Abstract
Early approaches to surgical implantation of electronic tags in fish were often through trial and error, however, in recent years there has been an interest in using scientific research to identify techniques and procedures that improve the outcome of surgical procedures and determine the effects of tagging on individuals. Here we summarize the trends in 108 peer-reviewed electronic tagging effect studies focused on intracoleomic implantation to determine opportunities for future research. To date, almost all of the studies have been conducted in freshwater, typically in laboratory environments, and have focused on biotelemetry devices. The majority of studies have focused on salmonids, cyprinids, ictalurids and centrarchids, with a regional bias towards North America, Europe and Australia. Most studies have focused on determining whether there is a negative effect of tagging relative to control fish, with proportionally fewer that have contrasted different aspects of the surgical procedure (e.g., methods of sterilization, incision location, wound closure material) that could advance the discipline. Many of these studies included routine endpoints such as mortality, growth, healing and tag retention, with fewer addressing sublethal measures such as swimming ability, predator avoidance, physiological costs, or fitness. Continued research is needed to further elevate the practice of electronic tag implantation in fish in order to ensure that the data generated are relevant to untagged conspecifics (i.e., no long-term behavioural or physiological consequences) and the surgical procedure does not impair the health and welfare status of the tagged fish. To that end, we advocate for (1) rigorous controlled manipulations based on statistical designs that have adequate power, account for inter-individual variation, and include controls and shams, (2) studies that transcend the laboratory and the field with more studies in marine waters, (3) incorporation of knowledge and techniques emerging from the medical and veterinary disciplines, (4) addressing all components of the surgical event, (5) comparative studies that evaluate the same surgical techniques on multiple species and in different environments, (6) consideration of how biotic factors (e.g., sex, age, size) influence tagging outcomes, and (7) studies that cover a range of endpoints over ecologically relevant time periods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biotelemetry and biologging devices are increasingly being used to study the spatial ecology and survival of a variety of taxa in the wild (Lucas and Baras 2000; Cooke et al. 2004b; Gibbons and Andrews 2004; Block 2005; Ropert-Coudert and Wilson 2005; Nielsen et al. 2009). In fisheries sciences, these tools have become particularly important given the inherent challenges with determining basic information on the natural history of free-swimming fish (Lucas and Baras 2000). On a routine basis, fisheries scientists rely on biotelemetry and biologging devices to determine migration routes and the timing of migration (e.g., Stokesbury et al. 2007; Keefer et al. 2008), to determine natural and human-influenced mortality (e.g., bycatch [e.g., Donaldson et al. 2008], interaction with hydroelectric dams and turbines [e.g., Stier and Kynard 1986; Brown et al. 2006]), to evaluate habitat use and distribution of fish spatially and temporally (e.g., Lucas and Baras 2000), to understand energetic (e.g., Cooke et al. 2004a) and environmental relationships (e.g., Lucas and Baras 2000; Newell and Quinn 2005), and to study fish in aquaculture settings (e.g., Baras and Lagardère 1995). In fact, one could argue that the process of electronic tagging and the results of these efforts have and continues to revolutionize fisheries science, contributing to the understanding of natural history and biological phenomena (Lucas and Baras 2000), as well as for addressing applied management and conservation problems (Cooke 2008). Although there are a range of approaches for the attachment of electronic tags including gastric insertion, external mounting (using backpacks or darts), and intracoelomic surgical implantation, if feasible, intracoelomic implantation via laparotomy (i.e., incision through abdominal wall to access the coelom) is generally regarded as the approach that is most appropriate for long-term biotelemetry and biologging applications (Jepsen et al. 2002; Bridger and Booth 2003; Brown et al. 2009), depending the study’s objectives. Any surgical procedure, no matter how minor, has the potential to impair health, introduce infection, alter behaviour, cause physiological imbalances, and even lead to mortality, either directly via surgical error or indirectly via infection or post-release predation.
Over the past decade there have been numerous advances in the science supporting the practice of surgical implantation of biotelemetry and biologging devices in fish. The rationale for such studies was initially related to the need to ensure that data collected from fish implanted with devices was representative of the larger population of untagged conspecifics (Bridger and Booth 2003). Did tagged fish exhibit altered behaviour? Did tagged fish experience tagging-related mortality? More recently, the growing interest in fish welfare (Mulcahy 2003a) and the need to maintain the welfare status of fish involved in research, including those implanted with electronic tags, has promoted even greater scrutiny of surgical procedures on a range of taxa (Wilson and McMahon 2006). From a research perspective one wants to ensure that the surgical procedures and techniques produce negligible impacts on the fish and that they recover from surgery in a timely manner such that their welfare status is maintained and that their behaviour and fate is similar to untagged conspecifics.
Historically, innovations in surgical techniques arose from observations associated with tagging (i.e., trial and error; Cooke and Wagner 2004). Beginning in the 1970s as more fish were being tagged, researchers began to contrast outcome (usually survival and wound healing) of tagged fish to controls and shams (surgical procedure where no tag(s) are left in coelom). The majority of the comparative studies involved contrasting different tagging techniques such as gastric, external and intracoelomic implantation. It was more recently that researchers began to actually compare different aspects of surgical implantation, such as wound closure techniques and materials. Paralleling the research underway by fisheries scientists, there was growing interest in aquatic animal medicine and the development of techniques for use in veterinary practice (Stoskopf 1993; Harms and Lewbart 2000; Mulcahy 2003b; Harms 2005; Lewbart and Harms this issue). Despite this growing body of work, most practicing surgeons that routinely implant electronic tags in fish acknowledge that there is both need and opportunity to further improve surgical procedures for successful tagging fish in the field (Cooke and Wagner 2004).
One could argue that the body of literature supporting the practice of surgical implantation of electronic tags is salmon-centric, with a particular focus on juvenile or hatchery fish in freshwater. Indeed, the innovations arising from this work are well-summarized in the literature (e.g., Mulcahy 2003b) and arguably represent the most comprehensive data on the topic of surgical implantation of electronic tags. This is in large part due to the availability of these fish for experimental work, but even more so, the fact that there are tens of thousands of salmon smolts tagged each year in the Pacific Northwest with acoustic and radio transmitters to estimate mortality and evaluate behaviour of outmigrating fish relative to hydropower facilities and operations (Adams et al. 1999). Having one group of fish for which one can effectively summarize all available data to help inform future tagging is certainly useful, however, is it reasonable to expect that what works for a salmon smolt in freshwater will be entirely relevant to a fish that lives in the Antarctic, a nutrient-rich river in the rainforest, or a reef-associated fish in the tropics?
The objective of this paper is to briefly summarize the entire body of peer reviewed literature related to the intracoelomic implantation of electronic tags in fish. We characterize the existing body of work, synthesize the topics addressed in these studies, and summarize the various endpoints and research methods used. Where appropriate, we also identify where research gaps still exist and provide a research agenda specific to different tag types, species, and environments. To achieve our objectives we conduct a quantitative literature review and use a need-gap analysis to identify the current state of surgical implantation of electronic tags in fish and the scientific gaps that exist and need to be addressed in order to advance the practice of surgical implantation of electronic tags into fish from a range of different families and environments.
Approach
On November 15, 2009 a Boolean search of Web of Science (ISI Inc.) was conducted using the search terms “surg*” and “tag*” or “transmit” or “archival” and “fish*” within the subcategories of Limnology, Zoology, Fisheries and Aquatic Sciences, and Veterinary Medicine. This search string generated a list of 189 records that were then examined individually to determine if they were appropriate for inclusion. The majority of the records manually expunged were field telemetry studies on fish using surgical implantation as the tagging method but provided no specific analysis of surgical techniques or procedures. To supplement the list and ensure that the search was comprehensive, a Cited Reference Search in Web of Science on November 15, 2009 was also used with a focus on Jepsen et al. (2002) and Bridger and Booth (2003), two of the most-cited review papers on surgical techniques. The reference list in each of those papers was also examined to further identify additional resources.
In the end, 108 papers were identified that were explicitly focused on evaluating the effects of different tagging procedures for intracoelomic implantation. Studies that evaluated aspects of surgical procedures, if the primary rationale for the study was not for intracoelomic implantation of electronic tags, were excluded. For example, Hurty et al. (2002) compared five different suture materials for closing incisions on koi, but did so in the context of gonadectomy and tumor removal, so it was excluded. Only papers that were externally peer reviewed and published in English (i.e., journal articles and edited books or symposia) were included. Review papers (e.g., Bridger and Booth 2003; Jepsen et al. 2002), solely technical papers (i.e., “how to”; Winter 1983; Harms 2005), or survey papers used to identify trends among fish surgeons (i.e., Cooke and Wagner 2004; Wagner and Cooke 2005) were all excluded. Papers that focused solely on anesthesia were intentionally avoided. While, anesthesia is a critical component of intracoelomic tag implantation, many of the studies on fish anesthesia were germane to a variety of fisheries procedures. Several of the papers that were included in the final list did vary anesthetic, but they also varied some other aspect of surgical procedures (e.g., suture type, tag size). In addition, given the varied legal requirements among jurisdictions, the type of anesthesia used in different studies tends to reflect regional norms. Readers are referred to Iwama and Ackerman (1994), Ross and Ross (1999), and Carter et al. (this issue) for detailed overviews of fish anesthesia, recognizing that the scientific community is still in search of the ideal anesthetic that is safe for fish, humans, and the environment (Schnick 2006).
All data extraction was conducted by a single individual using standardized pre-defined criteria. The reader extracted general characteristics of the study including: the year in which the study was published, the journal in which it was published, the country in which the study was conducted, the number of species studied, the taxa, the location of the study (i.e., laboratory, field), the type of electronic tag (i.e., radio, acoustic, PIT, archival, electromyogram), and the duration of the study period (in weeks). Studies were also evaluated to determine if the sole focus was on documenting if there was a “tagging effect” or if it was designed in a way such that the study could refine future surgery studies (e.g., there was a comparison of different treatments such as suture type or tag size). Other aspects of study design such as the use of shams or power analyses were also extracted. To evaluate trends in study objectives, each study was categorized with respect to the following topics, realizing that a single study could have multiple objectives; comparison with other tagging approaches (e.g., external, gastric), tag size (or manipulations in fish size to evaluate tagging size), biotic factors (e.g., sex, stage of maturation), environmental correlates (e.g., evaluations of water temperature, salinity, etc.), tag coatings, antenna configurations (only for radio tag studies), incision and wound closure, sterility and antibiotics, and surgeon characteristics. Finally, each paper was also examined for the endpoints that were studied and categorized into one or more of the following: mortality, incision healing, growth and feeding, behaviour, swimming performance, physiology, fitness, and tag retention. The category of “temporal aspects” was also included as an endpoint for studies that compared how long different surgical techniques took to complete.
General characteristics of tagging studies
Of the 108 surgical or tagging effects studies conducted since 1975, 73 have occurred between 2000 and 2009. Overall, there is a general trend of increased efforts devoted towards evaluating surgical and tagging effects through time, particularly after 1997 (Fig. 1). The most papers published in a single year (n = 18) was in 2009. However, the journal Marine and Freshwater Research published a special issue (Volume 60, Issue 4), on the development of tagging protocols for freshwater animals in 2009, which contributed 7 papers for that year (see Ebner 2009).
The 108 papers appeared in 30 different outlets, with the majority appearing in journals. Most papers to date (n = 80) have been published in five journals: North American Journal of Fisheries Management (n = 30), Transactions of the American Fisheries Society (n = 21), Journal of Fish Biology (n = 18), Marine and Freshwater Research (n = 7), and Hydrobiologia (n = 4).
The 108 studies were conducted in 16 different countries. The majority of studies were conducted in the United States (n = 56), Canada (n = 17), Belgium (n = 8), and Australia (n = 7). In addition, the majority (n = 96) of studies were conducted in the northern hemisphere which would limit the species studied. Only four studies were conducted in developing countries (i.e., Namibia [Okland et al. 2003], South Africa [Thorstad et al. 2009], Brazil [Schulz 2003] and Thailand [Mitamura et al. 2006]). The majority of telemetry studies conducted to date were undertaken in the developed world. There have been efforts, however, to build capacity for telemetry studies in developing countries (Baras et al. 2002), but little information has been published in the scientific literature. Given that the biology of fish species in developing countries tends to be poorly understood, it is important to conduct tagging validation studies to ensure that the tagging procedures are effective given that he techniques used may be more rudimentary and the species different than those in other regions. It is not unreasonable that most of the “core” principles of fish surgery could be refined and tested in developed countries, but those working in developing countries should be encouraged to conduct validation studies to further refine techniques. Moreover, given that most studies have occurred in the northern hemisphere, there is also need to include models from the southern hemisphere where the fish taxa are quite different.
Of the 108 studies, 87% (n = 94) were conducted in freshwater, while only 13% (n = 14) were conducted in saltwater. The majority of the studies (n = 73) were conducted in the laboratory. Fewer studies were conducted in the field (n = 26) and even fewer included both laboratory and field components (n = 10; e.g., Cooke et al. 2003; Stakenas et al. 2009). Studies that combine both laboratory and field components potentially can greatly benefit the science of surgical tag implantation, despite there being few examples in the literature. This is because laboratory studies enable controlled manipulations while field studies provide ecological realism. Moser et al. (2007) detected differences in the field that were not detected in the lab emphasizing the potential benefits of combining lab and field studies and the problem with relying solely on lab studies to set criteria for study design and applications in the field.
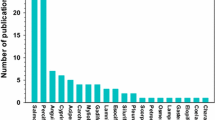
Most studied examined a single species (n = 96), while fewer studies examined 2 (n = 8), 3 (n = 1; O’Connor et al. 2009), 4 (n = 2; Stakenas et al. 2009), 5 (n = 1; Ross and Kleiner 1982), or 6 (n = 1; Starr et al. 2000) species at a time. Forty-seven studies focused on surgical implantation of salmonids (e.g., rainbow trout, Oncorhynchus mykiss [Lucas 1989; Brown et al. 1999]; Chinook salmon, Oncorhynchus tshawytscha [Adams et al. 1998a, b]; cutthroat trout, Oncorhynchus clarkii [Sanderson and Hubert 2007]; brown trout, Salmo trutta [Jepsen et al. 2008]; Atlantic salmon, Salmo salar [Robertson et al. 2003]; coho salmon, Oncorhynchus kisutch [Chittenden et al. 2009]; masu salmon, Oncorhynchus masu [Makiguchi and Ueda 2009]; sockeye salmon, Oncorhynchus nerka [Brown et al. 2006]). Cyprinids have been the focus on 14 studies (e.g., common carp, Cyprinus carpio [Okland et al. 2003; Bauer 2005]; nase, Chondrostoma nasus [Bauer et al. 2005]; northern pikeminnow, Ptychocheilus oregonensis [Tyus 1988]; silvery minnow, Hybognathus amarus [Archdeacon et al. 2009]), ictalurids (e.g. channel catfish, Ictalurus punctatus [Marty and Summerfelt 1986] giant catfish, Pangasianodon gigas [Mitamura et al. 2006]; African catfish, Clarias gariepinus [Baras and Westerloppe 1999]) and percids (e.g., pikeperch, Sander lucioperca [Jepsen 2003]; walleye, Sander vitreus [Ross and Kleiner 1982]) the focus of ten studies and centrarchids the focus of seven studies (largemouth bass, Micropterus salmoides [Cooke et al. 2003]; smallmouth bass, Micropterus dolomieu [Cooke and Bunt 2001]; bluegill, Lepomis macrochirus [Paukert et al. 2001]; pumpkinseed, Lepomis gibbosus [Stakenas et al. 2009]; black crappie, Pomoxis nigromaculatus [Petering and Johnson 1991]). Fewer studies have been conducted on esocids (n = 5; e.g., muskellunge, Esox masquinongy [Mangan 1998]; northern pike, Esox lucius [Jepsen and Aarestrup 1999]), moronids (n = 4; e.g., striped bass, Morone saxatilis [Mulford 1984]), and petromyzontids (n = 3; e.g., Pacific lamprey, Lampetra tridentata [Close et al. 2003]). There were an additional 26 families that were only included in two studies (e.g., Atlantic cod, Gadus morhua [Cote et al. 1999]) or a single study (e.g., bluefin trevally, Caranx melampygus [Meyer and Honebrink 2005]).
To date there is a clear pattern with respect to the types of electronic tags that are typically the focus of surgical studies. Only two of the studies have been conducted on archival loggers (i.e., Block et al. 1998; Campbell et al. 2005), whereas 106 studies have focused on a biotelemetry device including radio tags (n = 60), acoustic tags (n = 36), and PIT tags (n = 8). One study compared radio and PIT (Hockersmith et al. 2003); while another compared radio and acoustic transmitters (Stakenas et al. 2009). The reason for so few studies focused on archival loggers may simply be a function of the relative time of technological development, tag size, and initially the cost. Archival logger technology has advanced greatly in the last decade (Block 2005; Nielsen et al. 2009) whereas radio telemetry in particular was exceptionally common in the 1990 s and may in fact be waning in popularity (Cooke and Thorstad in press). Another factor which may influence researchers from using the loggers is that the logger must be returned for data download so they tend to be applied externally.
Trends in study design
Every study examined was at some level focused on evaluating the “effects” of the surgical procedure and/or the presence of the tag on the fish. However, only 56 of the 108 studies were designed in a way such that they could actually yield data that had the potential to improve future studies and inform the discipline. An alternate study design that has been rarely used is the before-after control treatment approach. Connors et al. (2002) used such an approach to evaluate changes in social dominance in Atlantic salmon after tagging. Use of appropriate shams and controls is necessary in such a study design. This approach recognizes the substantial level of inter-individual variability in factors such as swimming performance (Kolok 1999) and behaviour (Hanson et al. 2008b).
The majority of studies (n = 84) failed to include a sham control (i.e., fish for which surgery is performed but no tag is implanted), while the remainder (n = 25) included sham controls. Sham controls were used in studies to isolate the effects of surgical procedures from the presence of the transmitter (e.g., Brown et al. 1999; Fabrizio and Pessutti 2007; Daniel et al. 2009). Failure to include sham controls reduces the ability of experiments to resolve the factors that contribute to any negative effects on endpoints. Some studies adopted more of an adaptive approach where continual refinement led to the development of what are believed to be “best practices” even in the absence of controls or shams. For example, Block et al. (1998) developed the handling and surgical procedures necessary for intracoelomic implantation of archival tags in bluefin tuna up to 234 kg and Holland et al. (2006) describe the first successful surgical implantation of electronic tags in billfish. Obtaining controls and shams from animals that are not easy to maintain in captivity (such as marine pelagics) limits the ability to conduct experiments, although Block et al. (1998) describe ongoing studies that make use of captive tuna populations at aquaria where controls may be possible.
One of the key problems with many of the existing intracoelomic tagging studies is that they lack adequate statistical power (i.e., the potential for making a type II error whereby the researcher fails to detect a “true” effect; Peterman 1990). Use of a priori power analysis can help to dictate appropriate sample sizes for a study thus providing the researcher with the opportunity to address the issue of low power. A posteriori, power analysis will dictate the extent to which the data set has adequate power. Only a single study was located that indicated that they conducted an a priori power analysis (i.e., Anglea et al. 2004). However, several studies provided a posteriori power analysis (e.g., Brown et al. 1999; Cooke and Bunt 2001; Brown et al. 2006). Given that so many tagging effects studies have failed to document negative effects (see Bridger and Booth 2003 for overview), power analysis, even if only retrospective, seems to be particularly important. Indeed, Moser et al. (2007) mused that an alternative hypothesis to the fact that most tagging effects studies fail to document significant effects is because sample sizes are too small. Another notable experimental design aspect is the frequent use of pseudoreplication in tagging effect studies. Few experiments are truly factorial in design and most lack adequate replicates. Often times fish from all treatments are held in a single tank. Such analyses means that the relative differences among treatments are likely valid, but the actual differences may not be relevant to field settings due to potential problems with disease outbreak or other tank effects. Future studies would benefit greatly from more statistical rigor and better experimental design.
Of the studies that provided sufficient detail that clearly identified the duration of the project, the modal duration was 1 month (Fig. 2). Several studies that approached more than a year (e.g., Jepsen and Aarestrup 1999; Caputo et al. 2009) tended to be opportunistic where several animals were recaptured after being at liberty enabling the researcher to evaluate the incision and provide observational and/or anecdotal comments on healing. For example, Mangan (1998) recaptured an adult muskellunge that had been implanted with a telemetry transmitter 13 years prior. Tyus (1988) recaptured twelve northern pikeminnow, which had retained radio transmitters for 3 months to 8 years. Jepsen (2003) reported on the recapture of radio-tagged pikeperch in a reservoir that were captured by anglers 52–55 months after tagging. We advocate for more long-term studies that are experimental rather than purely opportunistic and anecdotal.
Trends in study objectives
Studies were characterized by objectives. Where appropriate, studies may have addressed a number of objectives. We summarize key papers associated with each of the objectives and provide a brief analysis.
Comparisons with other tagging techniques
The majority (n = 82) of the studies we examined did not compare surgical implantation to other tagging techniques. Of the 26 studies that did compare surgical implantation to other techniques, they tended to contrast either external (e.g., Bégout-Anras et al. 2003; Campbell et al. 2005; Mitamura et al. 2006) or gastric tagging (e.g., Jepsen et al. 2001; Hockersmith et al. 2003; Brown et al. 2009) techniques. In general, these studies concluded that internal surgical implantation was the best approach for long-term tagging and tended to have fewer negative consequences on the fish. Nonetheless, a creative study by Campbell et al. (2005) revealed that black cod affixed with external tags tended to have lower heart rates than fish with intracoelomic implants. It should be mentioned that the study suffered from low sample sizes and is inconsistent with the larger body of literature, which suggests that external tags are more energetically costly. We are not suggesting that external, gastric and even oviduct tagging approaches should not be considered. However, when the objective of a study is long-term and it is desirable to have minimal negative impacts on the fish, then intracoelomic implantation, if done properly, is usually preferable based on results of this and other studies.
Tag size
Of the 108 studies that were examined, 16 studies explicitly identified testing size (usually mass) of the electronic tag as an objective and systematically manipulated tag size to test that objective. Eleven studies varied fish size while holding tag size constant. One study combined those two questions in a single analysis (i.e., comparing tags of different size for fish among different size classes; see Zale et al. 2005). Few studies manipulated other aspects of tag size aside from mass (see Penne et al. 2007 for a study that varied tag volume).
Detailed analyses of tag size are addressed in detail elsewhere (Jepsen et al. 2005) so the focus here is on several important ideas. First off, tag size as reflected by the ratio of tag mass to fish mass in air is just one of the means by which one can determine tag size. The “2%” rule has become arbitrarily accepted within the fisheries science community after being proposed by Winter (1983). Several studies have challenged the 2% rule (e.g., Brown et al. 1999) not to suggest the intentional use of bigger tags, but to encourage the scientific community thinking that the “rule” needs to be flexible according to animal’s biology and the a study’s objectives. Relying solely on tag:fish mass relationships can be misleading. For example, the physical dimensions or volume of a tag may be more important for slender fish (e.g., anguilliform) where a long tag may interfere with swimming undulations (Moser et al. 2007) or in fish with small body cavities. Lacroix et al. (2004) used retention, growth and swimming performance studies to determine that the length of an electronic tag should not be greater than 16% of total body length for Atlantic salmon smolts. There is a growing body of literature that some species of fish do not exhibit significant mortality, tag loss or sublethal impacts when tagged with devices that approach as much as 8–12% of body mass (e.g., Brown et al. 1999; Lacroix et al. 2004). However, some studies have documented impairments in swimming and growth at less than 2% of tag mass to body mass (Zale et al. 2005). A general rule of thumb is that one should use the smallest tag (in both mass and volume) possible in order to reduce the chance of generating an outcome that is undesirable.
Biotic factors
The majority of studies (n = 77) were conducted on adults, while 30 studies were conducted on juveniles. One study failed to indicate the stage of the animals studied. Aside from fish size and life-stage (juvenile versus adult), very few studies have examined how intrinsic biotic factors influence surgical outcome. For example, despite the fact that male and female fish often vary with respect to their life-history characteristics, behaviour and physiology (Hanson et al. 2008a), sex or stage of sexual development (aside from juvenile versus adult; see above) is rarely used as a factor in analyses or explicitly contrasted. Baras and Westerloppe (1999) were one of the few to include sex and state of reproductive development in their analysis and found no significant effect of those factors. One author (i.e., Moore et al. 1990) studied the role of smoltification on surgical outcome using Atlantic salmon, another example of a biotic factor, and failed to find any impairment as a result of surgery and tagging or any differences between tagging effects on parr and smolts.
Another biotic factor studied has been the source of fish. Peake et al. (1997) studied hatchery and wild Atlantic salmon smolts and revealed that wild-tagged fish tested 1 or 16 h after internal or external attachment had lower swimming performance than that of wild controls, whereas no differences were noted among hatchery treatment groups. As such, the authors caution that the reaction of hatchery-reared fish to tagging can differ from that of wild fish.
Environmental correlates
Seven of the 108 studies explicitly studied environmental correlates of surgical success or systematically varied environmental conditions. The most commonly studied environmental variant was not surprisingly water temperature (e.g., Bunnell and Isely 1999; Knights and Lasee 1996; Walsh et al. 2000). Water temperature is regarded as the “controlling variable” in the biology of fish. From a surgical perspective, water temperature can influence not only other physical–chemical parameters, such as dissolved oxygen and pH, numerous attributes of the tagging procedure including the magnitude of the stress response from capture and handling, efficacy of anesthesia, recovery rate, incision healing rate, and suture performance. In general, the literature reveals that tagging fish at unusually warm water temperatures or temperatures that near their upper sub-lethal thermal tolerances should be avoided. It is worth noting that laboratory-determined thermal tolerances may differ from field conditions so extrapolations are problematic. Moreover, the term “warm” is subjective and temperature adaptation varies by species and even populations, reflecting different thermal tolerances. Even fish that are regarded as “hardy” such as common carp experience significant mortality when tagged at what are regarded as high water temperatures. Okland et al. (2003) tagged common carp in a reservoir in Namibia between 24° and 25°C and found 100% mortality for fish implanted intracoelomically, whereas all externally tagged fish survived.
Another factor that has been studied is the depth at which surgery is conducted. Starr et al. (2000) developed a method of tagging rockfish at depth to minimize barotrauma arising from bringing these deepwater dwelling fish to the surface. The researchers used self contained underwater breathing apparatus (SCUBA) to surgically implant acoustic transmitters in greenspotted rockfish (Sebastes chlorostictus) and bocaccio (S. paucispinis) in Monterey Bay, California. Fish were captured at depths of 100–200 m and reeled up to a depth of approximately 20 m where surgery was conducted. The researchers selected that depth to reduce temperature and pressure stress that would be caused by removing fish from the water. However, the authors failed to study the physiological consequences of this tagging protocol on fish. In an effort to understand the consequences of pressure change arising from turbine passage, researchers have also compared pressure change influences on surgical outcomes. Perry et al. (2001) studied Chinook salmon smolts in a laboratory using a hyperbaric chamber. The authors revealed that although fish compensated for the transmitters following implantation, changes in depth affected the buoyancy of tagged fish more than that of untagged fish. Reductions in buoyancy at depth may affect the behavior of tagged fish.
More recently, Brown et al. (2009) also studied Chinook salmon smolts using a similar approach, which involved exposing fish to simulated pressure changes associated with passage through a large Kaplan turbine. The authors reported that mortality and injury varied depending on whether a fish was carrying a transmitter, the method of transmitter implantation, the depth of acclimation, and the size of the fish, and nadir exposure. Juvenile Chinook salmon implanted with radio transmitters were more likely than those without to die or sustain injuries during simulated turbine passage. The results of that study are significant in that estimates of turbine passage survival for juvenile Chinook salmon obtained with radiotelemetry tags may be negatively biased.
Tag coatings
Only 4 of 108 studies evaluated the effects of different tag coating. Marty and Summerfelt (1986) studied retention of polystyrene tags in channel catfish that were either coated in silicone-rubber or paraffin wax. The authors determined that the kind of transmitter coating had no significant effect on expulsion, though reported expulsion rates were uniformly high (exceeding 50% expulsion overall within several weeks of tagging). Helm and Tyus (1992) studied the influence of tag coating on retention of tags by rainbow trout. The authors determined that tags with a beeswax coating were rarely expelled (3%). However, tags coated with paraffin wax (13%) and those with silicone coatings (40%) were commonly expelled. Helm and Tyus (1992) also identified gross tissue responses to the different coatings. Beeswax-coated transmitters usually were encapsulated, while other coatings were typically not encapsulated and were free in the body cavity. Sakaris and Jesien (2005) compared retention of tags coated with paraffin wax and Scotchcast (an inert epoxy resin) in brown bullheads (Ameiurus nebulosus) tagged with acoustic transmitters. All bullhead implanted with paraffin-coated implants retained their transmitters for the duration of the experiment (i.e., 75 days), whereas two fish with Scotchcast implants expelled their transmitters within 50 days. In a more recent study, Daniel et al. (2009) applied a polymer coating to acoustic tags and compared the retention and healing relative to non-coated tags (standard epoxy) in common carp. However, the authors failed to document a significant benefit of the coating as expulsion rates were 60% for non-coated tags and 50% for coated tags. Bacterial infection associated with the wound appeared to be the primary mechanism for expulsion independent of transmitter coating. It is worth noting that coatings that allow for sterilization may be beneficial and to our knowledge; this has not been explicitly studied in the context of implanting fish with electronic tags. The “standard” epoxy coating used in most electronic tags appears to be sufficient for most species. Nonetheless, for some species that are plagued with poor retention (e.g., ictalurids, common carp), there seems to be a need for more work on tag coatings. Paraffin wax coating appear to have some benefit relative to other coatings (e.g., Helm and Tyus 1992; Sakaris and Jesien 2005) but may be difficult to adequately disinfect paraffin coatings due to their low melting point and relative porosity (compared to epoxy).
Antenna configurations
Six of the 62 studies that were focused on radio telemetry (note—acoustic and PIT technology do not require trailing antennas) included an evaluation of issues specific to radio transmitter antennas. Murchie et al. (2004) systematically varied antenna length and compared the swimming performance of juvenile rainbow trout. Although the authors tested a variety of different antenna lengths up to a maximum of 300 mm, only the longest antenna significantly impaired swimming performance relative to control fish. In addition, when held in laboratory tanks, fish with the three longest antennas (150, 225 and 300 mm) frequently became entangled with the standpipe. That observation was consistent with other radio tagging studies (e.g., Adams et al. 1998a, b). We concur with Murchie et al. (2004) that antenna length is an important issue for small fish (relative to tag and antenna length). Additional research is needed to understand the effects of antenna length on fish survival, infection rate or pathogen loading, tag retention and behaviour, and to also identify the consequences of different tag lengths on signal reception range.
Four studies have compared the effects of having radio transmitter antennas exit the body cavity relative to having the antenna coiled within the body cavity. Cooke and Bunt (2001) found no differences in behaviour in the field or swimming performance in the lab between fish with radio antennas that either exited the body cavity or were coiled within. However, transmitter signals were attenuated with the internal antenna configuration. Collins et al. (2002) compared internally coiled antennas and externally trailing antennas in shortnose sturgeon (Acipenser brevirostrum). The internal coiled antennas yielded 100% survival and retention over the study period. Sturgeon with trailing antennas all survived 3 months but the antennas created severe cuts in the body wall as the transmitters migrated posterior. Eventually transmitters were lost through these wounds and this led to mortality of all fish in the trailing antenna treatment. Gosset and Rives (2004) conducted a similar study on brown trout and found that internal antenna coiling was more stable inside the abdomen with lower expulsion rates. Isely et al. (2002) compared the effects of two antenna placements (trailing and nontrailing) on mortality and transmitter loss in hybrid striped bass (Morone saxatilis × M. chrysops). The authors failed to detect an effect of antenna type on the time to first mortality, but cumulative mortality was higher in the trailing antenna groups (50%) than in the nontrailing antenna groups (12%). In addition, three transmitters were expelled during the study, all from trailing-antenna treatment groups, indicating a significant effect of antenna placement on the level of transmitter expulsion. Although just an anecdotal report, Connors et al. (2002) reported that antennas elicited attacks by conspecifics in Atlantic salmon smolts. Collectively, these studies suggest that when possible, antennas should be coiled internally, but this will depend on the necessary detection range, body size of the fish, social interactions, and the potential for entanglement.
Only one study was located as part of the structured search that has compared the exit site of the antenna. Walsh et al. (2000) compared the shielded needle technique (whereby the antenna exits through a separate antenna exit as per Ross and Kleiner 1982) and a more simple technique where the antenna simply trails out of the primary incision site used to insert the radio tag for hybrid striped bass (Morone saxitilis × Morone chrysops). Antenna placement did not affect transmitter loss, mortality, or growth. Although there is little science aside from this single paper to support antenna placement decisions, it is believed that creating a secondary exit site is less likely to yield expulsion and should facilitate healing of the primary incision site. Clearly additional work is needed on this topic.
It is worth noting that several other studies have reported anecdotal findings regarding antennas, although the experiments were not conducted in a manner to specifically test different antenna configurations. For example, Bauer et al. (2005) recaptured a single nase that was at liberty for 7 months with an external trailing antenna. The trailing antenna had migrated approx 3 cm to the posterior edge of the incision. Even when antennas exit from site typically posterior to the primary incision, fish that are held for extended periods or recaptured after being at liberty often show signs of redness and sometimes infection at the antenna exit site (e.g., Adams et al. 1998a, b). Indeed, the antenna can serve as a permanent source of irritation and the exit site could serve as an entry site for pathogens. Addition experimental work is needed to better optimize antenna placement and issues associated with trailing antennas. Some researchers have placed sutures on the antenna exit site, but this likely has little benefit and may lead to more opportunities for infection. The issue of antenna exit sites is also of relevance to any electronic tags that use external sensors such as light stalks often included on archival loggers (e.g., see Arnold and Dewer 2001).
Incision and wound closure
Making the incision and closing the wound are two fundamental components of intracoelomic implantation of electronic tags in fish. Six of the 108 studies compared elements of the incision. For example, Wagner and Stevens (2000) examined the effects of midline and off midline, but still on the ventral surface, incision locations on the behaviour of rainbow trout. When the incision was made on the midline, fish had reduced swimming activity relative to fish tagged off midline. Schramm and Black (1984) compared the radio tags implanted via midventral incisions compared to lateral incisions (on the side of the body wall) and determined that midventral incisions had less incidences of puncturing the ovaries of female fish and the operation was easier for the surgeon. Using brown trout as a model, Gosset and Rives (2004) compared incisions placed anterior and posterior to the pelvic girdle and reported no differences in survival, risk of expulsion, healing and situation of tags inside the abdomen.
Beyond the question of where to make the incision, several researchers have explored other aspects of the incision or implantation procedure. For example, Berejikian et al. (2007) explored the potential of using subdermal implantation of transmitters rather than complete laparotomy to surgically implant tags in salmonids with developed gonads. Bauer (2005) examined a question relevant to fish with scales on their ventral surface—should scale be removed to facilitate making the incision? Using common carp as a model, Bauer (2005) revealed that fish that had scales removed prior to surgery exhibited severe tissue necrosis at the incision site whereas fish with intact scales did not.
Thirteen of 108 studies compared different means of closing incisions, the majority of which focused on comparing different suture materials. For example, Wagner and Stevens (2000) evaluated the influence of monofilament and braided silk suture on the swimming behaviour of tagged rainbow trout and noted no differences. In a study of juvenile largemouth bass, there was no influence of suture type (i.e., absorbable monofilament versus braided silk) on fish survival (Cooke et al. 2003). Interestingly, however, braided silk sutures were easier to tie, reducing surgery time relative to monofilament. In general, however, there is a tendency for monofilament sutures to cause less inflammation than braided silk (see Wagner et al. this issue). Walsh et al. (2000) compared absorbable versus nonabsorbable monofilament for closing incisions in hybrid striped bass. Absorbable sutures were lost more quickly than were nonabsorbable sutures, but they persisted beyond incision closure. At temperatures between 22 and 29°C, 50% suture loss occurred by 30 days for absorbable sutures and by 60 days for nonabsorbable sutures.
Beyond comparing suture materials, other studies have evaluated alternative wound closure methods. For example, Petering and Johnson (1991) compared healing rates of incisions in black crappie closed with sutures or cyanoacrylate adhesive. Although use of adhesive reduced surgery time by 38%, sutured incisions healed faster. In addition, 70% of fish with incisions closed by the adhesive lost their transmitters whereas none were lost in sutured fish. Baras and Jeandrain (1998) evaluated different wound closure methods for eel (Anguilla anguilla). The incisions were either left open, or closed with sutures (either absorbable or non absorbable) or commercial-grade cyanoacrylate adhesive. Interestingly, no transmitter was expelled over a 12-week period, even in eels with unclosed incisions, of which 50% healed within 28 days. The cyanoacrylate suppressed the inflammatory response and yielded high survival rates. However, eels actively bit and removed the adhesive within hours, although that behaviour was suppressed when a freshly cut fragment of the eel dorsal fin was applied as a biological bandage over the drying cyanoacrylate, leading to the most rapid healing rate of all closure techniques. Other researchers have examined incision healing in fish where the incision is left open, typically for PIT tag implantation. Baras et al. (1999) determined that for nile tilapia (Oreochromis niloticus), suturing reduced the risks of PIT tag expulsion and protrusion of the viscera through the open incision within the first several days following surgery (10% risk in the non-sutured fish).
Staples are another technique used for wound closure. Swanberg et al. (1999) compared incision healing and long-term fish growth of rainbow trout where incisions were closed with either braided silk sutures or steel staples. Surgeries with staples were performed twice as quickly as suture surgeries and had less epidermal infection than sutured incisions. Fish with staples lost fewer tags and had less abdominal bloating, but there were no differences in growth between the two treatments. Sanderson and Hubert (2007) revealed that for cutthroat trout tagged while under CO2 anesthesia, survival rates were dramatically higher for individuals that had incisions closed by staples than by braided silk, presumably because of the speed at which staples can be applied. There were no differences in survival between incision closure methods when AQUI-S anesthetic was used. At present, there have been sufficiently few studies that it is difficult to make general conclusions about incision closure types aside from on salmonids (see Wagner et al. this issue). There is a need for additional work focused on comparing multiple incision closure methods on different species and in different environments, particularly saltwater and very warm or very cold freshwater.
Of the 108 studies, only one compared different suture styles (i.e., surgical knots). Wagner et al. (2000) used two separate experiments to investigate the effects of different suture patterns on wound healing in rainbow trout. The authors used absorbable and nonabsorbable monofilament and as well as braided silk sutures in simple interrupted and vertical mattress patterns to close 3 cm incisions. The authors revealed that vertical mattress suture patterns caused significantly more tissue inflammation. However, there were no differences in the histology or strength of the wounds related to the type of suture material or the type of suture pattern used. There is much room for additional research on suture patterns.
Sterility and antibiotics
The topics of sterility and use of antibiotics have been poorly addressed for fish surgery (see Mulcahy 2003b and Mulcahy this issue for details). Indeed, only one of the 108 studies that met our search criteria addressed sterilization. Wagner et al. (1999) evaluated the influence of preparing surgical incision sites of rainbow trout with antiseptic swabs. The providone-iodine solution was applied both pre- and post-surgery to the incision sites. There was no histological difference between control and treated incisions suggesting that the antiseptic did not improve wound healing or alter healing rate. In terms of antibiotics, we also found only a single study (out of 108) that addressed this issue experimentally. Isely et al. (2002) evaluated the effectiveness of antibiotic treatment (i.e., 0.5 mg/kg gentamicin sulfate injected intramuscularly) when tagging hybrid striped bass and revealed that it was effective in preventing initial postsurgical infection. Several other studies have made comments about the use of antibiotics (e.g., Archdeacon et al. 2009) but have failed to adequately test their use experimentally. Clearly there is need for additional research on different levels of sterility and use of antibiotics on fish for the intracoelomic implantation of electronic tags.
Surgeon characteristics
Given that the surgeon has the potential to induce injury and stress to the point where fish may die from surgical implantation of electronic tags, very few studies have explicitly considered the role of the surgeon expertise or training. Our analysis revealed only a single paper that experimentally manipulated the expertise of the surgeon. Cooke et al. (2003) compared an expert surgeon and a novice surgeon and their ability to implant telemetry tags into largemouth bass. The expert surgeon was faster and had greater precision with wound closure. In addition, the expert surgeon had lower mortality and better wound healing. Unfortunately this study only compared two individual surgeons so it is difficult to make too many generalizations based on the work. Hart and Summerfelt (1975) anecdotally noted that surgeon speed and ability seemed to improve over time. Another study that was not part of our formal analysis because it was published after our review (i.e., Deters et al. 2010), provided more direct insight into the role of the surgeon. Deters et al. (2010) found a significant effect of surgeon on suture retention, incision openness, and tag retention. The majority (62%) of surveyed fisheries researchers performing surgical implantation in telemetry projects felt effects of surgeon performance were large enough to include surgeon as a variable in analyses (Wagner and Cooke 2005). Although experience can be a predictor of surgical competence (Cooke et al. 2003), this study suggests surgical volume (number of surgeries performed) should not be the only measure of aptitude. Training for potential surgeons should include constructive feedback whenever possible. In addition, only the Cooke et al. (2003) study described above provided an explicit description of the training protocols used for surgeons. Training protocols and a call for greater reliance on training are reported elsewhere (see Cooke et al. this issue). There is much room for additional research on the role of surgeon experience, practice and training.
Endpoints
There are a number of different endpoints or other means of evaluating “success” or effects of surgical procedures. The majority of the endpoints used in studies are biological and focus on metrics such as survival, incision healing, growth, behaviour, physiology, swimming performance, and fitness. Other relevant metrics for evaluating surgical procedures include the duration of the surgery and whether or not the tags are retained. Here we provide an overview of the different endpoints or metrics that have been evaluated and the specific research techniques that are used.
Mortality
The ultimate endpoint is mortality and it was used in 102 of the 108 studies. Indeed, mortality was likely an endpoint in all studies, but those that failed to identify mortality as an endpoint in their objective or methods likely failed to document any mortality. Mortality was measured on a variety of time scales ranging from failure to revive from the surgical procedure (immediate mortality; e.g., Gries and Letcher 2002) through to days (Bateman and Gresswell 2006) or weeks (e.g., Cote et al. 1999; Walsh et al. 2000) after tagging. Some studies report cumulative mortality through time whereas others only “count” survivors at a single time period (e.g., when a pond is drained; Cooke et al. 2003). Similar to the overall trend towards most studies being conducted in the lab, most studies held fish in tanks (e.g., Thoreau and Baras 1997; Sakaris and Jesien 2005; Welch et al. 2007; Daniel et al. 2009). Studies in the field typically involved holding fish in experimental ponds/mesocosms (Anras et al. 2003; Cooke et al. 2003; Mitamura et al. 2006; Moser et al. 2007) or releasing fish with active and/or passive electronic tags and then comparing the survival of fish tagged (e.g., Hockersmith et al. 2003). Of course it is not possible to determine survival rates of non-tagged controls, so it is not possible to generate control mortality levels unless the experiments include replicate mesocosms/cages of control fish. Indeed, electronic tagging, and telemetry in particular, are regarded as effective means of quantifying post-release mortality rates for fish that are captured and released by anglers (Donaldson et al. 2008), so it is not surprising that these techniques are also relevant to evaluating effects of tagging. Nonetheless, challenges remain with obtaining control data or using sham controls.
Incision healing
Incision healing was a common metric (i.e., 79 of 108 studies) used in studies of intracoelomic implantation for obvious reasons. However, the exact means by which incision healing was evaluated varied extensively among studies. The default was to simply determine whether the incision was closed by a predetermined time or alternatively checking the incision sites routinely in order to identify when healing had occurred. Several studies had a far more involved approach to evaluating incision healing, including histological evaluations (e.g., Wagner et al. 2000; Bauer and Loupal 2007). Some of the most thorough and innovative studies on incision healing were by Wagner and Stevens (e.g., Wagner et al. 1999, 2000). In a study of suture material and patterns on wound healing in rainbow trout, Wagner et al. (2000) developed macroscopic and histological criteria, and ranking scales that can be used for evaluating healing. The authors also quantified the strength of the wound by mechanical testing with an Instron testing system where the tissue (from fish with and without incisions) was stretched at 10 cm/min until failure. Breaking strength (maximum force required to break apart the incision) and breaking energy (total amount of energy required to break apart the incision) were calculated providing a direct evaluation of wound healing strength. Although most incision healing studies have been conducted on the order of days to weeks, several recent studies have been longer term (e.g., Jepsen et al. 2008; Caputo et al. 2009).
Growth and feeding
Beyond mortality and failed healing, there are many sublethal impairments that could eventually lead to mortality or reduce organismal fitness. For example, growth is a commonly used metric to evaluate tagging procedures and effects (60 of 108 studies measured growth). Studies either measure fish size at time of tagging and again at a single time point to mark the end of an experiment (e.g., Martin et al. 1995; Cooke et al. 2003) or repeatedly measure individuals (of subsets of individuals) throughout the experimental period (e.g., Adams et al. 1998a; Lacroix et al. 2004). Although most growth studies are conducted in the lab where fish may not be forced to search or compete for food as they would in the wild, there are also some studies that have measured growth in the wild. For example, Jepsen et al. (2008) examined growth rates of brown trout in the wild that were recaptured after months of being at liberty. Jepsen and Aarestrup (1999) adopted a similar approach in a study of northern pike where they compared growth of dye-marked and surgically implanted fish. Caputo et al. (2009) compared the relative change in growth rate for wild largemouth bass tagged with different sized tags over a several year period and then recaptured by angling. Baras et al. (2000) reported that although tagged fish experienced an initial growth decline, they eventually caught up (i.e., compensatory growth) emphasizing the need for studies that evaluate performance across a range of time periods such that a study does not just provide a snapshot in time.
Related to growth, several studies have evaluated feeding activity, which can provide more specific information on mechanisms underlying growth differences. For example, Robertson et al. (2003) compared food consumption rates of wild Atlantic salmon. Interestingly, the food consumption rates were similar between tagged and control fish and did not explain differences in growth that were observed. As such, the growth impairments must be associated with differential activity levels or other metabolic costs. Another metric relevant to growth is condition factor (i.e., the relative plumpness of fish), which has been used in several studies (e.g., Martinelli et al. 1998). One study (i.e., Baras et al. 2000) used ability of perch to store body lipid as a metric of energetic condition.
Physiology
There are a number of relevant sublethal physiological metrics that can be used for evaluating surgical procedures and tagging effects ranging from biochemical indicators of stress to hematological indicators of disease and immune function. However, only 11 of the 108 studies we evaluated included physiological endpoints (e.g., Mesa et al. 2003; Caputo et al. 2009), and almost all of them have occurred in the last few years. Collection of blood samples was the most common means of obtaining physiological data. For example, Jepsen et al. (2001) examined the physiological response of Chinook salmon smolts to gastric and intracoelomic radio-tagging. Plasma levels of the stress hormone cortisol, and the metabolites glucose and lactate were measured before tagging and at 3 h, 24 h, 7, and 14 days after tagging. Compared to control fish, significant increases in all three physiological indicators occurred within 3 h of tagging. Even 24 h post-tagging cortisol levels were still elevated in both groups of tagged fish. However, levels of glucose and lactate had returned to control levels for the surgically implanted fish, but not for the gastrically tagged fish. By 7 days all groups were comparable to controls. The authors failed to document any relationships between body size and the magnitude of physiological response. Martinelli et al. (1998) measured stress indicators in the blood for Chinook salmon smolts and compared gastic and intracoelomic tagging with controls. Mean hematocrit values were significantly lower in the surgical and gastric groups as compared to controls at 5 days post surgery, but no differences were detected by day 21. No differences in leucocyte values were noted. Both tagged groups had significantly lower plasma protein levels relative to controls at day 5, but there were no differences between control and surgically implanted fish by day 21. Makiguchi and Ueda (2009) examined physiological response to external tagging and intracoelomic implantation (as well as controls) for juvenile masu salmon and failed to detect any differences in plasma cortisol, plasma glucose and haematocrit among groups.
The studies above involved holding fish in laboratory environments and then having to handle fish in order to collect blood samples. Given the potential to induce undesirable handling effects, Lower et al. (2005) developed a new method for obtaining data on physiological status (i.e., cortisol) of fish held in small tanks without having to directly sample the fish. Using non-invasive measurements of cortisol from the tank water, the authors were able to evaluate the effects of tagging procedures on common carp and roach that had been surgically implanted with tags. Fish responded to the surgical implantation with an immediate (between 1 and 4 h) increase in cortisol concentrations in the water. Compared to sham controls (handled only and handled and anesthetized), tagging elicited a larger cortisol response. After 4 h, cortisol levels for tagged fish were comparable to controls suggesting that there was not a long-term stress response to the presence of a tag in the body cavity in either of the species that were studied. Close et al. (2003) was also able to obtain some physiological information without handling fish. The authors included an assessment of ventilation rates in their study of surgical effects on Pacific lamprey. Ventilation rates of tagged and control lampreys did not differ at 1, 24, and 168 h after surgery, although ventilation rates are not regarded as being the most effective indicator of fish stress (see Barreto and Volpato 2004). The other study that has examined the physiological consequences of tagging without physically taking a blood sample from the fish was done using heart rate loggers (i.e., Campbell et al. 2005).
Several studies included immune function or disease metrics in their analyses. For example, Wagner et al. (1999) investigated the effects of preparing surgical incision sites with a topical antiseptic on hematological response in rainbow trout. The authors conducted erythrocyte counts, determined the percentage of dividing erythrocytes, and differential leucocyte counts. They also conducted postmortem, pathogenic bacterial assays on the kidney and spleen. Knights and Lasee (1996) studied bluegill that had been radio-tagged. Using microbiological cultures, they determined that tagged fish did not appear to be more susceptible than control fish to bacterial infection. In general, studies of immune function related to tagging were poorly represented in the literature.
Swimming performance
Swimming performance is a fairly common method for evaluating tagging impacts. For the purpose of this section we consider swimming performance to be forced swim trials (i.e., u-crits or bursting), excluding field studies that compare swimming activity. Of the 108 studies examined, 22 included measures of swimming performance. Swimming performance is regarded as being a sensitive metric of overall animal health given that it requires the integration of all major body systems (e.g., sensory, cardio-respiratory, locomotion) and is an ecologically relevant measure as locomotion is needed to locate food and avoid predators (Beamish 1978). Critical swimming speed, whereby fish are placed in a swimming flume and exposed to step-wise increased in velocity (Beamish 1978), was the most common means of evaluating fish swimming performance (e.g., Brown et al. 1999; Chittenden et al. 2009). For example, Anglea et al. (2004) measured critical swimming speeds of intracoelomically-tagged, sham-control, and control fish, and determined that performance was similar among treatment groups at 1- and 21-days post-surgery. Koed and Thorstad (2001) conducted a long-term swimming performance evaluation of pikeperch. Control fish and surgically implanted fish were released into the wild and then recaptured by electrofishing after 1 year at liberty. No differences in swimming performance were noted.
Mueller et al. (2006) was the only researcher to evaluate burst swimming ability and did so for Pacific lamprey. The authors failed to document any differences between tagged and untagged fish. Wagner and Stevens (2000) studied several aspects of swimming behaviour (i.e., the number of C-turns performed, the number of sprints performed, and the total distance travelled) in rainbow trout relative to different suture types and the location of the incision. There was no significant variation in the swimming performance metrics used, leading the authors to caution that the metrics used may not be sufficiently sensitive to detect effects. In general, swimming performance studies have been subject to significant criticism in recent years, particularly for generating absolute swimming speeds (Plaut 2001). However, swimming challenges are still regarded as useful for comparing relative differences among treatments and thus still have a place in evaluation of tagging effects and different surgical procedures.
Behaviour
Behavioural metrics were incorporated into 25 of the 108 studies. A number of studies released fish in the wild (e.g., with gastric versus surgical implantation) and compared aspects of fish movement. For example, Cooke and Bunt (2001) compared the field swimming activity of smallmouth bass tagged with internally coiled radio transmitter antennas and internal tags where the antenna exited the body and did not find any difference.
A number of behavioural studies have also been conducted in laboratory settings. For example, Thoreau and Baras (1997) used motion-sensitive transmitters in an aquaculture tank to evaluate the activity of four tilapias during the recovery from anaesthesia and surgical procedures. The authors reported that all four fish exhibited normal diurnal activity rhythm patterns (based on control fish) throughout the study. However, activity levels were low during the first 12–24 h post surgery. Jadot et al. (2005) used a computerized video tracking system to evaluate the behavior of salema (Sarpa salpa) tagged with tags representing 2 and 6% of the fish’s mass. They found that the larger tag resulted in altered behavior.
Predator avoidance studies have been used to evaluate consequences of implantation. Anglea et al. (2004) studied predator avoidance of juvenile Chinook salmon implanted with active acoustic tags to determine whether tagged fish were impaired by the surgical implantation of tags or predators were attracted to the signals from the acoustic tags. Surgical implantation of acoustic tags did not result in greater predation susceptibility in tagged fish than in untagged fish. Adams et al. (1998a) and Jepsen et al. (2008) also conducted predator prey studies. Jepsen et al. (2008) suggest that predator avoidance is one of the most relevant metrics of performance impairments and should be included in future tagging effects studies. It is worth noting that it is becoming increasingly difficult to secure permission from animal care and welfare bodies to conduct predator prey trials, which is unfortunate given the apparent sensitivity and ecological relevance of this metric.
Several studies have also evaluated the effects of tagging on various aspects of social behaviour. For example, Connors et al. (2002) examined the agonistic behaviour, dominance, distance to nearest neighbor, and distance from substrate in Atlantic salmon smolts before and after surgical implantation of radio tags. The authors determined that social ranking changed for some fish, but that no significant differences were found in any of the behavioural parameters that the researchers studied. Swanberg and Geist (1997) examined social interactions of rainbow trout to evaluate consequences of tagging. The authors revealed that dominant fish with dummy transmitters retained their rank and showed no significant differences from control fish in amounts of agonism and interaction time with subdominant fish. Several authors have also compared behaviour of fish tagged in different ways in the field by incorporating different tagging techniques into actual field movement studies (e.g., Hockersmith et al. 2003; Moser et al. 2007).
Reproductive fitness
Reproductive fitness metrics have rarely been used as endpoints in tagging effects studies (i.e., 4 of 108). Baras et al. (2000) studied perch and found no evidence that tags implanted into fish of varying size impacted gonadal development. Berejikian et al. (2007) conducted a study with the objective being to determine if subdermal tagging (i.e., surgery but not intracoelomic) would reduce egg retention, a phenomenon that the authors had anecdotally observed before for maturing female anadromous salmonids receiving intracoelomic tags. Using steelhead as a model, the authors revealed that internally tagged females retained more eggs than did the subdermally tagged and nontagged control groups. However, the onset of sexual activity did not differ significantly among treatments. In a study of Pacific lamprey, Close et al. (2003) did not observe any differences in development of secondary sexual characteristics or gonads between control and intracoelomic tagged individuals. Clearly there is much opportunity and need for more research on reproductive fitness consequences of tagging, particularly given that many telemetry studies focus on tagging mature animals prior to spawning periods.
Tag retention
Tag expulsion (or the inverse—retention) is often included as an endpoint in studies of tagging effects (i.e., 71 of 108 studies) given that an expelled tag indicates failure of the wound closure method or some form of active expulsion (e.g., transintestinal, transabdominal; see Summerfelt and Mosier 1984; Marty and Summerfelt 1986; Baras and Westerloppe 1999). Beyond tag expulsion potentially implying that the animal has an open wound (e.g., suture loss, failed healing or pressure necrosis; Walsh et al. 2000; Welch et al. 2007; Daniel et al. 2009), it also will affect the ability of a study to achieve its objective. For example, tag loss can lead to underestimates of movement or overestimates of mortality. Usually tag expulsion is evaluated by holding tagged fish in tanks (e.g., Zale et al. 2005; Welch et al. 2007; Broadhurst et al. 2009a, b; Daniel et al. 2009) or ponds/mesocosms (e.g., Cooke et al. 2003; Mitamura et al. 2006) and evaluating tag loss at either a single period or over time. If a single period, the researcher may euthanize the fish and perform an autopsy during which time it is possible to determine the presence/absence of the tag and its location (e.g., Knights and Lasee 1996). If the retention is being evaluated through time, it is necessary to determine if and when tags have been shed. If the fish has been tagged with a radio transmitter with an antenna that exits the body cavity, tag retention may be obvious. One can also simply look for expelled tags (which may be labeled) in the bottom of a tank although they may be consumed by fish. For PIT telemetry studies one can simply scan the fish with a hand-held PIT receiver to determine if the tag is still present while also identifying an individual or use a wand-type metal detector. It is also possible to embed PIT tags within “dummy” radio, acoustic, or archival tags as Welch et al. (2007) and Chittenden et al. (2009) have done for retention, growth and mortality studies on Pacific salmon smolts. It is also possible to use radiographs (i.e., x-rays) to determine if tags are still retained as well as their position within the body cavity which is relevant for PIT detections (e.g., Baras et al. 2000).
Temporal aspects
Beyond biological endpoints, several studies (12 of 108) have also evaluated various temporal aspects of surgical procedures as endpoints. For example, Cooke et al. (2003) compared the time required for surgeries to be completed by a novice versus experienced fish surgeon. Swanberg et al. (1999) revealed that use of staples was more rapid than suturing. At present it is unclear whether speed of conducting a procedure is indeed a good endpoint. Additional work is needed to better understand the consequences of different durations of anesthesia on biological endpoints to help determine the amount of effort that should be devoted to surgical speed.
Research agenda
Although we identified 108 peer reviewed studies on tagging effects, with another estimated 10–20 non-peer reviewed studies that likely exist but did not fit the criteria for our review, there are still many outstanding research questions. In order to further elevate the practice of electronic tag implantation in fish, we generated a table that provides a relative evaluation of our knowledge of various aspects of the tagging process for several key groups of fish including “juvenile salmonid fish”, “other freshwater fish”, “coastal marine fish”, and “marine pelagic fish” (Table 1). Although “juvenile salmonid fish” is very focused, nearly 1/3rd of existing studies have been conducted on that group and they are also the focus of a recent synthesis (i.e., Brown et al. 2010). From this somewhat subjective exercise, it is evident that even for the most studied group of fish (i.e., juvenile salmonids), there are still many unknowns with respect to tagging effects and advancing surgical procedures. For other inland fish there is much less known and for marine fish it could be argued that there is virtually nothing known about how they respond to tagging or how to optimize tagging procedures. There are a number of inherent challenges with tagging marine fish, and in particular marine pelagics (see Block et al. 1998). This exercise is not intended to be a criticism. Instead it is a candid reality check and starting point for moving forward to address knowledge deficiencies.
Further, we generated a research agenda that lists what we regard as key questions that we hope will be used by researchers in developing future studies (Table 2). There is some level of redundancy in the list as some topics were identified under more than one topical area. Nonetheless, if we are able to address these outstanding research questions, it is more likely that the data generated from fish tagged intracoelomically will be relevant to untagged conspecifics (i.e., no long-term behavioural or physiological consequences) and the surgical procedure will not impair the health and welfare status of the tagged fish. Just because there has been a single study on a topic, it is not prudent to assume that those findings will be consistent across all taxa, environments and situations (Ebner et al. 2009). We are not advocating “stamp collecting”, but it is clear that we have a rudimentary understanding of how different surgical procedures and tagging effects vary among species and environments. Similarly, the optimal tag size (both mass and dimensions) will certainly vary among species, particularly those with vastly different morphology and anatomy. Extrapolation of size effect results from one study to fish that are outside the size range studied may be problematic.
Recommendations for design of future studies
In addition to identifying specific research needs, this synthesis also revealed a number of systematic problems and limitations with existing studies. Based on those issues we generated a list of seven recommendations for future studies. If researchers incorporate some or all of these recommendations into the specific research questions identified above, the potential for the individual studies to achieve their objectives will be more likely to be realized and the collective body of research generated will be more likely to advance the science and practice of intracoelomic implantation of electronic tags. Our seven recommendations follow:
-
1.
rigorous controlled manipulations based on statistical designs that have adequate power, account for inter-individual variation, and include appropriate controls and shams
-
2.
studies that transcend the laboratory and the field with more studies in marine waters and on a diversity of taxa,
-
3.
incorporation of techniques, tools, and knowledge emerging from the medical and veterinary disciplines where there are frequent innovations in surgical procedures and materials,
-
4.
addressing all components of the surgical event including pre-operative handling and post-operative care,
-
5.
comparative studies that evaluate the same surgical techniques on multiple species and in different environments (e.g., salinities, temperature) that have the potential to influence recovery and healing,
-
6.
considering how biotic factors such as sex and stage of maturation influence tagging outcomes, and
-
7.
studies that cover a range of endpoints and are conducted over ecologically-relevant time periods.
Conclusions
Our review of peer reviewed research papers that have addressed tagging effects or attempted to improve surgical procedures for intracoelomic implantation of electronic tags revealed a number of interesting trends. For example, of the 108 studies that we located, almost all of the studies were conducted in freshwater. In addition, most studies have occurred in laboratory environments or other pseudo-field settings such as experimental ponds or mesocosms. Most studies have focused on biotelemetry devices (radio, acoustic and PIT technology), with very few studies examining tagging effects for archival loggers. The majority of studies have focused on salmonids, cyprinids, ictalurids and centrarchids, primarily freshwater or anadromous taxa. There was also a regional bias towards North America, Europe and Australia. In fact, almost all of the studies were conducted in developed countries. Most studies have focused on determining whether there is a negative effect of tagging relative to control fish, with proportionally fewer that have contrasted different aspects of the surgical procedure (e.g., methods of sterilization, incision location, wound closure material) that could advance the discipline. Most studies included routine endpoints such as mortality, growth, healing and tag retention, with fewer addressing sublethal measures such as swimming ability, predator avoidance, physiology, or fitness.
Intracoelomic implantation of electronic tags has many advantages over other tagging techniques for long-term applications. However, as with all tagging techniques, it is essential to understand the potential consequences of tagging procedures on fish. Beyond documenting problems, it is also critical to identify opportunities for improving surgical procedures. There are a number of questions that remain (see Table 2). Beyond summarizing current trends in order to synthesize information for immediate application, this exercise also played an important role in identifying needs and opportunities for research. We produced a detailed research agenda that we hope will be embraced by the scientific community. It is remarkable how little we really know about how to tag many groups of fish. Continued research is needed to further elevate the practice of electronic tag implantation in fish in order to ensure that the data generated are relevant to untagged conspecifics (i.e., no long-term behavioural or physiological consequences) and the surgical procedure does not impair the health and welfare status of the tagged fish. In reviewing the existing body of literature, we also identified a number of opportunities for improving future tagging effects and surgical refinement studies. We encourage the scientific community to adopt some of the general characteristics that have identified for future studies as these should improve the scientific rigor and potential to yield information that will truly advance the science of intracoelomic implantation. Failure to improve and advance the practice of surgical implantation of electronic tags and to better understand the consequences of different tagging procedures will reduce the reliability of data that are generated from electronic tagging studies and ultimately reduce the potential benefit and application of this powerful technology.
References
Adams NS, Rondorf DW, Evans SD, Kelly JE (1998a) Effects of surgically and gastrically implanted radio transmitters on growth and feeding behavior of juvenile Chinook salmon. Trans Am Fish Soc 127:128–136
Adams NS, Rondorf DW, Evans SD, Kelly JE, Perry RW (1998b) Effects of surgically and gastrically implanted radio transmitters on swimming performance and predator avoidance of juvenile Chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 55:781–787
Adams NS, Shively RS, Rondorf DW (1999) Advances in biotelemetry technology in the Columbia River Basin and how they are providing behavioral data used to shape fisheries management. In: Eiler JE, Alcorn DJ, Neuman MR (eds) Biotelemetry 15: proceedings of the 15th international symposium on biotelemetry. Juneau, Alaska, pp 259–268
Anglea SM, Geist DR, Brown RS, Deters KA, McDonald RD (2004) Effects of acoustic transmitters on swimming performance and predator avoidance of juvenile Chinook salmon. N Am J Fish Manage 24:162–170
Archdeacon TP, Remshardt WJ, Knecht TL (2009) Comparison of two methods for implanting passive integrated transponders in Rio Grande silvery minnow. N Am J Fish Manage 29:346–351
Arnold G, Dewer H (2001) Electronic tags in marine fisheries research: A 30-year perspective. In: Sibert J, Nielsen J (eds) Methods and technologies in fish biology and fisheries vol 1. Academic Press, Dordecht, pp 7–64
Baras E, Jeandrain D (1998) Evaluation of surgery procedures for tagging eel Anguilla anguilla (L.) with biotelemetry transmitters. Hydrobiologia 372:107–111
Baras E, Lagardère J-P (1995) Fish telemetry in aquaculture: review and perspectives. Aquac Int 3:77–102
Baras E, Westerloppe L (1999) Transintestinal expulsion of surgically implanted tags by African catfish Heterobranchus longifilis of variable size and age. Trans Am Fish Soc 128:737–740
Baras E, Westerloppe L, Mélard C, Philippart J-C, Bénech V (1999) Evaluation of implantation procedures for PIT-tagging juvenile Nile Tilapia. N Am J Aquac 61:246–251
Baras E, Malbrouck C, Houbart M, Kestemont P, Mélard C (2000) The effect of PIT tags on growth and some physiological factors of age-0 cultured Eurasian perch Perca fluviatilis of variable size. Aquaculture 185:159–173
Baras E, Benech V, Marmulla G (2002) Outcomes of a pilot fish telemetry workshop for developing countries. Hydrobiologia 483:9–11
Barreto RE, Volpato GL (2004) Caution for using ventilatory frequency as an indicator of stress in fish. Behav Processes 66:43–51
Bateman DS, Gresswell RE (2006) Survival and growth of age-0 steelhead after surgical implantation of 23-mm passive integrated transponders. N Am J Fish Manage 26:545–550
Bauer C (2005) Potential problems with removing scales before surgical transmitter implantation. J Fish Biol 66:847–850
Bauer C, Loupal G (2007) Common carp tissue reactions to surgically implanted radio tags with external antennas. J Fish Biol 70:292–297
Bauer C, Unfer G, Loupal G (2005) Potential problems with external trailing antennae: antenna migration and in growth of epithelial tissue, a case study from a recaptured Chondrostoma nasus. J Fish Biol 67:885–889
Beamish FWH (1978) Swimming capacity. In: Hoar WH, Randall DJ (eds) Fish physiology, vol VII. Academic Press, New York, pp 101–187
Bégout-Anras ML, Coves D, Dutto G, Laffargue P, Lagardere F (2003) Tagging juvenile seabass and sole with telemetry transmitters: medium-term effects on growth. ICES J Mar Sci 60:1328–1334
Berejikian BA, Brown RS, Tatara CP, Cooke SJ (2007) Effects of telemetry transmitter placement on egg retention of naturally spawning captively reared steelhead. N Am J Fish Manage 27:659–664
Block BA (2005) Physiological ecology in the 21st century: advancements in biologging science. Integr Comp Biol 45:305–320
Block BA, Dewar H, Williams T, Prince E, Farwell C, Fudge D (1998) Archival tagging of Atlantic bluefin tuna (Thunnus thynnus thynnus). Mar Tech Soc J 32:37–46
Bridger CJ, Booth RK (2003) The effects of biotelemetry transmitter presence and attachment procedures on fish physiology and behavior. Rev Fish Sci 11:13–34
Broadhurst BT, Ebner BC, Clear RC (2009a) Radio-tagging flexible-bodied fish: temporary confinement enhances radio-tag retention. Mar Freshw Res 60:356–360
Broadhurst BT, Ebner BC, Clear RC (2009b) Effects of radio-tagging on two-year-old, endangered Macquarie perch (Macquaria australasica: Percichthyidae). Mar Freshw Res 60:341–345
Brown RS, Cooke SJ, Anderson WG, McKinley RS (1999) Evidence to challenge the “2% rule for biotelemetry. N Am J Fish Manage 19:867–871
Brown RS, Geist DR, Mesa MM (2006) The use of electromyogram (EMG) telemetry to assess swimming activity and energy use of adult spring Chinook salmon migrating past a Columbia River dam. Trans Am Fish Soc 135:281–287
Brown RS, Carlson TJ, Welch AE, Stephenson JR, Abernethy CS, Ebberts BD, Langeslay MJ, Ahmann ML, Feil DH, Skalksi JR, Townsend RL (2009) Assessment of barotrauma from rapid decompression of depth-acclimated juvenile Chinook salmon bearing radiotelemetry transmitters. Trans Am Fish Soc 138:1285–1301
Brown RS, Cooke SJ, Wagner GN, Eppard MB (2010) Methods for surgical implantation of acoustic transmitters in juvenile salmonids: a review of literature and guidelines for techniques. U.S. Army Corps of Engineers, Portland, OR
Bunnell DB, Isely JJ (1999) Influence of temperature on mortality and retention of simulated transmitters in rainbow trout. N Am J Fish Manag 19:152–154
Campbell HA, Bishop C, Egginton S (2005) Recording long-term heart rate in the Black cod (P. angustata) using an electronic datalogging device. J Fish Biol 67:1150–1156
Caputo M, O’Connor C, Hasler CT, Hanson KC, Cooke SJ (2009) Do surgically implanted telemetry transmitters have long-term consequences on the nutritional physiology and condition of wild fish? Dis Aquatic Org 84:35–41
Chittenden CM, Butterworth KG, Cubitt KF, Jacobs MC, Ladouceur A, Welch DW, McKinley RS (2009) Maximum tag to body size ratios for an endangered coho salmon (O. kisutch) stock based on physiology and performance. Environ Biol Fishes 84:129–140
Close DA, Fitzpatrick MS, Lorion CM, Li HW, Schreck CB (2003) Effects of intraperitoneally implanted radio transmitters on the swimming performance and physiology of Pacific lamprey. N Am J Fish Manage 23:1184–1192
Collins MR, Cooke DW, Smith TIJ, Post WC, Russ DC, Walling DC (2002) Evaluation of four methods of transmitter attachment on shortnose sturgeon, Acipenser brevirostrum. J Appl Icthyol 18:491–494
Connors KB, Scruton D, Brown JA, McKinley RS (2002) The effects of surgically-implanted dummy radio transmitters on the behaviour of wild Atlantic salmon smolts. Hydrobiologia 483:231–237
Cooke SJ (2008) Biotelemetry and biologging in endangered species research and animal conservation: relevance to regional, national, and IUCN Red List threat assessments. Endanger Species Res 4:165–185
Cooke SJ, Bunt CM (2001) Assessment of internal and external antenna configurations of radio transmitters implanted in smallmouth bass. N Am J Fish Manage 21:236–241
Cooke SJ, Thorstad EB (in press) Is radio telemetry getting washed downstream? The changing role of radio telemetry in studies of freshwater ichthyofauna relative to other tagging and telemetry technology. Am Fish Soc Symp
Cooke SJ, Wagner GN (2004) Training, experience, and opinions of researchers who use surgical techniques to implant telemetry devices into fish. Fisheries 29(12):10–18
Cooke SJ, Graeb BDS, Suski CD, Ostrand KG (2003) Effects of suture material on incision healing, growth and survival of juvenile largemouth bass implanted with miniature radio transmitters: case study of a novice and experienced fish surgeon. J Fish Biol 62:1366–1380
Cooke SJ, Thorstad EB, Hinch SG (2004a) Activity and energetics of free-swimming fish: insights from electromyogram telemetry. Fish Fish 5:21–52
Cooke SJ, Hinch SG, Wikelski M, Andrews RD, Wolcott TG, Butler PJ (2004b) Biotelemetry: a mechanistic approach to ecology. Trends Ecol Evol 19:334–343
Cote D, Scruton DA, Cole LA, McKinley RS (1999) Swimming performance and growth rates of juvenile Atlantic Cod intraperitoneally implanted with dummy acoustic transmitters. N Am J Fish Manage 19:1137–1141
Daniel AJ, Hicks BJ, Ling N, David B (2009) Acoustic and radio transmitter retention in common carp (Cyprinus carpio L.) in New Zealand. Mar Freshw Res 60:328–333
Deters KA, Brown RS, Carter KM, Boyd JW, Eppard MB, Seaburg AG (2010) Performance assessment of suture type in juvenile chinook salmon surgically implanted with acoustic transmitters. Trans Am Fish Soc 139:888–889
Donaldson MR, Arlinghaus R, Hanson KC, Cooke SJ (2008) Enhancing catch-and-release science with biotelemetry. Fish Fish 9:79–105
Ebner BC (2009) Tagging for telemetry of freshwater fauna. Mar Freshw Res 60:281–283
Ebner BC, Lintermans M, Jekabsons M, Dunford M, Andrews W (2009) A cautionary tale: surrogates for radio-tagging practice do not always simulate the responses of closely related species. Mar Freshw Res 60:371–378
Fabrizio MC, Pessutti JP (2007) Long-term effects and recovery from surgical implantation of dummy transmitters in two marine fishes. J Exp Mar Biol Ecol 351:243–254
Gibbons JW, Andrews KM (2004) PIT tagging: simple technology at its best. Bioscience 54:447–454
Gosset C, Rives J (2004) Anesthésie et procédures chirurgicales pour l’implantation de radio e′metteurs dans la cavité ventrale de truites communes adultes (Salmo trutta). Bull Fran Peche Prot Pisc 374:21–34
Gries G, Letcher B (2002) Tag retention and survival of age-0 Atlantic salmon following surgical implantation with passive integrated transponder tags. N Am J Fish Manage 22:219–222
Hanson KC, Gravel MA, Graham A, Shoji A, Cooke SJ (2008a) Intersexual variation in fisheries research and mangement: when does sex matter? Rev Fish Sci 18:421–436
Hanson KC, Cooke SJ, Hinch SG, Crossin GT, Patterson DA, English KK, Donaldson MR, Shrimpton JM, Van Der Kraak G, Farrell AP (2008b) Individual variation in migration speed of upriver migrating sockeye salmon in the Fraser River in relation to their physiological and energetic status at marine approach. Physiol Biochem Zool 81:255–268
Harms CA (2005) Surgery in fish research: common procedures and postoperative care. Lab Anim 34:28–34
Harms CA, Lewbart GA (2000) Surgery in fish. Exot Anim Pract 3:759–774
Hart LG, Summerfelt RC (1975) Surgical procedures for implanting ultrasonic transmitters into flathead catfish (Pylodictis olivaris). Trans Am Fish Soc 104:56–59
Helm WT, Tyus HM (1992) Influence of coating type on retention of dummy transmitters implanted in rainbow trout. N Am J Fish Manage 12:257–259
Hockersmith EE, Muir WD, Smith WG, Sandford BP, Perry RW, Adams NS, Rondorf DW (2003) Comparison of migration rate and survival between radio-tagged and PIT-tagged migrant yearling Chinook salmon in the Snake and Columbia rivers. N Am J Fish Manage 23:404–413
Holland KN, Itano DG, Domeier M (2006) First successful surgical implantation of electronic tags in marlin. Bull Mar Sci 79:871–874
Hurty CA, Brazik DC, Law JM, Sakaoto K, Lewbart GA (2002) Evaluation of the tissue reactions in the skin and body wall of koi (Cyprinus carpio) to five suture materials. Vet Rec 151:324–328
Isely JJ, Young SP, Jones TA, Schaffler JJ (2002) Effects of antenna placement and antibiotic treatment on loss of simulated transmitters and mortality in hybrid striped bass. N Am J Fish Manage 22:204–207
Iwama GK, Ackerman PA (1994) Anaesthesia. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes, vol 3. Elsevier, Amsterdam, pp 1–15
Jadot C, Donnay A, Ylieff M, Poncin P (2005) Impact implantation of a transmitter on Sarpa salpa behaviour: study with a computerized video tracking system. J Fish Biol 67:589–595
Jepsen N (2003) Long-term retention of surgically implanted radio transmitters in pikeperch. J Fish Biol 63:260–262
Jepsen N, Aarestrup K (1999) A comparison of the growth of radio-tagged and dye-marked pike. J Fish Biol 55:880–883
Jepsen N, Davis LE, Schreck CB, Siddens B (2001) The physiological response of chinook salmon smolts to two methods of radio-tagging. Trans Am Fish Soc 130:495–500
Jepsen N, Koed A, Thorstad EB, Baras E (2002) Surgical implantation of telemetry transmitters in fish: how much have we learned? Hydrobiologia 483:239–248
Jepsen N, Schreck C, Clements S, Thorstad EB (2005) A brief discussion of the 2% tag/bodymass rule of thumb. In: Spedicato MT, Lembo G, Marmulla G (eds) Aquatic telemetry: advances and applications. FAO/COISPA, Rome, Italy, pp 225–260
Jepsen N, Mikkelsen JS, Koed A (2008) Effects of tag and suture type on survival and growth of brown trout with surgically implanted telemetry tags in the wild. J Fish Biol 72:594–602
Keefer ML, Peery CA, Caudill CC (2008) Migration timing of Columbia River spring Chinook salmon: effects of temperature, river discharge, and ocean environment. Trans Am Fish Soc 137:1120–1133
Knights BC, Lasee BA (1996) Effects of implanted transmitters on adult bluegills at two temperatures. Trans Am Fish Soc 125:440–449
Koed A, Thorstad EB (2001) Long-term effect of radio-tagging on the swimming performance of pikeperch. J Fish Biol 58:1753–1756
Kolok AS (1999) Interindividual variation in the prolonged locomotor performance of ectothermic vertebrates: a comparison of fish and herptofaunal methodologies and a brief review of the recent fish literature. Can J Fish Aquat Sci 56:700–710
Lacroix GL, Knox D, McCurdy P (2004) Effects of implanted dummy acoustic transmitters on juvenile Atlantic salmon. Trans Am Fish Soc 133:211–220
Lower N, Moore A, Scott P, Ellis T, James JD, Russell IC (2005) A noninvasive method to assess the impact of electronic tag insertion on stress levels in fishes. J Fish Biol 67:1202–1212
Lucas MC (1989) Effects of implanted dummy transmitters on mortality, growth and tissue reaction in rainbow trout, Salmo gairdneri Richardson. J Fish Biol 35:577–587
Lucas MC, Baras E (2000) Methods for studying the spatial behaviour of freshwater fishes in the natural environment. Fish Fish 1:283–316
Makiguchi Y, Ueda H (2009) Effects of external and surgically implanted dummy radio transmitters on mortality, swimming performance and physiological status of juvenile masu salmon Oncorhynchus masou. J Fish Biol 74:304–311
Mangan BP (1998) Long-term retention of a radio transmitter by a muskellunge. J Freshwater Ecol 13:485–487
Martin SW, Long JA, Pearsons TN (1995) Comparison of survival, gonad development, and growth between rainbow trout with and without surgically implanted dummy radio transmitters. N Amer J Fish Manage 15:494–498
Martinelli TL, Hansel HC, Shively RS (1998) Growth and physiological responses to surgical and gastric radio-transmitter implantation techniques in subyearling chinook salmon. Hydrobiologia 371/372:79–87
Marty GD, Summerfelt RC (1986) Pathways and mechanisms for expulsion of surgically implanted dummy transmitters from channel catfish. Trans Am Fish Soc 115:577–589
Mesa MG, Bayer JM, Seelye JG (2003) Swimming performance and physiological responses to exhaustive exercise in radio-tagged and untagged Pacific lampreys. Trans Am Fish Soc 132:483–492
Meyer CG, Honebrink RR (2005) Transintestinal expulsion of surgically implanted dummy transmitters by bluefin trevally: implications for long-term movement studies. Trans Am Fish Soc 134:602–606
Mitamura H, Mitsunaga Y, Arai N, Viputhanumas T (2006) Comparison of two methods of attaching telemetry transmitters to the Mekong giant catfish, Pangasianodon gigas. Zool Sci 23:235–238
Moore A, Russell IC, Potter ECE (1990) The effects of intraperitoneally implanted dummy acoustic transmitters on the behaviour and physiology of juvenile Atlantic salmon, Salmo salar L. J Fish Biol 37:713–721
Moser ML, Ogden DA, Sandford BP (2007) Effects of surgically implanted transmitters on anguilliform fishes: lessons from lamprey. J Fish Biol 71:1847–1852
Mueller RP, Moursund RA, Bleich MD (2006) Tagging juvenile Pacific lamprey with passive integrated transponders: methodology, short-term mortality, and influence on swimming performance. N Am J Fish Manage 26:361–366
Mulcahy DM (2003a) Does the Animal Welfare Act apply to free-ranging animals? ILAR J 44:252–258
Mulcahy DM (2003b) Surgical implantation of transmitters into fish. ILAR J 44:295–306
Mulford CJ (1984) Use of a surgical skin stapler to quickly close incisions in striped bass. N Am J Fish Manage 4:571–573
Murchie KJ, Cooke SJ, Schreer JF (2004) Effects of radio-transmitter antenna length on swimming performance of juvenile rainbow trout. Ecol Freshw Fish 13:312–316
Newell JC, Quinn TP (2005) Behavioral thermoregulation by maturing adult sockeye salmon (Oncorhynchus nerka) in a stratified lake prior to spawning. Can J Zool 83:1232–1239
Nielsen JL, Arrizabalaga H, Fragoso N, Hobday A, Lutcavage M, Sibert J (2009) Tagging and tracking of marine animals with electronic devices. Reviews: methods and technologies. Fish Biol Fish (REME) 9
O’Connor JP, Koehn JD, Nicol SJ, O’Mahony DJ, McKenzie JA (2009) Retention of radio tags in golden perch (Macquaria ambigua), silver perch (Bidyanus bidyanus) and carp (Cyprinus carpio). Mar Freshw Res 60:334–340
Okland F, Hay CJ, Naesje TF, Nickandor N, Thorsstad EB (2003) Learning from unsuccessful radio tagging of common carp in a Namibian reservoir. J Fish Biol 62:735–739
Paukert CP, Chvala PJ, Heikes BL, Brown ML (2001) Effects of implanted transmitter size and surgery on survival, growth, and wound healing of bluegill. Trans Am Fish Soc 130:975–980
Peake S, McKinley RS, Scruton DA, Moccia R (1997) Influence of transmitter attachment procedures on swimming performance of wild and hatchery-reared Atlantic salmon, Salmo salar, smolts. Trans Am Fish Soc 125:707–714
Penne CR, Ahrens NL, Summerfelt RC, Pierce CL (2007) Effect of relative volume on radio transmitter expulsion in subadult common carp. N Am J Fish Manage 27:986–991
Perry RW, Adams NS, Rondorf DW (2001) Buoyancy compensation of juvenile Chinook salmon implanted with two different size dummy transmitters. Trans Am Fish Soc 130:46–52
Petering RW, Johnson DL (1991) Suitability of a cyanoacrylate adhesive to close incisions in black crappies used in telemetry studies. Trans Am Fish Soc 120:535–537
Peterman RM (1990) Statistical power analysis can improve fisheries research and management. Can J Fish Aquat Sci 47:2–15
Plaut I (2001) Critical swimming speed: its ecological relevance. Comp Biochem Physiol A 131:41–50
Robertson MJ, Scruton DA, Brown JA (2003) Effects of surgically implanted transmitters on swimming performance, food consumption and growth of wild Atlantic salmon parr. J Fish Biol 62:673–678
Ropert-Coudert Y, Wilson RP (2005) Trends and perspectives in animal-attached remote sensing. Front Ecol Environ 3:437–444
Ross MJ, Kleiner CF (1982) Shielded needle technique for surgically implanting radio frequency transmitters in fish. Prog Fish Cult 44:41–43
Ross LG, Ross B (1999) Anaesthetic and sedative techniques for aquatic animals, 2nd edn. Blackwell, Oxford
Sakaris PC, Jesien RV (2005) Retention of surgically implanted ultrasonic transmitters in the brown bullhead catfish. N Am J Fish Manage 25:822–826
Sanderson TB, Hubert WA (2007) Assessment of gaseous CO2 and AQUI-S as anesthetics when surgically implanting radio transmitters into cutthroat trout. N Am J Fish Manage 27:1053–1057
Schnick RA (2006) Zero withdrawal anesthetic for all finfish and shellfish: need and candidates. Fisheries 31:122–126
Schramm HL, Black DJ (1984) Anaesthesia and surgical procedures for implanting radio transmitters into grass carp. Prog Fish Cult 46:185–190
Schulz UH (2003) Effects of surgically implanted dummy transmitters on the South American catfish Jundiá (Rhamdia quelen). Braz J Biol 63:345–348
Stakėnas S, Copp GH, Scott DM (2009) Tagging effects on three non-native fish species in England (Lepomis gibbosus, Pseudorasbora parva, Sander lucioperca) and of native Salmo trutta. Ecol Fresh Fish 18:167–176
Starr RM, Heine JN, Johnson KA (2000) Techniques for tagging and tracking deepwater rockfishes. N Am J Fish Manage 20:597–609
Stier DJ, Kynard B (1986) Use of radio telemetry to determine the mortality of Atlantic salmon smolts passed through a 17-Mw Kaplan turbine at a low-head hydro-electric dam. Trans Am Fish Soc 115:771–775
Stokesbury MJW, Cosgrove R, Teo SLH, Browne D, O’Dor RK, Block BA (2007) Movement of Atlantic bluefin tuna from the eastern Atlantic Ocean to the western Atlantic Ocean as determined with pop-up satellite archival tags. Hydrobiologia 582:91–97
Stoskopf MK (1993) Fish medicine. W.B. Saunders, Philadelphia
Summerfelt RC, Mosier D (1984) Transintestinal expulsion of surgically implanted dummy transmitters by channel catfish. Trans Am Fish Soc 113:760–766
Swanberg TR, Geist DR (1997) Effects of intraperitoneal transmitters on the social interaction of rainbow trout. N Amer J Fish Manage 17:178–181
Swanberg TR, Schmetterling DA, McEvoy DH (1999) Comparison of surgical staples and silk sutures for closing incisions in rainbow trout. N Am J Fish Manage 19:215–218
Thoreau X, Baras E (1997) Evaluation of surgery procedures for implanting telemetry transmitters into the body cavity of tilapia Oreochromis aureus. Aquat Living Resour 10:207–211
Thorstad EB, Kerwath SE, Attwood CG, Økland F, Wilke C, Cowley PD, Næsje TF (2009) Long-term effects of two sizes of surgically-implanted acoustic transmitters on a predatory marine fish species (Pomatomus saltatrix). Mar Freshw Res 60:183–186
Tyus HM (1988) Long-term retention of implanted transmitters in Colorado squawfish and razorback sucker. N Am J Fish Manage 8:264–267
Wagner GN, Cooke SJ (2005) Methodological approaches and opinions of researchers involved in the surgical implantation of telemetry transmitters in fish. J Aquat Anim Health 17:160–169
Wagner GN, Stevens ED (2000) Effects of different surgical techniques: Suture material and location of incision site on the behaviour of rainbow trout (Oncorhynchus mykiss). Mar Freshw Behav Phy 33:103–114
Wagner GN, Stevens ED, Harvey-Clark C (1999) Wound healing in rainbow trout Oncorhynchus mykiss following surgical site preparation with a povidone-iodine antiseptic. J Aquat Anim Health 11:373–382
Wagner GN, Stevens ED, Byrne P (2000) Effects of suture type and patterns on surgical wound healing in rainbow trout. Trans Am Fish Soc 129:1196–1205
Walsh MG, Bjorgo KA, Isely JJ (2000) Effects of implantation method and temperature on mortality and loss of simulated transmitters in hybrid striped bass. Trans Am Fish Soc 129:539–544
Welch DW, Batten SD, Ward BR (2007) Growth, survival, and tag retention of steelhead trout (O. mykiss) surgically implanted with dummy acoustic tags. Hydrobiologia 582(1):289–299
Wilson RP, McMahon CR (2006) Measuring devices on wild animals: what constitutes acceptable practice? Front Ecol Environ 4:147–154
Winter JD (1983) Underwater biotelemetry. In: Nielsen LA, Johnson DL (eds) Fisheries Techniques. American Fisheries Society, Bethesda, pp 371–395
Zale AV, Brooke C, Fraser WC (2005) Effects of surgically implanted transmitter weights on growth and swimming stamina of small adult westslope cutthroat trout. Trans Am Fish Soc 134:653–660
Acknowledgments
Cooke was supported by the United States Army Corps of Engineers, Portland District under contract from the Pacific Northwest National Laboratory (Contract # DE-AC05-76RL01830). Additional support was provided by Carleton University, the Canada Research Chairs Program, and the Ocean Telemetry Network Canada. Chris Holbrook, Karen Murchie and an anonymous referee kindly provided comments on the manuscript. Any reference to trade names does not indicate endorsement by the US Federal Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cooke, S.J., Woodley, C.M., Brad Eppard, M. et al. Advancing the surgical implantation of electronic tags in fish: a gap analysis and research agenda based on a review of trends in intracoelomic tagging effects studies. Rev Fish Biol Fisheries 21, 127–151 (2011). https://doi.org/10.1007/s11160-010-9193-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-010-9193-3