overall demand for thiamine. If an individual is already thiamine deficient, diverting thiamine from its role as a cofactor for thiamine-dependent enzymes to the role of an antioxidant may intensify the symptoms of thiamine deficiency. Second, oxidative stress may decrease the activity of thiamine-dependent enzymes (Park et al. 1999) as well as levels of available thiamine (Gibson and Zhang 2002).
As early as 1942, herring fat content was considered as a possible cause of a fatal disorder in herring-fed trout (later recognized as thiamine deficiency), but dietary fat content alone did not induce presentation of the disorder (Wolf 1942). Similarly, decreased antioxidant vitamin concentrations in salmonines (astaxanthin and vitamin E) and in forage species (low vitamin E in alewife; Honeyfield et al. 2012) have been associated with thiamine deficiency in salmonines, but these correlations have all been detected in the presence of dietary thiaminase I. Furthermore, treatment of thiamine-deficient eggs with astaxanthin does not affect fry mortality rate in steelhead trout (Hornung et al. 1998). Occurrences of TDC in the Baltic Sea may therefore be due to thiaminase I consumption and compounded by consumption of low-thiamine prey or oxidative stress, but no evidence to date supports oxidative stress or low dietary antioxidants as direct, causative agents of TDC.
Thiamine deficiency complex in the Great Lakes
The first documentation of a thiamine deficiency in Great Lakes fishes was an investigation of a “dietary disease” observed in hatcheries and associated with the usage of particular forage fish species (Wolf 1942). Brown trout, brook trout (Salvelinus fontinalis) and rainbow trout (Oncorhynchus mykiss) fed diets containing varying proportions of herring (Clupea sp.) developed symptoms of thiamine deficiency (Wolf 1942), similar to feeding trials with farm-raised foxes that were fed raw fish diets and developed Chastek paralysis, a known thiamine deficiency disorder (Green and Evans 1940). Wolf (1942) concluded that certain forage fish contained a “vitamin B1 destroying” capability (later identified as thiaminase I) that was able to induce thiamine deficiency in hatchery reared trout fed raw forage fish.
In 1968, a few years prior to the first description of M74, emergence of TDC in populations of salmon and trout from the Great Lakes was documented for the first time in fishery agency reports. The condition manifested primarily as increased rates of mortality in hatchery culture prior to swim-up (the developmental stage where yolk sac absorption is complete and the fry become completely dependent on exogenous feeding) in coho and Chinook salmon, brown trout, and steelhead trout, and was originally named “early mortality syndrome” (EMS, now known as TDC). From 1968 through 1992, rates of TDC-induced mortality varied among species, but did not exceed 30% for families in hatchery culture. In 1993, mortality reached up to 90% for juvenile coho salmon in Lake Michigan hatcheries, and mortality also began to occur earlier at the sac fry stage in addition to mortality at the swim-up stage (Marcquenski and Brown 1997).
Initially, research in the Great Lakes aimed to determine whether environmental contaminants—such as polychlorinated biphenyls (PCBs), pesticides, dioxins, furans, and polynuclear aromatic hydrocarbons (PAHs)–were associated with the observed mortality patterns for affected species, which included lake trout, Chinook salmon, coho salmon, steelhead trout, and brown trout (Skea et al. 1985; Mac et al. 1985, 1993; Fitzsimons et al. 1995; Marcquenski and Brown 1997). Maternal exposure of salmonine eggs to dioxins and other contaminants that activate the aryl hydrocarbon receptor causes a similar syndrome that results in mortality of sac fry (King-Heiden et al. 2012). However, egg contaminant levels in the Great Lakes decreased below levels expected to cause mortality by the early 1990s and could not explain the observed rates of juvenile mortality (see Tillitt et al. 2008 for a review). In 1995, attention turned to a nutritional basis for TDC in wild salmonine populations when thiamine treatments for sac fry (immersion and injection) were shown to significantly decrease mortality, whereas treatment with four other B-vitamins proved ineffective (Fitzsimons 1995). The identification of thiamine deficiency in sac fry effectively refocused the search for the cause of TDC (Fitzsimons 1995; Fisher et al. 1995).
Thiamine loss due to dietary thiaminase I
By the 1960s, two nonnative species—alewife and rainbow smelt (Osmerus mordax)—were well established throughout the Great Lakes (Miller 1957; MacCallum and Regier 1970) and comprised a large portion of Great Lakes salmonine diets (Stewart et al. 1981; Jude et al. 1987), a trend which continues to the present (Ray et al. 2007; Jacobs et al. 2013; Roseman et al. 2014; Happel et al. 2018). Because alewife, rainbow smelt, and other thiaminase I-containing prey species supply an otherwise sufficient amount of dietary thiamine to consumers (Tillitt et al. 2005; Honeyfield et al. 2012), TDC in the Great Lakes is likely caused by thiaminase I consumption, rather than insufficient tissue thiamine concentrations in the salmonine forage base. Lake trout diets dominated by alewife and smelt have been correlated with an 89–94% decrease in lake trout egg thiamine concentrations (Fitzsimons and Brown 1998), indicating consumption of these two species—and thiaminase I—as a potential cause of TDC in the Great Lakes.
Experimental studies have confirmed that consumption of thiaminase I (Honeyfield et al. 2005; Houde et al. 2015) and diets comprised entirely of alewife (Honeyfield et al. 2005) can decrease tissue and egg thiamine concentrations and induce TDC. Salmonine egg thiamine concentrations have also been inversely correlated with alewife abundance (Riley et al. 2011), providing further evidence that the consumption of alewife (and thereby thiaminase I) may be driving salmonine TDC occurrences in the Great Lakes.
Sources and activity of thiaminase I
In the presence of an appropriate electron donor (nucleophile), thiaminase I degrades thiamine into a thiazole-nucleophile moiety and a pyrimidine moiety (Fig. 2). The first bacterium to be isolated and identified as a thiaminase I producer, Bacillus thiaminolyticus, was named to reflect this property (Kuno 1951). Now reclassified as Paenibacillus thiaminolyticus, this bacterium has been successfully cultured from entire homogenized viscera (intestines, liver, heart, and spleen) of alewife (Honeyfield et al. 2002), indicating intestinal tissues of forage fishes as potential sources of thiaminase I. This hypothesis is consistent with later studies that demonstrated that thiaminase I activity is highest in alewife splenic and bacteria-rich intestinal tissues compared to muscle and gill tissues (Zajicek et al. 2005; Kraft et al. 2014). Interestingly, a similar observation has been made for a plant species: bracken fern rhizome tissue, which contains much greater diversity and abundance of symbiotic bacteria than other plant tissues (Berendsen et al. 2012), exhibits higher thiaminase I activity than stipe or leaf tissues, consistent with a potential microbial source of thiaminase I (Kraft et al. 2014). Six other microbes are known to produce a thiaminase I-type enzyme (Kraft and Angert 2017). One of these (Clostridium sporogenes) is commonly found in the GI tracts of ruminant mammals that commonly die from thiamine deficiency associated with changes in their GI tracts (Hatheway 1990), but these microbes have not been described in fish intestinal tracts. Gene sequences of additional bacterial species indicate that they are capable of producing thiaminase I enzymes (C. E. Kraft, personal observation).
Degradation of thiamine by thiaminase into its constituent thiazole-nucleophile and pyrimidine moieties. The initiation of this reaction in food consumed by a thiamine auxotroph irreversibly decreases the amount of thiamine available to the consumer, as auxotrophs cannot recycle the products of the reaction for thiamine synthesis
Thiaminase I may also be produced by fishes endogenously. Identifications of putative thiaminase I enzymes with distinct N-terminal protein sequences in carp (Cyprinus sp.; Boś and Kozik 2000) and red cornetfish (Fistularia petimba; Nishimune et al. 2000) liver homogenates prompted further investigation into possible production of thiaminases by fishes. Richter et al. (2012) found that thiaminase I activity in the tissues of multiple fish, zooplankton, and mussel species was not related to P. thiaminolyticus cell abundance or to the concentration of P. thiaminolyticus thiaminase I. This result suggests that P. thiaminolyticus may not be the primary thiaminase I producer in salmonine prey species. It is possible that other bacterial species contribute to thiaminase I production or that alewife and other fishes produce their own thiaminase I enzymes de novo. The hypothesis that fishes produce thiaminase I is also interesting from an evolutionary perspective. Animals cannot produce their own thiamine and therefore cannot recycle the products of thiamine degradation for the purpose of thiamine synthesis. That any animal would produce an enzyme capable of destroying an essential nutrient seems counterintuitive. Highly speculative hypotheses include that thiaminase I may play a role in innate immunity by restricting the thiamine available to bacteria in the gut or that thiaminase I protects fishes from toxic thiamine analogs in the diet. Production of extracellular thiamine analogs is hypothesized to be important in ecological competition for thiamine (Kraft and Angert 2017).
Even though thiaminase I activity has been detected in a variety of Great Lakes species, highly variable thiaminase I activities among and within species complicate efforts to describe the origin(s) of thiaminase I. In alewife tissues, thiaminase I activity has been shown to vary by year, season, sampling location, and individual size (Ji and Adelman 1998; Tillitt et al. 2005; Honeyfield et al. 2012). In rainbow smelt, thiaminase I activity varies significantly between years in some studied populations (Ji and Adelman 1998; Honeyfield et al. 2012), but not others (Tillitt et al. 2005). Activity has also been shown to vary by season and collection depth in invasive dreissenid mussels (Tillitt et al. 2009) and relative to reproductive status in native unionid mussels (Unionidae; Blakeslee et al. 2015). Atlantic herring and alewife have exhibited substantial increases in thiaminase I activity levels when transferred to captivity from the wild, but these increases were not associated with any other explanatory variable (Lepak et al. 2008; Wistbacka and Bylund 2008). Despite descriptions of these patterns, no single environmental variable has been found to predict variation in thiaminase I activity across multiple systems or time points for any species.
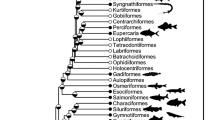
Although environmental predictors of thiaminase I activity are not well established, trophic patterns have emerged across studies examining thiaminase I activity in fishes. Fishes feeding at lower trophic levels are more likely to exhibit thiaminase I activity than fishes feeding at higher trophic levels (Riley and Evans 2008). Taxonomic patterns have also been described, with basal teleosts (i.e., Anguilliformes, Clupeiformes, Cypriniformes, and Siluriformes) more likely to exhibit thiaminase I activity than basal euteleosts (i.e., Esociformes, Osmeriformes, and Salmoniformes) or neoteleosts (i.e., Gadiformes, Gasterosteiformes, Perciformes, and Scorpaeniformes; Riley and Evans 2008). Specifically, clupeids including Atlantic herring, alewife, and sprat have been implicated in emergence of increased TDC, and these species often exhibit high thiaminase I activity in their tissues (Wistbacka and Bylund 2008; Kraft et al. 2014). Thiaminase I activity has also been detected in other clupeids, including hickory shad (Alosa mediocris), threadfin shad (Dorosoma petenense), and gizzard shad (Dorosoma cepedianum; Tillitt et al. 2005; Honeyfield et al. 2007; Kraft et al. 2014), suggesting that either endogenous thiaminase I production or symbiosis with thiaminase I-producing bacteria is common to members of Clupeidae (Fitzsimons et al. 2012). Several members of Cyprinidae including the carps (Cyprinus spp.), blacknose dace (Rhinichthys atratulus), longnose dace (Rhinichthys cataractae), central stoneroller (Campostoma anomalum), common shiner (Luxilus cornutus), spottail shiner (Notropis hudsonius), creek chub (Semotilus atromaculatus), cutlips minnow (Exoglossum maxilingua), and fallfish (Semotilus corporalis) also exhibit thiaminase I activity (Boś and Kozik 2000; Tillitt et al. 2005; Kraft et al. 2014). Thiaminase I activity has also been observed in species belonging to Catostomidae (white sucker, Catostomus commersonii, Kraft et al. 2014) and Osmeridae (rainbow smelt, Tillitt et al. 2005), but the families Clupeidae and Cyprinidae are regularly recognized as exhibiting high thiaminase I activities that are associated with thiamine deficiency in consumers.
Effects of thiamine deficiency
The majority of research examining TDC in fishes has relied on data collected from the offspring of spawning adults caught in the wild and reared in hatcheries or laboratories. Observing the development of thiamine-deficient eggs and fry in culture or in the laboratory has allowed for detailed descriptions of the physical and behavioral signs of thiamine deficiency, but these data may not accurately describe developmental outcomes for deficient offspring in the wild. In hatcheries or laboratory environments, fry in culture are usually first given powdered feed near the time of yolk sac absorption (approximately 5 weeks after hatch, depending on water temperature). However, wild lake trout fry begin feeding within 2 weeks of hatching, with nearly all individuals (98%) successfully feeding on live prey by the completion of yolk sac absorption (Swedberg and Peck 1984; Ladago et al. 2016). This behavior indicates that some offspring of thiamine-deficient parents may be able to mitigate deficiency by feeding early. In Lake Champlain, invasion of alewife led to significant declines in egg thiamine in lake trout and Atlantic salmon but was followed by the onset of substantial and sustained recruitment of wild juvenile lake trout (Marsden et al. 2018). However, in cases of severe thiamine deficiency, fry may be unable to forage regardless of food availability, as lake trout egg thiamine concentrations of 6.9 and 2.9 nmol thiamine/g egg are associated with 20% and 50% reductions in foraging rate, respectively (Fitzsimons et al. 2009).
Lethal and sublethal effects in fry
The physical and behavioral signs of thiamine deficiency are similar among salmonines. Thiamine-deficient fry exhibit a suite of physical signs, including hydrocephalus, vascular congestion, diminished yolk sac conversion efficiency, and large yolk sacs with opacities, edema, and hemorrhaging (Fisher et al. 1995; Lundström et al. 1999a; Fitzsimons et al. 2001a, b). Perhaps even more striking than the physical consequences of thiamine deficiency are the resulting behavioral abnormalities. These behaviors include lethargy, ataxia, and unusual swimming patterns such as bouts of uncoordinated swimming followed by variable intervals of passive drifting and swimming in a “corkscrew” or “wiggling” manner (Fisher et al. 1995; Marcquenski and Brown 1997; Fitzsimons et al. 2005a). Experimental induction of thiamine deficiency leads to similar physiological and behavioral shifts in juveniles of other species, including sterlet (Acipenser ruthenus) (Ghiasi et al. 2017), Nile tilapia (Oreochromis niloticus) (Lim et al. 2011), and Japanese eel (Anguilla japonica) (Hashimoto et al. 1970). In salmonine fry, death usually occurs after the onset of behavioral signs and prior to swim-up.
In addition to TDC-induced mortality, sublethal effects of TDC have been described in lake trout. For some of these effects, egg thiamine thresholds required to produce effects of specific magnitudes have been determined. For example, an egg thiamine concentration of 5.1 nmol/g was associated with a 50% reduction in growth rate of lake trout fry relative to thiamine-replete fry (Fitzsimons et al. 2009). Reduced growth due to thiamine deficiency can profoundly impact fry survival, as larger body size allows larvae to achieve and sustain faster swimming speeds, which may in turn decrease predation vulnerability and increase prey capture efficiency (Miller et al. 1988). The threshold concentration for 50% reduced growth is more than three times higher than the egg thiamine concentration associated with 50% TDC-induced mortality in lake trout (ED50 = 1.6 nmol/g, 95% CI [1.1, 2.1]) (Fitzsimons et al. 2007), suggesting that thiamine deficiency can impact long-term survival at egg thiamine concentrations far above those required to induce direct mortality. Additional sublethal effects of diminished egg thiamine concentrations on fry include reduced foraging rate (Carvalho et al. 2009; Fitzsimons et al. 2009), decreased visual acuity (Carvalho et al. 2009), and altered immune responses (Ottinger et al. 2012). Many of these sublethal effects likely translate into reduced survival and fitness later in life.
Thresholds for thiamine deficiency
Many studies have focused on determining how much thiamine must be allocated to an egg to prevent TDC-induced mortality of the hatched fry. Although egg threshold determination methods differ across studies, concentrations of free (unphosphorylated) egg thiamine required to avoid mortality from TDC during development range from 0.3 nmol/g (in Lake Michigan steelhead trout, Hornung et al. 1998) to 0.8–1.0 nmol/g (in Lake Michigan lake trout, Czesny et al. 2009). In many cases, variability has been observed among sampling locations, sampling years, and species for the relationship between egg thiamine concentrations and subsequent rates of mortality (Hornung et al. 1998; Honeyfield et al. 2005; Fitzsimons et al. 2007; Futia et al. 2017). This variation may result from differences in environmental characteristics during egg development and maturation (i.e., maternal effects), such as maternal diet, or may relate to uncharacterized patterns of adaptive genetic or epigenetic variation among families or populations.
Even when thiamine levels are not sufficiently low to result in TDC-related mortality, sub-lethal effects may result in demographic declines at the population level. Although increasing awareness of TDC has resulted in increased monitoring efforts for lake trout in the Great Lakes (Riley et al. 2011) and for other species in other systems (Honeyfield et al. 2016), thresholds for sublethal effects in other species have never been empirically established. Reliably assessing the risk of sublethal effects for species other than lake trout is not currently possible. Establishing lethal and sublethal effect thresholds for other species would expand the capacity for more accurate demographic projections for declining populations potentially affected by TDC.
Because of the negative relationship described between egg thiamine concentration and rates of TDC-related mortality for salmonines, applied studies have sought to define diagnostic maternal characteristics that can be used to predict egg thiamine concentrations. For females that are sufficiently thiamine-depleted to produce offspring with TDC, no relationships have been detected between maternal tissue thiamine concentrations and egg thiamine concentration (Fisher et al. 1998; Futia et al. 2017). This absence of correlation may be common in a heavily impacted population, as maternal tissue and egg thiamine concentrations may be uniformly low with insufficient variation among individuals to describe a relationship that could be found if a population contained individuals with a wider range of thiamine statuses. Some data from affected females indicate an inverse relationship between maternal weight or length and egg thiamine concentrations (Brown et al. 2005a; Wolgamood et al. 2005; Werner et al. 2006; Honeyfield et al. 2008; Futia et al. 2017), suggesting possible variation in salmonine diet composition related to age and size (e.g., older, longer females may consume alewife in higher proportions than younger females). However, this hypothesis has not been experimentally tested, and questions regarding maternal thiamine allocation priorities and the physiological mechanisms of egg thiamine deposition still need to be addressed.
Thiamine deficiency in adults
While much research has focused on how developmental outcomes for eggs and fry are affected by TDC, fewer experiments have examined how thiamine deficiency directly impacts adult fish health. In Atlantic salmon sampled for hatchery rearing while returning to the River Luleälven from the Baltic Sea, females exhibiting “wiggling, sideways swimming, [and] a lack of coordination” were found to have significantly lower ovarian thiamine concentrations than returning females with normal behavior (Amcoff et al. 1998). Of 103 females displaying abnormal swimming behavior, 27 died before being spawned, with mortality attributed to TDC (Amcoff et al. 1998). While severe thiamine deficiency can directly lead to adult mortality (Amcoff et al. 1998; Brown et al. 2005c; Fitzsimons et al. 2005a), sublethal effects of deficiency may also reduce the fitness of affected populations. In 2-year-old (subadult) Atlantic salmon, individuals fed a high-thiaminase I diet for 8 months exhibited decreased tissue thiamine (as measured in red blood cells, white muscle, and liver), pronounced changes in body morphology and pigmentation, and decreased swimming performance (Houde et al. 2015). Although the experimental thiaminase I diet did not impact survival over the course of the study, the traits that were affected by the thiaminase I diet have previously been shown to impact Atlantic salmon survival. Thiamine deficiency can also impact the ability of migrating salmonines to ascend cascades (Ketola et al. 2005) and decrease the number of attempts an individual fish will make to traverse challenging river reaches (Harbicht et al. 2018).
Thiamine deficiency in non-salmonine species
It may be particularly difficult to forecast demographic effects of reduced tissue thiamine concentrations for non-salmonine species that have not been frequent subjects of thiamine deficiency research, such as anguillids. An assessment of the Lake Ontario-upper St. Lawrence River population of American eels indicated that for some individuals, muscle thiamine concentrations approached thresholds associated with TDC-induced behaviors in Japanese eels (Hashimoto et al. 1970; Fitzsimons et al. 2013). However, thresholds may differ interspecifically for anguillids, as they do for salmonines, making direct comparisons between American and Japanese eels challenging. Resolving thresholds for American eels might be particularly important given that adult eels cease feeding and assimilating nutrients prior to migration (Pankhurst and Sorensen 1984) as do many salmonines (Quinn 2005). Migratory individuals must therefore rely on endogenous energy stores throughout most of migration and spawning—two metabolically demanding activities—and evidence suggests that swimming endurance is reduced in thiamine deficient American yellow eels (Balk et al. 2016). The quantity of thiamine required for a migrating individual to successfully reproduce is unknown, but determination of species specific thresholds could help predict overall spawning rates for populations affected by thiamine deficiency.
Conservation and management of fishes in TDC affected systems
Evolutionary rescue
In some systems, the introduction of a prey species known to cause TDC in consumers may threaten native populations or interfere with native species reintroduction efforts (Fisher et al. 1996; Marsden and Hauser 2009; Riley et al. 2011; but see Marsden et al. 2018). In these scenarios, it could be helpful to ascertain whether populations could evolve to tolerate low dietary thiamine availability (i.e., prey with thiaminase I activity or with low thiamine:lipid ratios). Susceptibility to TDC is known to vary across salmonine species, with adult Chinook salmon remaining asymptomatic at lower muscle thiamine concentrations (Honeyfield et al. 2008) than coho salmon, lake trout, and steelhead trout (Brown et al. 2005c). Egg thiamine thresholds for fry thiamine deficiency are also lower in Chinook than in coho salmon and lake trout (Fitzsimons et al. 2007), further suggesting that some species may be genetically adapted to require less thiamine than other species. Recent empirical evidence from a comparison of three Atlantic salmon strains fed diets high in thiaminase I suggests that some locally adapted salmon populations may be able to tolerate lower thiamine concentrations than populations with less exposure to thiaminase I-containing prey (Houde et al. 2015). Two landlocked strains that typically consume thiaminase I-containing prey in their native habitats experienced smaller reductions in tissue thiamine than an anadromous strain with a more diverse diet lacking thiaminase I (Houde et al. 2015). This result indicates that adaptation to low available thiamine may be possible at the population level.
One mechanism by which populations could adapt to low thiamine conditions is by responding to selection on genes associated with thiamine-dependent biochemical pathways. Sequence variation in the genes encoding the four TDP-dependent enzymes necessary for carbohydrate and branched-chain amino acid metabolism (Fig. 1) (Depeint et al. 2006) could result in enzymes with differing binding affinities for TDP. This variation could be subject to selection if altered cofactor affinities facilitate more efficient exploitation of limited thiamine resources. For example, genetic mutations in one thiamine-dependent enzyme complex (branched-chain α-ketoacid dehydrogenase, BCKDH; Fig. 1) results in maple syrup urine disease in humans, with symptoms including ataxia, neurologic impairments, and blindness (Ames et al. 2002). A missense mutation in the gene encoding one subunit of the BCKDH complex decreases the complex’s affinity for TDP and, in turn, increases cellular requirement for TDP and thiamine (Chuang et al. 1982; Fisher et al. 1991; Ames et al. 2002). Variation in protein coding sequences (exons) of genes encoding TDP-dependent enzymes could therefore correspond to variation in thiamine requirements among individuals, populations, or species.
Non-protein coding (intronic) variation in thiamine-related genes could also contribute to differing patterns of thiamine use among individuals. For example, specific point mutations in an intron of the gene encoding thiamine pyrophosphokinase (TPK)—the enzyme that converts thiamine to TDP (Jurgenson et al. 2009)—are associated with variation in human birth weight (Fradin and Bougneres 2007). This relationship may be due to the ability of intron sequences to affect gene expression (Nott et al. 2003). Variation in TPK intron sequence could lead to varying patterns of TPK expression, altered availability of TDP, and metabolic shifts related to TDP abundance. Thiamine transporters, which are responsible for cellular and mitochondrial thiamine uptake (Bettendorff 2013), could also be subject to selection. In humans, the SLC23A1 gene encodes an active transporter protein that facilitates the absorption of L-ascorbic acid (vitamin C, an essential vitamin) in the intestine and kidneys. A single missense mutation in one SLC23A1 exon is associated with decreased blood concentrations of vitamin C. This effect is hypothesized to result from a conformational change in the transporter protein that impairs uptake of vitamin C (Timpson et al. 2010). The efficacy of thiamine transporters could be similarly affected by mutations in corresponding genes.
Genes associated with gut microbiome composition could also play a role in host thiamine intake requirements. Host genotypes that promote the presence of beneficial microbes are plausible targets of positive selection if those microbes perform tasks (e.g., vitamin synthesis) that increase host fitness (Goodrich et al. 2016). If fishes exploit thiamine produced by the gut microbiome (Ji et al. 1998), fish genotypes associated with increased abundance of thiamine-producing microbial taxa could reasonably be subject to positive selection in ecosystems where thiamine uptake via piscivory is limited by the presence of thiaminase I-containing prey. The majority of microbiome thiamine production occurs in the lower intestine in fishes (Ji et al. 1998), but the upper intestine is the primary site of thiamine absorption (Bettendorff 2013). Although epithelial cells in the human lower intestine are known to express thiamine transporter proteins and are capable of absorbing thiamine (Said et al. 2001; Nabokina and Said 2012), the degree to which fishes can absorb thiamine at the site of production in the lower intestine is unknown. Coprophagy may also provide fishes with access to thiamine produced in the lower intestine, and has been observed in Atlantic salmon smolts (Nylund et al. 1994), but the prevalence of this behavior among salmonine species and developmental stages is also unknown. Nevertheless, research dedicated to identifying associations between host genotype, diet, and microbiome composition (e.g., Goodrich et al. 2014; Carmody et al. 2015) could open promising avenues of research for investigating thiamine-related genetic adaptations in fishes.
In scenarios of evolutionary rescue, genetic adaptation allows for the recovery of a declining population that would have otherwise been extirpated due to environmental factors (Carlson et al. 2014). If individuals that can tolerate low available thiamine successfully reproduce at rates higher than those that cannot tolerate low thiamine, this process could lead to an increase in the proportion of individuals adapted to low available thiamine. The strength of the response to selection would depend on the degree to which low thiamine tolerance has a heritable, genetic basis. If selection on thiamine requirement is possible, management efforts for an affected population should prioritize conservation of adaptive genetic variation in that population. In natural populations, this could be achieved by maximizing effective population sizes (Waples 1990), limiting additional sources of mortality (e.g., fishing pressure) and maximizing spawning and rearing opportunities (e.g., increasing or improving spawning habitat). Facilitating genetic adaptation to low available thiamine could be achieved in hatchery-dependent populations by modifying existing egg thiamine treatment regimes. For example, withholding supplemental thiamine from a portion of each family of eggs would allow for differential survival among untreated individuals with varying tolerances to low thiamine conditions. Over several generations, this approach could result in an increase in the proportion of the population capable of tolerating low thiamine availability. However, adaptation to low thiamine may not be possible for all populations and species and this procedure would likely result in an unintentional response to selection for other differences between the hatchery and wild environments (Christie et al. 2014). Thus, in most cases, it will be important to first identify the proximate cause of thiamine deficiency in the population.
Diagnosis and mitigation of TDC in fish populations
In some systems, identifying the root cause of TDC may be straightforward. In Lake Champlain, for example, TDC was not observed in lake trout or landlocked Atlantic salmon until after the introduction and establishment of alewife (Ladago et al. 2016), a species that had already been associated with occurrences of TDC in lake trout and Atlantic salmon in the Great Lakes. In other cases, the identification of TDC may not be as clear cut. The signs of severe thiamine deficiency described for salmonines seem to be consistent among other fishes but may also be initially interpreted as evidence of a novel pathogen or contaminant in a previously unaffected system. In less severe cases, sublethal effects of TDC may be influencing demographic trends in the population, but could easily remain undetected or be misattributed, at least in part, to any co-occurring biotic or abiotic change (e.g., eutrophication, temperature shifts, changes in prey species demographics).
Once TDC has been identified as the most likely cause of observed signs or population decline, establishing the proximate cause(s) of the deficiency can guide management approaches. For example, in systems where establishment of an introduced species has led to TDC emergence, as with alewife in the Great Lakes and Lake Champlain, suppression of the exotic species would be expected to relieve the deficiency. Managing for diverse forage fish communities that include fishes with no or low thiaminase I activity may help to displace thiaminase I-containing prey from the affected species’ diet, but as in the Great Lakes, such decisions are often complex and occur in the context of a wide range of economic and social issues (Dettmers et al. 2012).
Research questions and needs
The ability to detect TDC in aquatic organisms as early as possible would give managers and researchers greater capacity to identify and mitigate the proximate cause. However, detection of mild to moderate TDC is a challenging task, as “normal” tissue thiamine concentrations for fishes remain undefined (but see Table 1 for examples of tissue thiamine concentrations in wild caught fishes without apparent TDC). Thiamine deficiency research in fish culture environments has allowed for the determination of thresholds for detrimental effects of TDC at the population level using thiamine levels in eggs but has not produced analytical approaches for determining where an individual falls on a continuum from thiamine replete to thiamine deficient. Other research needs relevant to management include identification of environmental variables affecting thiaminase I production in forage species and descriptions of thiamine deficient fry in natural environments (Table 2).
Several other research questions related to the molecular mechanisms and ecology of TDC are outlined in Fig. 3 and Table 2. For some topics, little to no research has been conducted in fishes. For example, changes in TDP-dependent enzyme activities following induction and reversal of thiamine deficiency have been documented in brown rats (Rattus norvegicus) (Gibson et al. 1984; Butterworth and Héroux 1989); similar experiments in fishes could help understand the mechanisms underlying sublethal effects in deficient fry and adults. Additional research needs, such as an efficient method of assessing thiamine concentrations in open water, are critical for understanding how rates of thiamine production impact nutritional status of species in higher trophic levels. Although not an exhaustive list, we present these questions as an outline for understanding ecological processes responsible for the development of TDC.
Summary of the processes and trophic levels involved in the ecology of thiamine deficiency. Processes are indicated by yellow arrows. Examples of how and why these processes may be impacted in an ecosystem where piscivorous fishes are thiamine deficient are indicated in red text. Numbers (1–8) correspond to table section headings in Table 2
Despite extensive observational and experimental evidence of low thiamine in eggs of feral salmonines and TDC in fry derived from feral adults, the linkage of TDC to population-level effects in the wild is primarily supported by correlations between abundance of thiaminase I-containing prey, egg thiamine data, and recruitment of wild salmonines (e.g., Fitzsimons et al. 2010; Riley et al. 2011; He et al. 2012). For example, lake trout egg thiamine concentrations in Lake Huron have been negatively correlated with alewife abundance, and increasing thiamine concentrations (Riley et al. 2011) and natural lake trout recruitment (Riley et al. 2007) were observed following the 2002-2004 collapse of the Lake Huron alewife population. After 2004, Lake Huron lake trout diets were no longer alewife-dominated, and this shift—rather than reduced alewife predation on lake trout fry—seems to be responsible for the increase in lake trout recruitment (Fitzsimons et al. 2010). However, testing causal relationships between ecological variables and rates of TDC in the field is more challenging because causal relationships cannot be evaluated by collecting correlational data. Capture or in situ observation of larval fishes and fry in the wild is difficult and biased—the individuals most susceptible to TDC are least likely to be caught or observable due to early life stage mortality. Consequently, studies of thiamine-deficient populations are conducted on eggs and fry in culture, and results are often extrapolated to wild environments (e.g., Fisher et al. 1996). Influence of TDC on mortality of wild fish is likely to be a result of a complex interaction of factors, including availability of thiamine-rich food, fat content of diet, density of predators, and availability of refuge (Czesny et al. 2009; Fitzsimons et al. 2009; Ladago et al. 2016). Continued research is needed to define the effect of TDC in wild populations and to determine whether TDC-related mortality is sufficient to affect recruitment and abundance of yearling and older year classes.
Conclusion
A recent horizon scan (a method designed to identify emerging issues in conservation) included thiamine deficiency in wildlife populations as a potential threat to multiple taxonomic groups (Sutherland et al. 2018), indicating a need for increased research and awareness. Although extensive data support thiaminase I consumption as a causative factor for TDC in fishes, research is still needed to identify and describe other ecological and environmental factors that may influence the manifestation of TDC. Although viable treatment options are available for mitigating existing thiamine deficiency in hatcheries, investigating why thiamine deficiency occurs in natural systems will allow development of predictive and preventative management strategies in situ. Increasing awareness of the potential causes of thiamine deficiency and of available treatment and management options represents a first step toward diminishing demographic losses in systems in which TDC occurs.
References
Amcoff P, Börjeson H, Lindeberg J, Norrgren L (1998) Thiamine concentrations in feral Baltic salmon exhibiting the M74 syndrome. In: McDonald DG, Fitzsimons JD, Honeyfield DC (eds) Early life stage mortality syndrome in fishes of the Great Lakes and Baltic Sea. American Fisheries Society, Bethesda, pp 82–89
Amend DF, Pietsch JP (1972) Virucidal activity of two iodophors to salmonid viruses. J Fish Res Board Can 29:61–65
Ames BN, Elson-Schwab I, Silver EA (2002) High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased K m): relevance to genetic disease and polymorphisms. Am J Clin Nutr 75:616–658
Balk L, Hägerroth P-Å, Åkerman G et al (2009) Wild birds of declining European species are dying from a thiamine deficiency syndrome. Proc Natl Acad Sci 106:12001–12006
Balk L, Hägerroth P-Å, Gustavsson H et al (2016) Widespread episodic thiamine deficiency in Northern Hemisphere wildlife. Sci Rep 6:38821
Bengtsson B-E, Hill C, Bergman Å et al (1999) Reproductive disturbances in Baltic fish: a synopsis of the FiRe project. Ambio 28:2–8
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Bettendorff L (2013) Thiamine. In: Zempleni J, Suttie JW, Gregory JF III, Stover PJ (eds) Handbook of vitamins, 5th edn. CRC Press, Boca Raton, pp 267–324
Bettendorff L, Hennuy B, Wins P, Schoffeniels E (1993) Thiamin and derivatives as modulators of rat brain chloride channels. Neuroscience 52:1009–1017
Bettendorff L, Lakaye B, Kohn G, Wins P (2014) Thiamine triphosphate: a ubiquitous molecule in search of a physiological role. Metab Brain Dis 29:1069–1082
Blakeslee CJ, Sweet SA, Galbraith HS, Honeyfield DC (2015) Thiaminase activity in native freshwater mussels. J Gt Lakes Res 41:516–519
Boś M, Kozik A (2000) Some molecular and enzymatic properties of a homogeneous preparation of thiaminase I purified from carp liver. J Protein Chem 19:75–84
Breves G, Hoeller H, Harmeyer J, Martens H (1980) Thiamin balance in the gastrointestinal tract of sheep. J Anim Sci 51:1177–1181
Breves G, Brandt M, Hoeller H, Rohr K (1981) Flow of thiamin to the duodenum in dairy cows fed different rations. J Agric Sci 96:587–591
Brown SB, Honeyfield DC, Vandenbyllaardt L (1998) Thiamine analysis in fish tissues. In: McDonald G, Fitzsimons J, Honeyfield DC (eds) Early life stage mortality syndrome in fishes of the Great Lakes and the Baltic Sea, vol 21. American Fisheries Society, Bethesda, Maryland, pp 73–81
Brown SB, Arts MT, Brown LR et al (2005a) Can diet-dependent factors help explain fish-to-fish variation in thiamine-dependent early mortality syndrome? J Aquat Anim Health 17:36–47
Brown SB, Fitzsimons JD, Honeyfield DC, Tillitt DE (2005b) Implications of thiamine deficiency in Great Lakes salmonines. J Aquat Anim Health 17:113–124
Brown SB, Honeyfield DC, Hnath JG et al (2005c) Thiamine status in adult salmonines in the Great Lakes. J Aquat Anim Health 17:59–64
Butterworth RF, Héroux M (1989) Effect of pyrithiamine treatment and subsequent thiamine rehabilitation on regional cerebral amino acids and thiamine-dependent enzymes. J Neurochem 52:1079–1084
Bylund G, Lerche O (1995) Thiamine therapy of M74 affected fry of Atlantic salmon Salmo salar. Bull Eur Assoc Fish Pathol 15:93–97
Carlson SM, Cunningham CJ, Westley PAH (2014) Evolutionary rescue in a changing world. Trends Ecol Evol 29:521–530
Carmody RN, Gerber GK, Luevano JM et al (2015) Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 17:72–84
Carvalho PSM, Tillitt DE, Zajicek JL et al (2009) Thiamine deficiency effects on the vision and foraging ability of lake trout fry. J Aquat Anim Health 21:315–325
Casteels M, Sniekers M, Fraccascia P et al (2007) The role of 2-hydroxyacyl-CoA lyase, a thiamin pyrophosphate-dependent enzyme, in the peroxisomal metabolism of 3-methyl- branched fatty acids and 2-hydroxy straight-chain fatty acids. Biochem Soc Trans 35:876–880
Christie MR, Ford MJ, Blouin MS (2014) On the reproductive success of early-generation hatchery fish in the wild. Evol Appl 7:883–896
Chuang DT, Ku LS, Cox RP (1982) Thiamin-responsive maple-syrup-urine disease: decreased affinity of the mutant branched-chain alpha-keto acid dehydrogenase for alpha-ketoisovalerate and thiamin pyrophosphate. Proc Natl Acad Sci 79:3300–3304
Cooper JR, Pincus JH (1979) The role of thiamine in nervous tissue. Neurochem Res 4:223–239
Czesny S, Dettmers JM, Rinchard J, Dabrowski K (2009) Linking egg thiamine and fatty acid concentrations of Lake Michigan lake trout with early life stage mortality. J Aquat Anim Health 21:262–271
Depeint F, Bruce WR, Shangari N et al (2006) Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact 163:94–112
Dettmers JM, Goddard CI, Smith KD (2012) Management of alewife using Pacific salmon in the Great Lakes: whether to manage for economics or the ecosystem? Fisheries 37:495–501
Fisher CW, Lau KS, Fisher CR et al (1991) A 17-bp insertion and a Phe215 → Cys missense mutation in the dihydrolipoyl transacylase (E2) mRNA from a thiamine-responsive maple syrup urine disease patient WG-34. Biochem Biophys Res Commun 174:804–809
Fisher JP, Spitsbergen JM, Iamonte T et al (1995) Pathological and behavioral manifestations of the “Cayuga syndrome”, a thiamine deficiency in larval landlocked Atlantic salmon. J Aquat Anim Health 7:269–283
Fisher JP, Fitzsimons JD, Combs GF, Spitsbergen JM (1996) Naturally occurring thiamine deficiency causing reproductive failure in Finger Lakes Atlantic salmon and Great Lakes trout. Trans Am Fish Soc 125:167–178
Fisher JP, Brown SB, Wooster GW, Bowser PR (1998) Maternal blood, egg and larval thiamin levels correlate with larval survival in landlocked Atlantic salmon (Salmo salar). J Nutr 128:2456–2466
Fitzsimons JD (1995) The effect of B-vitamins on a swim-up syndrome in Lake Ontario lake trout. J Gt Lakes Res 21:286–289
Fitzsimons J, Brown SB (1998) Reduced egg thiamine levels in inland and Great Lakes lake trout and their relationship with diet. In: McDonald G, Fitzsimons JD, Honeyfield DC (eds) Early life stage mortality syndrome in fishes of the Great Lakes and Baltic Sea. American Fisheries Society, Bethesda, pp 160–171
Fitzsimons JD, Huestis S, Williston B (1995) Occurrence of a swim-up syndrome in Lake Ontario lake trout in relation to contaminants and cultural practices. J Gt Lakes Res 21:277–285
Fitzsimons JD, Ketola G, Wooster GW, Brown SB (2001a) Use of a thiamine antagonist to induce Cayuga-Syndrome-like mortalities in larval Atlantic salmon. J Aquat Anim Health 13:151–157
Fitzsimons JD, Vandenbyllaardt L, Brown SB (2001b) The use of thiamine and thiamine antagonists to investigate the etiology of early mortality syndrome in lake trout (Salvelinus namaycush). Aquat Toxicol 52:229–239
Fitzsimons JD, Williston B, Amcoff P et al (2005a) The effect of thiamine injection on upstream migration, survival, and thiamine status of putative thiamine-deficient coho salmon. J Aquat Anim Health 17:48–58
Fitzsimons JD, Williston B, Zajicek JL et al (2005b) Thiamine content and thiaminase activity of ten freshwater stocks and one marine stock of alewives. J Aquat Anim Health 17:26–35
Fitzsimons JD, Williston B, Williston G et al (2007) Egg thiamine status of Lake Ontario salmonines 1995–2004 with emphasis on lake trout. J Gt Lakes Res 33:93–103
Fitzsimons JD, Brown SB, Williston B et al (2009) Influence of thiamine deficiency on lake trout larval growth, foraging, and predator avoidance. J Aquat Anim Health 21:302–314
Fitzsimons JD, Brown S, Brown L et al (2010) Increase in lake trout reproduction in Lake Huron following the collapse of alewife: relief from thiamine deficiency or larval predation? Aquat Ecosyst Health Manag 13:73–84
Fitzsimons JD, Wolgamood M, Madenjian CP, Bunnell DB (2012) Thiamine deficiency in aquatic food chains: the cumulative result of ecosystem disruption by clupeids? In: Norrgren L, Levengood J (eds) Ecology and animal health. Baltic University Press, Uppsala, pp 167–180
Fitzsimons JD, Brown SB, Brown LR et al (2013) Impacts of diet on thiamine status of Lake Ontario American eels. Trans Am Fish Soc 142:1358–1369
Fox GA, Allan LJ, Weseloh DV, Mineau P (1990) The diet of herring gulls during the nesting period in Canadian waters of the Great Lakes. Can J Zool 68:1075–1085
Fradin D, Bougneres P (2007) Three common intronic variants in the maternal and fetal thiamine pyrophosphokinase gene (TPK1) are associated with birth weight. Ann Hum Genet 71:578–585
Futia MH, Hallenbeck S, Noyes AD et al (2017) Thiamine deficiency and the effectiveness of thiamine treatments through broodstock injections and egg immersion on Lake Ontario steelhead trout. J Gt Lakes Res 43:352–358
Ghiasi S, Falahatkar B, Arslan M, Dabrowski K (2017) Physiological changes and reproductive performance of Sterlet sturgeon Acipenser ruthenus injected with thiamine. Anim Reprod Sci 178:23–30
Gibson GE, Zhang H (2002) Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem Int 40:493–504
Gibson GE, Ksiezak-Reding H, Sheu K-FR et al (1984) Correlation of enzymatic, metabolic, and behavioral deficits in thiamin deficiency and its reversal. Neurochem Res 9:803–814
Goodrich JK, Waters JL, Poole AC et al (2014) Human genetics shape the gut microbiome. Cell 159:789–799
Goodrich JK, Davenport ER, Waters JL et al (2016) Cross-species comparisons of host genetic associations with the microbiome. Science 352:532–535
Green RG, Evans CA (1940) A deficiency disease of foxes. Science 92:154–155
Hansson S (2001) Stomach analyses of Baltic salmon from 1959–1962 and 1994–1997: possible relations between diet and yolk-sac-fry mortality (M74). J Fish Biol 58:1730–1745
Happel A, Pattridge R, Walsh M, Rinchard J (2017) Assessing diet compositions of Lake Ontario predators using fatty acid profiles of prey fishes. J Gt Lakes Res 43:838–845
Happel A, Jonas JL, McKenna PR et al (2018) Spatial variability of lake trout diets in Lakes Huron and Michigan revealed by stomach content and fatty acid profiles. Can J Fish Aquat Sci 75:95–105
Harbicht AB, Castro-Santos T, Gorsky D et al (2018) Environmental, anthropogenic, and dietary influences on fine scale movement patterns of Atlantic salmon through challenging waters. Can J Fish Aquat Sci. https://doi.org/10.1139/cjfas-2017-0476
Harper CG, Giles M, Finlay-Jones R (1986) Clinical signs in the Wernicke–Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry 49:341–345
Hashimoto Y, Arai S, Nose T (1970) Thiamine deficiency symptoms experimentally induced in the eel. Bull Jpn Soc Sci Fish 36:791–797
Hatheway CL (1990) Toxigenic clostridia. Clin Microbiol Rev 3(1):66–98
He JX, Ebener MP, Riley SC et al (2012) Lake trout status in the main basin of Lake Huron, 1973–2010. North Am J Fish Manag 32:402–412
Honeyfield DC, Hinterkopf JP, Brown SB (2002) Isolation of thiaminase-positive bacteria from alewife. Trans Am Fish Soc 131:171–175
Honeyfield DC, Hinterkopf JP, Fitzsimons JD et al (2005) Development of thiamine deficiencies and early mortality syndrome in lake trout by feeding experimental and feral fish diets containing thiaminase. J Aquat Anim Health 17:4–12
Honeyfield DC, Vandergoot CS, Bettoli PW et al (2007) Thiamine and fatty acid content of walleye tissue from three southern U.S. reservoirs. J Aquat Anim Health 19:84–93
Honeyfield D, Peters A, Jones M (2008) Thiamine and fatty acid content of Lake Michigan Chinook salmon. J Gt Lakes Res 34:581–589
Honeyfield DC, Daniels ME, Brown LR et al (2012) Survey of four essential nutrients and thiaminase activity in five Lake Ontario prey fish species. J Gt Lakes Res 38:11–17
Honeyfield D, Murphy J, Howard K et al (2016) An exploratory assessment of thiamine status in western Alaska Chinook salmon (Oncorhynchus tshawytscha). North Pac Anadromous Fish Comm Bull 6:21–31
Hornung MW, Miller L, Peterson RE et al (1998) Efficacy of thiamine, astaxanthin, β-carotene, and thyroxine treatments in reducing early mortality syndrome in Lake Michigan salmonid embryos. In: McDonald G, Fitzsimons JD, Honeyfield DC (eds) Early life stage mortality syndrome in fishes of the Great Lakes and Baltic Sea. American Fisheries Society, Bethesda, pp 124–134
Houde ALS, Saez PJ, Wilson CC et al (2015) Effects of feeding high dietary thiaminase to sub-adult Atlantic salmon from three populations. J Gt Lakes Res 41:898–906
Jacobs GR, Madenjian CP, Bunnell DB et al (2013) Chinook salmon foraging patterns in a changing Lake Michigan. Trans Am Fish Soc 142:362–372
Jenkins AH, Schyns G, Potot S et al (2007) A new thiamin salvage pathway. Nat Chem Biol 3:492–497
Ji YQ, Adelman IR (1998) Thiaminase activity in alewives and smelt in Lakes Huron, Michigan, and Superior. In: McDonald G, Fitzsimons JD, Honeyfield DC (eds) Early life stage mortality syndrome in fishes of the Great Lakes and Baltic Sea. American Fisheries Society, Bethesda, pp 154–159
Ji YQ, Warthesen JJ, Adelman IR (1998) Thiamine nutrition, synthesis, and retention in relation to lake trout reproduction in the Great Lakes. In: McDonald G, Fitzsimons JD, Honeyfield DC (eds) Early life stage mortality syndrome in fishes of the Great Lakes and Baltic Sea. American Fisheries Society, Bethesda, pp 99–111
Jude DJ, Tesar FJ, Deboe SF, Miller TJ (1987) Diet and selection of major prey species by Lake Michigan salmonines, 1973–1982. Trans Am Fish Soc 116:677–691
Jurgenson CT, Begley TP, Ealick SE (2009) The structural and biochemical foundations of thiamin biosynthesis. Annu Rev Biochem 78:569–603
Karlsson L, Ikonen E, Mitans A, Hansson S (1999) The diet of salmon (Salmo salar) in the Baltic Sea and connections with the M74 syndrome. Ambio 28:37–42
Keinänen M, Uddström A, Mikkonen J et al (2012) The thiamine deficiency syndrome M74, a reproductive disorder of Atlantic salmon (Salmo salar) feeding in the Baltic Sea, is related to the fat and thiamine content of prey fish. ICES J Mar Sci 69:516–528
Ketola HG, Bowser PR, Wooster GA et al (2000) Effects of thiamine on reproduction of Atlantic salmon and a new hypothesis for their extirpation in Lake Ontario. Trans Am Fish Soc 129:607–612
Ketola HG, Chiotti TL, Rathman RS et al (2005) Thiamine status of Cayuge Lake rainbow trout and its influence on spawning migration. N Am J Fish Manag 25:1281–1287
King-Heiden TC, Mehta V, Xiong KM et al (2012) Reproductive and developmental toxicity of dioxin in fish. Mol Cell Endocrinol 354:121–138
Kitamura T, Seki N, Kihara A (2017) Phytosphingosine degradation pathway includes fatty acid α-oxidation reactions in the endoplasmic reticulum. Proc Natl Acad Sci 114:E2616–E2623
Koski P, Pakarinen M, Nakari T et al (1999) Treatment with thiamine hydrochloride and astaxanthine for the prevention of yolk-sac mortality in Baltic salmon fry (M74 syndrome). Dis Aquat Organ 37:209–220
Kraft CE, Angert ER (2017) Competition for vitamin B1 (thiamin) structures numerous ecological interactions. Q Rev Biol 92:151–168
Kraft CE, Gordon ER, Angert ER (2014) A rapid method for assaying thiaminase I activity in diverse biological samples. PLoS ONE 9:e92688
Kuno Y (1951) Bacillus thiaminolyticus, a new thiamin-decomposing bacterium. Proc Jpn Acad 27:362–365
Ladago BJ, Marsden JE, Evans AN (2016) Early feeding by lake trout fry. Trans Am Fish Soc 145:1–6
Lee B-J, Jaroszewska M, Dabrowski K et al (2009) Effects of vitamin B1 (thiamine) deficiency in lake trout alevins and preventive treatments. J Aquat Anim Health 21:290–301
Lepak JM, Kraft CE, Honeyfield DC, Brown SB (2008) Evaluating the effect of stressors on thiaminase activity in alewife. J Aquat Anim Health 20:63–71
Lim C, Yildirim-Aksoy M, Barros MM, Klesius P (2011) Thiamin requirement of Nile tilapia, Oreochromis niloticus. J World Aquac Soc 42:824–833
Lukienko PI, Mel’nichenko NG, Zverinskii IV, Zabrodskaya SV (2000) Antioxidant properties of thiamine. Bull Exp Biol Med 130:874–876
Lundström J, Börjeson H, Norrgren L (1999a) Histopathological studies of yolk-sac fry of Baltic salmon (Salmo salar) with the M74 syndrome. Ambio 28:16–23
Lundström J, Carney B, Amcoff P et al (1999b) Antioxidative systems, detoxifying enzymes and thiamine levels in Baltic salmon (Salmo salar) that develop M74. Ambio 28:24–29
Mac MJ, Edsall CC, Seelye JG (1985) Survival of lake trout eggs and fry reared in water from the upper Great Lakes. J Gt Lakes Res 11:520–529
Mac MJ, Schwartz TR, Edsall CC, Frank AM (1993) Polychlorinated biphenyls in Great Lakes lake trout and their eggs: relations to survival and congener composition 1979–1988. J Gt Lakes Res 19:752–765
MacCallum WR, Regier HA (1970) Distribution of smelt, Osmerus mordax, and the smelt fishery in Lake Erie in the early 1960’s. J Fish Board Can 27:1823–1846
Madenjian CP, Fahnenstiel GL, Johengen TH et al (2002) Dynamics of the Lake Michigan food web, 1970–2000. Can J Fish Aquat Sci 59:736–753
Marcquenski S, Brown S (1997) Early mortality syndrome (EMS) in salmonid fishes from the Great Lakes. In: Rolland RM, Gilbertson M, Peterson RE (eds) Chemically induced alterations in functional development and reproduction of fishes. SETAC Press, Pensacola, pp 135–152
Marsden JE, Hauser M (2009) Exotic species in Lake Champlain. J Gt Lakes Res 35:250–265
Marsden JE, Kozel CL, Chipman BD (2018) Recruitment of lake trout in Lake Champlain. J Gt Lakes Res 44:166–173
Miller RR (1957) Origin and dispersal of the alewife, Alosa pseudoharengus, and the gizzard shad, Dorosoma cepedianum, in the Great Lakes. Trans Am Fish Soc 86:97–111
Miller TJ, Crowder LB, Rice JA, Marschall EA (1988) Larval size and recruitment mechanisms in fishes: toward a conceptual framework. Can J Fish Aquat Sci 45:1657–1670
Mimouni-Bloch A, Goldberg-Stern H, Strausberg R et al (2014) Thiamine deficiency in infancy: long-term follow-up. Pediatr Neurol 51:311–316
Monteverde DR, Gómez-Consarnau L, Cutter L et al (2015) Vitamin B1 in marine sediments: pore water concentration gradient drives benthic flux with potential biological implications. Front Microbiol 6:434
Nabokina SM, Said HM (2012) A high-affinity and specific carrier-mediated mechanism for uptake of thiamine pyrophosphate by human colonic epithelial cells. AJP Gastrointest Liver Physiol 303:G389–G395
Nishimune T, Watanabe Y, Okazaki H, Akai H (2000) Thiamin is decomposed due to Anaphe spp. entomophagy in seasonal ataxia patients in Nigeria. J Nutr 130:1625–1628
Nott A, Meislin SH, Moore MJ (2003) A quantitative analysis of intron effects on mammalian gene expression. RNA 9:607–617
Nylund A, Hovland T, Hodneland K et al (1994) Mechanisms for transmission of infectious salmon anaemia (ISA). Dis Aquat Organ 19:95–100
Ottinger CA, Honeyfield DC, Densmore CL, Iwanowicz LR (2012) Impact of thiamine deficiency on T-cell dependent and T-cell independent antibody production in lake trout. J Aquat Anim Health 24:258–273
Palace VP, Brown SB, Baron CL et al (1998) An evaluation of the relationships among oxidative stress, antioxidant vitamins and early mortality syndrome (EMS) of lake trout (Salvelinus namaycush) from Lake Ontario. Aquat Toxicol 43:195–208
Pankhurst NW, Sorensen PW (1984) Degeneration of the alimentary tract in sexually maturing European Anguilla anguilla (L.) and American eels Anguilla rostrata (LeSueur). Can J Zool 62:1143–1149
Park LCH, Zhang H, Sheu K-FR et al (1999) Metabolic impairment induces oxidative stress, compromises inflammatory responses, and inactivates a key mitochondrial enzyme in microglia. J Neurochem 72:1948–1958
Pettersson A, Lignell Å (1999) Astaxanthin deficiency in eggs and fry of Baltic salmon (Salmo salar) with the M74 syndrome. Ambio 28:43–47
Quinn TP (2005) The behavior and ecology of Pacific salmon and trout, 1st edn. University of Washington Press, Seattle
Ray BA, Hrabik TR, Ebener MP et al (2007) Diet and prey selection by Lake Superior lake trout during spring, 1986–2001. J Gt Lakes Res 33:104–113
Richter CA, Evans AN, Wright-Osment MK et al (2012) Paenibacillus thiaminolyticus is not the cause of thiamine deficiency impeding lake trout (Salvelinus namaycush) recruitment in the Great Lakes. Can J Fish Aquat Sci 69:1056–1064
Riley SC, Evans AN (2008) Phylogenetic and ecological characteristics associated with thiaminase activity in Laurentian Great Lakes fishes. Trans Am Fish Soc 137:147–157
Riley SC, He JX, Johnson JE et al (2007) Evidence of widespread natural reproduction by lake trout Salvelinus namaycush in the Michigan waters of Lake Huron. J Gt Lakes Res 33:917–921
Riley SC, Rinchard J, Honeyfield DC et al (2011) Increasing thiamine concentrations in lake trout eggs from Lakes Huron and Michigan coincide with low alewife abundance. N Am J Fish Manag 31:1052–1064
Roseman EF, Schaeffer JS, Bright E, Fielder DG (2014) Angler-caught piscivore diets reflect fish community changes in Lake Huron. Trans Am Fish Soc 143:1419–1433
Said HM, Ortiz A, Subramanian VS et al (2001) Mechanism of thiamine uptake by human colonocytes: studies with cultured colonic epithelial cell line NCM460. AJP Gastrointest Liver Physiol 281:G144–G150
Sannino DR, Dobson AJ, Edwards K et al (2018a) The Drosophila melanogaster gut microbiota provisions thiamine to its host. mBio 9:e00155-18
Sannino DR, Kraft CE, Edwards KA et al (2018b) Thiaminase I provides a growth advantage by salvaging precursors from environmental thiamin and its analogs in Burkholderia thailandensis. Appl Environ Microbiol. https://doi.org/10.1128/AEM.01268-18)
Sechi G, Serra A (2007) Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol 6:442–455
Skea JC, Symula J, Miccoli J (1985) Separating starvation losses from other early feeding fry mortality in steelhead trout Salmo gairdneri, Chinook salmon Oncorhynchus tshawytscha, and lake trout Salvelinus namaycush. Bull Environ Contam Toxicol 35:82–91
Stewart DJ, Kitchell JF, Crowder LB (1981) Forage fishes and their salmonid predators in Lake Michigan. Trans Am Fish Soc 110:751–763
Sutherland WJ, Butchart SHM, Connor B et al (2018) A 2018 horizon scan of emerging issues for global conservation and biological diversity. Trends Ecol Evol 33:47–58
Swedberg DV, Peck JW (1984) Food of young-of-the-year lake trout (Salvelinus namaycush) in Presque Isle Harbor, Lake Superior. J Gt Lakes Res 10:280–285
Sylvander P, Häubner N, Snoeijs P (2013) The thiamine content of phytoplankton cells is affected by abiotic stress and growth rate. Microb Ecol 65:566–577
Tillitt DE, Zajicek JL, Brown SB et al (2005) Thiamine and thiaminase status in forage fish of salmonines from Lake Michigan. J Aquat Anim Health 17:13–25
Tillitt DE, Cook PS, Giesy JP et al (2008) Reproductive impairment of Great Lakes lake trout by dioxin-like chemicals. In: Di Giulio RT, Hinton DE (eds) The toxicology of fishes. CRC Press, Boca Raton, FL, pp 819–876
Tillitt DE, Riley SC, Evans AN et al (2009) Dreissenid mussels from the Great Lakes contain elevated thiaminase activity. J Gt Lakes Res 35:309–312
Timpson NJ, Forouhi NG, Brion M-J et al (2010) Genetic variation at the SLC23A1 locus is associated with circulating concentrations of l-ascorbic acid (vitamin C): evidence from 5 independent studies with > 15,000 participants. Am J Clin Nutr 92:375–382
U.S. Department of Agriculture, Agricultural Research Service. 2018. USDA National Nutrient Database for Standar Reference, Legacy. Version Current: April 2018. Nutrient Data Laboratory Home Page. http://www.ars.usda.gov/nutrientdata. Accessed 25 July 2018
Waples RS (1990) Conservation genetics of Pacific salmon. II. Effective population size and the rate of loss of genetic variability. J Hered 81:267–276
Werner RM, Rook B, Greil R (2006) Egg-thiamine status and occurrence of early mortality syndrome (EMS) in Atlantic salmon from the St. Marys River, Michigan. J Gt Lakes Res 32:293–305
Weseloh DV, Ewins PJ, Struger J et al (1995) Double-crested cormorants of the Great Lakes: changes in population size, breeding distribution and reproductive output between 1913 and 1991. Colon Waterbirds 18:48
Whitfield KC, Bourassa MW, Adamolekun B et al (2018) Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs: thiamine deficiency-intervention roadmap. Ann N Y Acad Sci. https://doi.org/10.1111/nyas.13919
Widmann M, Radloff R, Pleiss J (2010) The Thiamine diphosphate dependent Enzyme Engineering Database: a tool for the systematic analysis of sequence and structure relations. BMC Biochem 11:9
Wistbacka S, Bylund G (2008) Thiaminase activity of Baltic salmon prey species: a comparision of net- and predator-caught samples. J Fish Biol 72:787–802
Wistbacka S, Heinonen A, Bylund G (2002) Thiaminase activity of gastrointestinal contents of salmon and herring from the Baltic Sea. J Fish Biol 60:1031–1042
Wolf LE (1942) Fish-diet disease of trout: a vitamin deficiency produced by diets containing raw fish. New York State Conservation Department, Bureau of Fish Culture
Wolgamood M, Hnath JG, Brown SB et al (2005) Temporal and spatial variation of early mortality syndrome in salmonids from Lakes Michigan and Huron. J Aquat Anim Health 17:65–76
Wooster GA, Bowser PR, Brown SB, Fisher JP (2000) Remediation of Cayuga Syndrome in landlocked Atlantic salmon Salmo salar using egg and sac-fry bath treatments of thiamine-hydrochloride. J World Aquac Soc 31:149–157
Zajicek JL, Tillitt DE, Honeyfield DC et al (2005) A method for measuring total thiaminase activity in fish tissues. J Aquat Anim Health 17:82–94
Funding
Avril Harder and Mark Christie were supported by funding from the Biological Sciences and Forestry and Natural Resources departments at Purdue University. Donald Tillitt and Cathy Richter were supported by the U.S. Geological Survey Ecosystems Mission Area.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Disclaimer Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The findings and conclusions in the article are those of the authors and do not necessarily represent the views of the USFWS.
Rights and permissions
About this article
Cite this article
Harder, A.M., Ardren, W.R., Evans, A.N. et al. Thiamine deficiency in fishes: causes, consequences, and potential solutions. Rev Fish Biol Fisheries 28, 865–886 (2018). https://doi.org/10.1007/s11160-018-9538-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-018-9538-x