Abstract
One of the major challenges facing fishery scientists and managers today is determining how fish populations are influenced by habitat conditions. Many approaches have been explored to address this challenge, all of which involve modeling at one level or another. In this paper, we explore a process-oriented model approach whereby the critical population processes of birth and death rates are explicitly linked to habitat conditions. Application of this approach to five species of Great Lakes fishes including: walleye (Sander vitreus), lake trout (Salvelinus namaycush), smallmouth bass (Micropterus dolomieu), yellow perch (Perca flavescens), and rainbow trout (Onchorynchus mykiss), yielded a number of insights into the modeling process. One of the foremost insights is that processes determining movement and transport of fish are critical components of such models since these processes largely determine the habitats fish occupy. Because of the importance of fish location, an individual-based model appears to be a nearly inescapable modeling requirement. There is, however, a paucity of field-based data directly relating birth, death, and movement rates to habitat conditions experienced by individual fish. There is also a paucity of habitat information at a fine temporal and spatial scale for many important habitat variables. Finally, the general occurrence of strong ontogenetic changes in the response of different life stages to habitat conditions emphasizes the need for a modeling approach that considers all life stages in an integrated fashion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The joint strategic plan for management of Great Lakes fisheries, first adopted in 1980 and subsequently revised in 1997 (JSP; GLFC 1997) is a central guide for fisheries management of the Laurentian Great Lakes. The JSP led to the creation of Lake Committees comprised of representatives from fishery management agencies with jurisdiction over each of the Great Lakes (Dochoda and Jones 2002). First among the charges to these Lake Committees in the JSP is that each committee should “define objectives for the structure of each of the Great Lakes fish communities” (GLFC 1994). These fish community objectives are intended to guide fisheries management decision-making by providing targets against which fisheries performance can be measured. The JSP also led to the creation of the Habitat Advisory Board, whose central purpose was to facilitate the development of environmental objectives to support the fish community objectives. Since the signing of the JSP in 1980, efforts to develop environmental objectives have met with limited success.
Many of the regulatory agencies responsible for the aquatic environments of the North American Great Lakes have recognized that the preservation of fish communities requires the preservation of fish habitat. However, development of effective habitat regulations has foundered on the failure of fisheries science to develop metrics of habitat quantity and quality that can be reliably used to assess the sustainability of fish populations. A primary reason for this failure is the absence of a strong scientific basis for linking habitat conditions to the fish populations and communities that depend on these habitats. Habitat management has generally proceeded without formal models that predict the response of fish populations or communities to changes in their habitat. Yet, such models are arguably necessary to help identify limitations imposed on fish populations by current habitat conditions as well as to guide the selection among potential habitat management sites or approaches to alleviate these limitations. Although numerous studies have been conducted on Great Lakes fish habitat (e.g., HabCARES conference proceedings, Kelso 1996), proven methodologies linking habitat supply to fish population dynamics have not yet been developed.
Over the course of several years, we had developed models intended to explicitly link habitat supply to fish population dynamics for a number of Great Lakes fishes to address the needs for developing habitat objectives. In the course of developing these models, we experienced numerous challenges, and felt that the guidance in the literature was lacking or scattered. The goal of this paper is twofold. First, we present general insights into the modeling process that we have gained through our collective experience. Our intent is not to provide an in-depth analysis of all the challenges we faced within these models, but rather to paint a picture of the fundamental challenges we faced across species within the context of our chosen modeling paradigm. Secondly, we provide a synopsis of commonalities we observed in factors limiting these fishes. We also provide a brief overview of some of the current approaches for linking fish populations to habitat conditions to set the context for our review.
Current approaches
Several approaches for linking fish population or community dynamics to habitat conditions have been developed for freshwater fisheries. The simplest models that do this are the well-known empirical habitat-yield models, such as the morphedaphic index (Ryder et al. 1974), regressions of yield on phosphorus concentrations (Hanson and Leggett 1982) or chlorophyll concentration (Oglesby 1977; Jones and Hoyer 1982). These models describe habitat in a highly aggregated manner, and thus are most useful when changes in habitat are of a pervasive nature, such as might occur when phosphorus loadings alter the nutrient loading of a large part of a lake basin. These models are also useful when spatially detailed data are absent. They are of limited value, however, for examining how changes in localized habitat conditions affect fish production or yield. They are also not well suited to the incorporation of additional habitat components that are thought to be important in particular situations.
Another approach for linking fish populations to habitat is embodied in habitat suitability index (HSI) models. These models usually treat habitat conditions at a relatively fine spatial scale. Habitat data are then combined with observations on the distribution of individual fish (often of a particular life stage) to develop utilization curves (e.g., Guay et al. 2000; Vilizzi et al. 2004). These curves, in conjunction with expert judgement of species’ habitat requirements, are used to convert habitat conditions into an aggregated measure of habitat quality, often referred to as weighted usable area (e.g., Williams et al. 1999). The HSI models are sometimes calibrated against abundance, but more commonly weighted usable area is assumed to be linearly related to abundance (e.g., Raleigh 1982; Stalnaker et al. 1995). These models permit explicit consideration of a variety of habitat components, but they do little to elucidate either the mechanism by which individual habitat components affect fish populations, or the relative importance of each component (i.e., which component is limiting). Moreover, much of the controversy surrounding these models (e.g., Acreman and Dunbar 2004) concerns the rules used to combine the individual suitability curves for different habitat components or for different life stages into an overall index (e.g., Roussel et al. 1999; Roloff and Kernohan 1999). For example, habitats that provide optimal conditions for one life stage may be sub-optimal for other life stages. Without the link provided by considering the species’ population dynamics, it is difficult to justify choices made when combining suitability measures for different life stages. Finally, because these models are calibrated against (or are designed to predict) aggregate indicators of population status such as abundance or biomass, rather than specific demographic parameters such as mortality rates, they are not well suited to explore the interaction between habitat supply and other factors that affect population dynamics such as biotic interactions or fishery exploitation.
Similar to HSI models, a number of empirical models have been developed that correlate multivariate measures of habitat conditions to fish distribution, habitat utilization, or to indicators of population status such as stock abundance or biomass (e.g., Bowlby and Roff 1986; Wagner and Austin 1999; Stoneman and Jones 2000). These methods provide useful insight into the ecology of fish and their relationship to habitat quality, but they suffer from many of the same limitations as HSI models, especially when used as predictive tools or incorporated into population or community models.
Another class of models attempts to explicitly link habitat conditions with the vital rates of populations (see review by Rose 2000) to provide predictions of fish response across a variety of scales. We have argued this approach may prove more successful in providing the foundations for a model-based habitat management system (Hayes et al. 1996; Minns et al. 1996). Although some exceptions exist (e.g., Shuter 1990; Marschall and Crowder 1996; Minns et al. 1996; Rose et al. 1999; McDermot and Rose 2000), few of these models cover all of the principal vital rates (i.e., birth rate, survival rate, individual growth rate), or follow through the entire life cycle of the model species. Frequently, such models have focused on a particular life stage or one vital rate (e.g., Brandt and Kirsch 1993; Mason et al. 1995).
In the research described here, we used the approach underlying these models as a basis for the development of models for several Great Lakes fish species that cover all vital rates and the full life history for the majority of these species. To test the robustness and feasibility of implementing this approach, we selected Great Lakes fish species with contrasting life histories. These species included: walleye (Sander vitreus), lake trout (Salvelinus namaycush), smallmouth bass (Micropterus dolomieu), yellow perch (Perca flavescens), and rainbow trout (Onchorynchus mykiss). Except rainbow trout, we modeled the full life cycle of the population of interest. In the next section we briefly describe these models in order to provide background supporting the set of insights and advice we present in our review. In these descriptions, we emphasize the major features of each of the species-specific models to provide an understanding of the common data requirements and modeling approaches used to link individual life stages with their surrounding habitat (Table 1).
Overview of individual species models
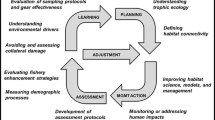
Details on the models developed for each species are available in Chu et al. (2006), Thompson (2004), Netto (2006), Jones et al. (2003), and Doka (2004). In the descriptions below, we emphasize the major features of each species-specific model. The approach we took in modeling the species selected is not fundamentally different than other approaches that have been previously used (e.g., Marschall and Crowder 1996; Minns et al. 1996; Rose et al. 1999). Rather, using these studies as a base, our choice of specific modeling methods was guided by the situation presented for each species. All models shared a number of common elements, however. A common theme was a focus on population processes of births, deaths and individual growth, leading to a cohort-based, age-structured description of dynamics. In all the models, at least one life stage was represented as an individual-based model, or a pseudo-individual based model where a cohort was broken into spatially or temporally explicit subgroups. This led us to use descriptions of habitat conditions and biological inputs at relatively fine scales. As our modeling efforts progressed, we found that in addition to the key population-level vital rates listed above, the incorporation of fish transport and movement behavior became a vital consideration. Therefore, the basic concept that links all our models is the idea that at a particular point in time, the state of an individual fish must be described in terms of its size and location, as well as other features such as sex and age. Between points in time, the fish can either survive or die, and it can remain at its current location or move. The probability of survival or movement, as well as the growth rate of individual fish is dependent on their state variable descriptors (e.g., size, age, location), the environmental conditions they are exposed to at their current location, and random chance (Fig. 1). From this basic building block, different life stages are linked in time and space, with vital rates being dependent on environmental conditions at each stage (Fig. 2).
In developing these models, we relied primarily on published studies to define suitable habitat conditions and to relate key processes to those habitat conditions; field sampling or experiments were conducted only for rainbow trout and smallmouth bass. When evaluating relationships presented in the literature, we made an attempt to explicitly characterize effects as density-dependent or density-independent, recognizing that factors operating in these different fashions have different implications for population dynamics (Hayes et al. 1996).
Western Basin of Lake Erie walleye (Jones et al. 2003)
An outline of the critical elements of the walleye model is provided in Fig. 3. One of the key details of the walleye population in the Western Basin of Lake Erie is that adults reside in the open lake during most of the year, but a portion of the population spawns in offshore reefs while another part of the population spawns in tributary rivers. At present, the proportion of the population spawning on reefs and in tributaries is unknown, but reef habitats are far more extensive. As such, we apportioned the population as 90% reef-spawning and 10% as tributary-spawners. The walleye model starts with adult walleye that produce eggs based on their sex ratio, size distribution and maturation schedule. Spawning times depend on water temperature.
For lake spawners, eggs are deposited in offshore reefs following observations of Roseman (2000). The development of eggs is a function of water temperature, and their numbers are reduced by a constant daily mortality rate, plus a depth-dependent episodic mortality during high wind events causing disturbance of the spawning habitat (Roseman 2000; Roseman et al. 2001). Following hatching, larval walleye are transported by wind-driven surface water currents (Roseman 2000). Larvae transported offshore are assumed to die, and larvae transported onshore remain in the nearshore zone where their growth and survival is modeled as a function of water temperature and prey abundance.
Selection of spawning habitat in the Sandusky and Maumee Rivers (the principal rivers used for spawning in the Western Basin) by adult walleye is modeled as a process where the best habitat (defined on the basis of substrate size) is selected first, and after saturation, less preferred habitats are selected secondarily. Survival rate of eggs varies with substrate size, and the development rate of eggs varies with water temperature. After hatching, survival rate and downstream transport of larvae depends on river discharge and temperature, following Mion et al. (1998). The growth of larvae while in the rivers is negligible because of the lack of zooplankton (Mion et al. 1998).
After reaching the lake itself, river-spawned larvae are assumed to be exposed to the same environmental conditions as reef-spawned larvae, although their size distribution frequently differs due to differences in spawning times. Larval and juvenile walleye growth and survival during the first year of life are modeled as a function of density and food abundance. We found no quantitative information in the literature to suggest how other habitat conditions (e.g., macrophytes, substrate size composition) affect the growth, survival or distribution of walleye during the first year of life.
Following the first winter of life, the principal habitat conditions determining the growth rate of juvenile and adult walleye are light, temperature, and prey abundance, with light and temperature defining the volume of the lake where habitat conditions are acceptable. Mortality rate was assumed to be a constant rate of natural mortality plus fishing mortality on fish of legal size/age.
Smallmouth bass (Chu et al. 2006)
Two habitat sub-models were defined for smallmouth bass based on the habitat requirements of the different life stages (Fig. 4). The first focuses on the nesting habitat and includes reproductive adults, eggs, hatchlings, swim-up fry and YOY (post-dispersal and for the remainder of the growing season). The second includes the juvenile and adult life stages. The adult life stage is defined as fish age ≥3 years because that is the age when these fish approach maturity.
Available habitat is represented as a grid, with temperature, substrate, depth, fetch and wave action described for each grid element. Using the habitat data, the model maps the suitability of different sites in a lake for nesting and for the juveniles/adults. Spawning timing is dependent on water temperature, and egg production by mature smallmouth bass is modeled as a function of their size distribution and sex ratio. Ideal free distribution theory (e.g., Fretwell and Lucas 1970) is used to determine the spatial distribution of nests, juveniles (age 1+ to maturity) and adults throughout the lake. Growth of the young-of-the-year is dependent on total dissolved solids, fetch and temperature, and is also density-dependent. Survival is size-dependent in the young-of-the-year, but is set at a constant annual rate for older fish. Growth of age 1+ smallmouth bass is dependent on an index of productivity, temperature, and density. A home range mechanism is used to model density-dependent effects on growth for all life stages.
Lake trout (Netto 2006)
Egg deposition by adult, wild-spawned lake trout is dependent on the sex, size and age distribution of the adult population (Fig. 5). The initiation of spawning behavior is triggered by suitable water temperature, and spawning sites are selected based on substrate size composition and water depth. Egg development is temperature-dependent, and survival of eggs and yolk-sac fry depends on the magnitude of wind-induced water current velocity and the amount of sediment resuspension. After emergence, early juveniles are transported by wind-induced currents, but are able to maintain their location within several weeks of emergence. The growth and survival of juvenile lake trout is modeled as a function of water temperature and food abundance. Conceptually, growth, survival and distribution of post-juvenile lake trout depends on prey abundance, temperature, and depth, but finding studies linking vital rates to environmental conditions proved to be problematic.
Yellow perch (Doka 2004)
As with the other models, egg deposition by yellow perch depends on the size structure and sex ratio of the population (Fig. 6). Spawning sites are selected based on substrate characteristics, water depth, and the presence of macrophytes (Weber and Les 1982; Fisher et al. 1996; Robillard and Marsden 2001), and the timing of spawning is based on water temperature. The development of eggs is also temperature-dependent, with survival dependent on temperature and wind-induced currents and wave action (as determined by wind speed, wind direction and fetch).
Following hatching, planktonic larval yellow perch are distributed by water currents, and their growth and survival is determined by water temperature and habitat-based, indirect measures of zooplankton prey abundance (Ross et al. 1977; Thorpe 1977). After reaching approximately 30 mm, juvenile yellow perch become demersal and are no longer vulnerable to relocation by water currents. Survival and growth are dependent on temperature, substrate (as an index of food availability), and macrophyte density (as a surrogate for relative predation risk). We also explored options for density-dependent growth and survival. As juvenile yellow perch age and grow, they become increasingly able to select preferred habitats, and the model allows for such movements. As adults, yellow perch are modeled as being able to select preferred habitats, and their growth is dependent on temperature and relative food availability in a density-dependent fashion. Mortality of juveniles and adults varies with growth, and also includes fishing mortality. The majority of the simulation work was focused on the stages from adult spawning to completion of the first season by new fry.
Rainbow trout (Thompson 2004)
Unlike our other target species, we did not construct a full life-cycle model for rainbow trout. Our approach for this species was somewhat different because we were able to conduct field sampling and experiments in the Pine River, Alcona County, Michigan (Thompson 2004). For this species, our focus was on the dynamics of age-1 rainbow trout (Fig. 7), emphasizing their movement dynamics and growth rate as a function of habitat conditions. The growth of age-1 rainbow trout is dependent on river branch-specific, empirically derived estimates of consumption rates and site-specific temperature regimes. These inputs were incorporated into the “Wisconsin” bioenergetics model (Kitchell et al. 1977) to model growth. Movement was represented as a stochastic process where the probability of movement among mesohabitat units was modeled as a function of mesohabitat designations (i.e., pool, riffle, run) and density of juvenile rainbow trout. We conducted a census of the mesohabitat units of over 70 km of the Pine River, classifying each unit as a pool, riffle, or run. Additional habitat variables such as river width, depth, and substrate composition were also collected, but were not incorporated into the final model.
Insights and perspectives
Individual-based models are needed
One characteristic we judged to be necessary for all of the models we developed was that key population processes (i.e., vital rates such as birth, death, individual growth rate, as well as movement) needed to be explicitly linked to environmental factors experienced by individual fish, or small groups of similar individuals. While other, more aggregated representations of these dynamical processes are possible (e.g., Leslie matrix), we found it necessary to use an individual-based approach because it allowed us to readily represent the spatial distribution of individuals among habitat patches, leading to important differences in their vital rates. Explicit incorporation of the spatial distribution was necessary to represent the range of environmental conditions experienced by individuals in the population, and to model changes in the spatial distribution of fish in response to environmental conditions. Individual-based models also allow for the incorporation of individual variation in important characteristics such as body size and hatch date. These variations, coupled with differences in habitat conditions experienced by individuals can lead to important implications for fish population dynamics. For example, in our walleye model, variations in hatch dates allows for the population to “sample” variations in river flows, thereby increasing the odds that some individuals will experience favorable conditions early in life (Jones et al. 2003). Likewise, variations in hatch dates were important components of our models for yellow perch and smallmouth bass. Similar results highlighting the importance of individual variation have been obtained in a variety of systems for numerous species (e.g., Miller 2007; Hook et al. 2007).
We had initially proposed to avoid the extreme reductionism and data demands of individual-based models, but were unable to find simpler, yet realistic alternatives for modeling these processes. As such, we agree with the recommendations of Lomnicki (1999), Juanes et al. (2000), and Rose (2000), among others, that individual-based models are generally the best approach for addressing questions regarding the linkage between (fish) populations and their habitat. We note, however, that there are serious implications of choosing an individual-based modeling approach. One of the major implications, which we discuss further below, is that data on the variance and shape of the statistical distribution, rather than just the mean, of many population processes is required to implement individual-based models to their full advantage. These data requirements are very difficult to meet, leading modelers to make assumptions on the parameters, or borrow data from other species, both of which may lead to substantial model uncertainty.
Another positive feature of the individual-based approach is that these models are amenable to the direct incorporation of factors such as fishery harvest or competition. Although we consciously excluded such factors in order to limit the complexity of our models, we feel that the modeling approach is robust enough to readily incorporate such factors. A final advantage of individual-based models is that they tend to favor conceptual simplicity over computational and analytical simplicity or elegance. This conceptual simplicity greatly facilitates the communication and discussion of model structure to scientists and policy makers (e.g., Beck et al. 2001).
Ontogenetic shifts and habitat juxtaposition are important
Another feature common to all of our models was the need to accommodate ontogenetic shifts in habitat requirements, and thus the spatial distribution and connectivity among habitats used by different life stages (Schindler and Scheuerell 2002). Because successive life stages often have different habitat requirements and limitations, simple HSI models are likely to be seriously deficient without a means to connect the dynamics of different life stages.
Although births and deaths are the only processes that ultimately determine abundance in closed populations, we found that the movement or transport of fish often turned out to be a key process affecting survival and reproductive success. For all species, representing this movement was one of the greatest challenges we faced in our modeling efforts. The movement of early life stages of many species occurs through passive transport by water currents. Thus, for example, we had to represent surface water currents in the Western Basin of Lake Erie to predict transport of reef-spawned larval walleye. Our approach was to use a simple rule, developed by Olson (1950) where surface current velocity is 10% of wind velocity and 10° to the left, to provide a first approximation.
More complex models of water currents exist for many areas in both marine (see in particular review by Miller 2007) and large freshwater systems such as the Great Lakes (e.g., Beletsky et al. 2007; Zhao et al. 2009). Such models have provided substantial insight into the transport processes of larval fishes, but in our situation, none were available for the particular regions inhabited by our target species at the time we developed these models. A further concern is that even if models are available, the prediction of fish transport may not be improved. This occurs because water currents in large lakes and the ocean have a complex three-dimensional pattern (e.g., Saylor and Miller 1987; Royer et al. 1987; Quinlan et al. 1999). Thus, at a given two-dimensional location (i.e., latitude and longitude), water current velocity and direction varies with depth and lake bathymetry (Schwab and Bennett 1987). For example, surface currents may run at 0.1 m/s in a SW direction, but the current at 5 m in depth may run 0.05 m/s in a NE direction. The vertical distribution of larval fishes is generally not well known, and further, can vary even on a diel basis (e.g., Houde 1969), possibly in response to water currents. Thus, the problem of predicting the passive transport of larval and juvenile fishes in the Great Lakes remains a major challenge, but the importance of such transport on the demographics of young fishes emphasizes the need to continue working in this area.
As fish age and are able to actively move against water currents, the situation becomes no less complex. A number of theories, such as optimal foraging theory (e.g., Mittelbach 1981) and the ideal free distribution (e.g., Tyler and Hargrove 1997), have been developed to predict the habitat choice and equilibrium distribution of fish in a heterogeneous environment. Unfortunately, the constantly changing mosaic of environmental conditions results in a transient state of habitat dynamics, a situation where these theories are not readily applicable. One strength of our modeling approach is that the models provide predictions of transient dynamics as well as equilibrium outcomes. For one species (rainbow trout), a major component of our field work was to perform tagging studies combined with experimental translocation to better understand the transient dynamics of fish movement and habitat selection. In general, we found that habitat selection at the mesohabitat (i.e., pool, riffle, run) level by river-dwelling fish was somewhat easier to handle than for lake-dwelling fish because rivers can be treated as linear geographic features with directional flows, whereas location within a lake is a three-dimensional geographic feature. For open lake and marine systems, some new approaches using mechanistic behavioral models show promise for simulating the movement of free-ranging fishes (Humston et al. 2004).
Paucity of published studies relating habitat conditions to vital rates
A major challenge we faced in all models was finding published studies relating population vital rates to habitat conditions. For example, a number of studies have documented that recruitment of walleye is correlated to the rate of springtime warming and the severity of wind events (Busch et al. 1975; Roseman 2000). While these studies suggest a linkage between aggregated descriptions of habitat conditions and recruitment success, they provide little information regarding how survival rate of juvenile walleye (for example) varies with water temperature, or how egg survival rate on reefs varies with wind and water velocity. Likewise, there are abundant data describing the habitat conditions where fish are collected, putatively indicating habitat selection, but these data also provide little insight into how population vital rates vary across habitats. However, some encouraging insights into these associations are provided by a recent study (Zhao et al. 2009) that used a three-dimensional hydrodynamic model to correlate cohort strength with current-driven, reef-to-shore movement of larval walleye in western Lake Erie.
We experienced similar difficulties with each of the species investigated, and for virtually all life stages. Even for adult stages, which are generally easier to capture and mark or follow using telemetry there is a dearth of data. Thus, we were often forced to make assumptions regarding how survival in particular varied across habitats.
It is difficult to determine the specific reasons why there are so few studies relating population processes to habitat conditions. We offer two explanations for this observation (1) scientists generally don’t conceptualize or frame fisheries problems this way; or (2) it is difficult and expensive to measure mortality and growth rates for fish over short enough time scales to assign these rates to particular habitat conditions. We hope that papers like this will help shape scientists’ thinking when developing studies of fish-habitat relations. Thus, where suitable data are collected to ascertain habitat-specific vital rates, we hope researchers pursue such opportunities. Studies using telemetry or acoustic tags appear particularly suited to estimating survival rates, for example, as a function of habitat conditions. The second impediment, however, remains a challenge to fishery scientists and fish ecologists. Our experience with field studies provides several insights. First, growth rate estimates are relatively easier to obtain over short intervals than other vital rates. Partly this occurs because the unit of observation is an individual fish. Individual tagging provides a means of estimating growth rate of individual fish, thereby providing point estimates and even the distribution of growth rates under specified habitat conditions. We were able to successfully apply this approach to rainbow trout in the Pine River, Michigan (Thompson 2004). We would note, however, that even in systems that are readily sampled, fish movement out of the study zone can limit recaptures, and that recapture rates of marked individuals rarely approaches 100%. Thus, even growth rates for individuals can be challenging to measure directly in the field. Estimating habitat-specific mortality rate has proven even more difficult. Partly this occurs because losses due to mortality are generally confounded with movement out of the habitat units. This is particularly a problem in “poor” habitat where fish often move away from such conditions before mortality takes place. Further, mortality rates are often estimated for the (sub) population as a whole, making the unit of observation the (sub) population; as a consequence, many experiments need to be conducted in order to determine variability among habitats. Finally, under “good” habitat conditions, mortality rates can be very low, and difficult to estimate precisely for short time intervals. As indicated above, telemetry studies hold promise to estimating mortality rates, but such studies are often expensive to conduct.
In addition to an understanding of the relation between vital rates and habitat conditions, knowledge of fish movement as a function of habitat conditions is also important. Developing movement rules for fish is an area where recent research highlights the utility of using individually marked fish (e.g., Railsback and Harvey 2002; Belanger and Rodriguez 2002). These approaches are particularly appealing within the modeling framework we present here because they focus on the behavior of individuals. Humston et al. (2004) present a useful summary of some mechanistic models relating fish movement to habitat conditions. In particular, movements based on kinesis or a gradient response based on a restricted-area search appear promising ways of connecting fish movement and location to habitat conditions. For smallmouth bass, we had sufficient information available to use the concept of the ideal free distribution to help guide our models of fish movement and habitat choice.
One linkage between the field experiments and our overall modeling approach is the use of inverse modeling techniques (e.g., Parker 1977; Nibbelink and Carpenter 1998) to infer vital rates that are consistent with field data. In particular, we were able to infer “movement rules” for rainbow trout in streams, differentiating probability of movement among pools, riffles and runs, as well as a function of temperature (Thompson 2004). In many cases, prior studies contain data that were collected at a more aggregated level than our level of treatment (e.g., growth and survival of individual fish depends on microhabitat conditions). In inverse modeling, trial values of system parameters are evaluated and adjusted to match observed data, thereby allowing inferences on processes that are not directly observed. This is a particular advantage if field data are not collected on a habitat-specific level, because modeling inferences can be developed to “explain” the observations. While this is a powerful and useful approach, several caveats must be kept in mind. First and foremost, it is often very difficult to resolve among competing model structures. Thus, the inferences being made are conditional on having the “right” model structure in place. Further, parameters are often highly confounded if the level of data resolution (spatial, temporal, or level of process description) does not match that represented in the model.
Paucity of relevant habitat data
In addition to a dearth of studies relating vital rates to habitat conditions, data for many of the critical environmental/habitat conditions are lacking at a “reasonable” temporal or spatial scale and sampling intensity to be useful. Technological advances in geographic information systems (GIS), monitoring devices such as flow gauges, and remote sensing are improving our ability to collect relevant habitat data but these data are still lacking in many situations. Temporally intensive data collections tend to be spatially very limited, and conversely spatially extensive data tend to be limited to few time periods. Because of this, we often had to use some means for describing habitat conditions based on sparse data. We used two approaches for describing the dynamics of habitats (1) a process-driven approach where we modeled the underlying factors driving habitat conditions (e.g., circulation modeling in lakes); and (2) a data-driven approach where habitat conditions are estimated by interpolation from surrounding times and locations.
In the first approach, mechanistic sub-models representing the underlying dynamics of habitat conditions were developed. An example of this is the wind-driven, water current sub-model we used for Lake Erie walleye. In this sub-model, water current velocity and direction were predicted from data available on wind speed and direction. Our representation was by necessity relatively simple; more sophisticated circulation models have since been developed (e.g., Zhao et al. 2009). One strength of this approach is that data on the habitat conditions of interest are not required if data on other, driving variables are available. A similar approach could be used in streams where landscape-scale features can be used to drive habitat models or can serve as proxies for habitat conditions present (e.g., Wiley et al. 1997). Often such approaches provide a means to estimate some driving factors, such as water temperature, that are driven by broad-scale factors, but are often not suitable to provide a picture of finer-scale habitat features such as the location of individual pools in a stream.
In the second approach, habitat conditions over the entire region are interpolated from existing data (Doka 2004; Kratzer et al. 2006). This too creates a model of habitat dynamics, making assumptions regarding how these conditions vary across space and time, but these assumptions are not based on mechanistic processes. For example, water temperature data for Long Point Bay were available at a limited number of locations and sampling dates. With these data and satellite imagery of the entire bay, an interpolation model predicting the water temperature at intermediate times and locations was constructed (Doka 2004). This model assumed substantial coherence in temperature patterns at a large spatial scale, but finer scale patterns (e.g., upwelling events) were generally preserved by the combined information from temperature dataloggers and spatially explicit thermal imagery. Interpolations further assume some regularity in the vertical temperature structure during the summer stratification period. Even a coarse estimate of temporal and spatial differences in physical variables (especially temperature) is more realistic than using a single profile to represent an entire water body. In general, we feel that the interpolation approach is particularly useful when data on habitat conditions are available at a reasonable sampling intensity to predict interpolated points, and when the habitat conditions themselves are relatively continuous and coherent across space and time. An example of where the interpolation approach is less useful is predicting water currents, which often show abrupt changes in time and space scales that are much shorter (e.g., hours to days) and finer (e.g., within 1–2 m vertically) than those represented in typical sampling designs.
As another example of how data intensive these models can be, we performed a “census” of mesohabitat conditions for over 70 km of the Pine River, Michigan to support our rainbow trout model (Thompson 2004). Even though it is a relatively straightforward task to collect such data, it is very time and labor intensive. Further, our data collections did not extend completely into the headwaters of this river system as this was beyond the scope of our study. Fortunately, such stream features are often relatively stable over time, allowing for habitat surveys to be conducted once over the course of several years, and then treated as “fixed” habitat features. We also collected transect-level habitat data (e.g., stream width, depth, substrate composition), however the scale of these data was finer than the mesohabitat scale we eventually chose. Even though these data would in principle have been useful in further detailing habitat condition available, implementation in our model would have required a much more intensive sampling of the entire river reach than would be feasible. As this example shows, there are obvious tradeoffs between the level of detail and scale of habitat information and the spatial extent that can be sampled with a given set of resources. It is not so obvious, however, which scale is “best”. For this species in this setting, we chose a greater spatial extent of sampling because preliminary sampling showed age-1 rainbow trout to be highly mobile, often covering distances greater than 1–2 km within 1 week or less.
The broad time scale across which habitats vary also presents several challenges. For example, temperature patterns in the environment often change over short time periods (e.g., days), but other features such as bathymetry or channel morphology often change slowly (e.g., years to decades). This can result in “stiff” systems (borrowing the term used for differential equations; Press et al. 1992) where the dynamics of different components need to be treated differently. The varying time scales for habitat variation also have implications for sampling intensity necessary to represent habitat conditions adequately. Some features, such as bathymetry, are likely to change relatively slowly, allowing for sampling to be temporally less intensive. Other features, such as stream water temperature, which may vary several degrees within a day, may require almost constant measurement. Most challenging, perhaps, are habitat conditions that rapidly vary both spatially and temporally. Water temperature in Great Lakes embayments is an example of such a situation. This was a particular problem for our yellow perch model because of their broad distribution within Long Point Bay. During periods of stratification development or decay, the water temperature at a given point can change within a few days, and the difference in temperature between adjacent points (e.g., points along a vertical thermal profile) may likewise change rapidly. Cases such as these are difficult to address with either mechanistic models or interpolation models because of the abrupt changes that may occur. Therefore, the choice of time step in habitat-based models is important, as well as the availability of data or physical models as input (Minns and Wichert 2005).
Common outcomes across species
Across our model species, several habitat features showed a pervasive effect on model outcomes. Foremost among these was water temperature (Magnuson et al. 1979). Water temperature has long been known to strongly affect growth (e.g., Kitchell et al. 1977; Hewett and Johnson 1992), survival and development rate of fishes (e.g., Allbaugh and Manz 1964; Hurley 1972), and further can strongly affect their spatial distribution (e.g., Mason et al. 1995). Because of its effect on multiple vital rates and fish behavior, changes in water temperature can have a disproportionate effect on aggregated outputs such as production, biomass and abundance. As such, obtaining adequate data on spatial and temporal variability in water temperature should be a priority for habitat investigations.
Another habitat feature that had an important role in each of our models was water currents. Although adult fish are generally able to avoid strong currents, or maintain their position against such currents, the survival of eggs and the distribution and survival of larvae and early juveniles is often strongly affected by water currents (e.g., Houde 1969; Clady 1976). Unfortunately, data directly measuring water current velocity in the Great Lakes or their tributaries are often lacking, and tend to be expensive to collect. Moreover, water velocity and direction in the Great Lakes is very dynamic, often changing over the course of hours to days, and over short spatial scales (e.g., 1–2 m within a vertical profile). Circulation models are a helpful tool to address this problem (e.g., Beletsky et al. 2007; Zhao et al. 2009), but are a major undertaking to construct, implement and validate.
Model scale and validation
We have focused on process-driven, mechanistic models, in contrast to aggregated, holistic models, such as the morphoedaphic index (Fig. 1). We do not mean to imply that the mechanistic modeling excludes aggregated modeling—rather, the two approaches should be able to inform one another. For example, the process-driven models can help identify mechanisms that give rise to the observed aggregate patterns. Likewise, the observed aggregate relationships are important validation data for the mechanistic models. A strength of our modeling approach is that the model explicitly makes predictions for many system attributes (Fig. 1), ranging from process rates (e.g., birth rate, death rate, individual growth rates) to detailed descriptions of system characteristics (e.g., distribution of fish), to more aggregated descriptions of the system (e.g., total biomass, abundance, population size structure). Comparison of model predictions across this range of scales with data helps to validate the models and point to the specific areas where there are problems. Further, such a modeling approach is well suited to utilizing the results of field experiments as a means of validating or calibrating specific parts of the model. This is in contrast to regression-based approaches where it is often difficult to pinpoint how and why the regression may not fit. Despite the conceptual appeal of performing model validations at several levels of aggregation, trying to validate our models has been a very challenging task because of the limited data available for validation. Further, a key use of these models is to predict the response of fish to changes in habitat conditions; conditions that may be outside the range of data used to construct the models. As such, validating models for this purpose may be virtually impossible. This does not imply that such models are useless; rather, the use of these models (or any other model used to make similar predictions) should recognize the uncertainty associated with such situations.
While constructing our models, we often made trade-offs between basing the model on data versus using assumptions. Because of the lack of published studies, we were often “forced” into making assumptions to develop even the most basic model. A hard question to answer is “when are there so many assumptions, or when are the assumptions so strong, that the model predictions are not informative?” An associated question is how to make good assumptions. One approach for making assumptions that we found particularly appealing was to use general principles from ecology or other basic sciences as the basis for making an assumption. For example, over long time periods, fish evolution is a shaping force. This can be a problem because fish can adapt (thereby altering the parameters describing how their vital rates vary with habitat conditions) to changing habitat conditions. In the short term, however, we can use the assumption that fish have adapted to present habitat conditions, and tend to behave in an optimal way (i.e., maximize fitness) to help constrain some of our modeling problems. For example, we used the idea that smallmouth bass spawning distribution will follow the ideal free distribution (e.g., Fretwell and Lucas 1970; Tyler and Hargrove 1997). We assume that they are behaving to maximize fitness, and that some principles of optimal habitat selection guide fish distribution if they are able to actively choose among potential habitats. Another example of using basic ecology to constrain our models is the application of life history theory (e.g., Jensen 1998) to help “tie together” different life stages in our models (Shuter et al. 1998). Constraints imposed by life history consideration helped us to infer ages and sizes at maturation of walleye (for example) under different growth regimes imposed by different habitat conditions. These theories help by creating constraints in the modeling problem, making it much more feasible to find reasonable solutions.
Conclusion
In summary, we feel that the modeling approach we describe holds promise for bringing forward a useful marriage between the tradition of fish population dynamics and habitat science. Many practical concerns remain, however, not the least of which is the lack of solid quantification of the response of population vital rates and fish behavior to habitat conditions, and the scarcity of data on habitat conditions at a broad temporal and spatial scale. Another challenge not listed above is how to bring this modeling approach into a true multi-species application, where fish populations interact fully with their prey, other populations of fish, and the habitat in which they all reside.
References
Acreman M, Dunbar MJ (2004) Defining environmental river flow requirements—a review. Hydrol Earth Syst Sci 8(5):861–875
Allbaugh CA, Manz JV (1964) Preliminary study of the effects of temperature fluctuations on developing walleye eggs and fry. Prog Fish-Cult 26:175–180. doi:10.1577/1548-8640(1964)26[175:PSOTEO]2.0.CO;2
Beck MW, Heck KL Jr, Able KW, Childers DL, Eggleston DB, Gillanders BM, Halpern B, Hays CG, Hoshino K, Minello TJ, Orth RJ, Sheridan PF, Weinstein MP (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51:633–641. doi:10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2
Belanger G, Rodriguez MA (2002) Local movement as a measure of habitat quality in stream salmonids. Environ Biol Fishes 64:155–164. doi:10.1023/A:1016044725154
Beletsky D, Mason DM, Schwab DJ, Rutherford ES, Janssen J, Clapp DF, Dettmers JM (2007) Biophysical model of larval yellow perch advection and settlement in Lake Michigan. J Great Lakes Res 33:842–866. doi:10.3394/0380-1330(2007)33[842:BMOLYP]2.0.CO;2
Bowlby JN, Roff JC (1986) Trout biomass and habitat relationships in southern Ontario streams. Trans Am Fish Soc 115:503–514. doi:10.1577/1548-8659(1986)115<503:TBAHRI>2.0.CO;2
Brandt SB, Kirsch J (1993) Spatially explicit models of striped bass growth potential in Chesapeake Bay. Trans Am Fish Soc 122:845–869. doi:10.1577/1548-8659(1993)122<0845:SEMOSB>2.3.CO;2
Busch WDN, Scholl RL, Hartman WL (1975) Environmental factors affecting the strength of walleye (Stizostedion vitreum vitreum) year-classes in western Lake Erie, 1960–70. J Fish Res Board Can 32:1733–1743
Chu C, Collins NC, Lester NP, Shuter BJ (2006) Population dynamics of smallmouth bass in response to habitat supply. Ecol Modell 195:349–362. doi:10.1016/j.ecolmodel.2005.11.024
Clady MD (1976) Influence of temperature and wind on the survival of early stages of yellow perch, Perca flavescens. J. Fish. Res. Bd. Can. 33:1887–1893
Dochoda MR, Jones ML (2002) Managing Great Lakes fisheries under multiple and diverse authorities. In: Lynch KD, Jones ML, Taylor WW (eds) Sustaining North American Salmon: perspectives across regions and disciplines. American Fisheries Society, Bethesda, pp 221–241
Doka SE (2004) Spatially-explicit habitat characterization, suitability analysis, verification, and modelling of the yellow perch, Perca Flavescens (Mitchell 1814), population in Long Point Bay, Lake Erie. Ph.D. Dissertation, McMaster University, Hamilton, Ontario, 250 p
Fisher SJ, Pope KL, Templeton LJ, Willis DW (1996) Yellow perch spawning habitats in Pickerel Lake, South Dakota. Prairie Nat. 28:65–75
Fretwell SD, Lucas HL (1970) On territorial behavior and other factors influencing habitat distribution in birds. I. Theoretical development. Acta Biotheor 9:16–36
Great Lakes Fishery Commission (1994) A joint strategic plan for management of Great Lakes fisheries. Ann Arbor, MI. (supersedes 1980 version.)
Great Lakes Fishery Commission (1997) A joint strategic plan for management of Great Lakes fisheries. Ann Arbor, MI. (supersedes 1994 version.)
Guay JC, Boisclair D, Rioux D, Leclerc C, Lapointe M, Legendre P (2000) Development and validation of numerical habitat models for juveniles of Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 57:2065–2075. doi:10.1139/cjfas-57-10-2065
Hanson JM, Leggett WC (1982) Empirical prediction of fish biomass and yield. Can J Fish Aquat Sci 39:257–263. doi:10.1139/f82-036
Hayes DB, Ferreri CP, Taylor WW (1996) Linking fish habitat to their population dynamics. Can J Fish Aquat Sci 53(Suppl. 1):383–390. doi:10.1139/cjfas-53-S1-383
Hewett SW, Johnson BL (1992) A generalized bioenergetics model of fish growth for microcomputers, version 2.0. University of Wisconsin Sea Grant Institute, Madison
Hook TO, Rutherford ES, Mason DM, Carter GS (2007) Hatch dates, growth, survival, and overwinter motality of age-0 alewives in Lake Michigan: implications for habitat-specific recruitment success. Trans Am Fish Soc 136:1298–1312. doi:10.1577/T06-194.1
Houde ED (1969) Distribution of larval walleyes and yellow perch in a bay of Oneida Lake and its relation to water currents and zooplankton. N Y Fish Game 16:185–205
Humston R, Olson DB, Ault JS (2004) Behavioral assumptions in models of fish movement and their influence on population dynamics. Trans Am Fish Soc 133:1204–1328. doi:10.1577/T03-040.1
Hurley DA (1972) Observations on incubating walleye eggs. Prog Fish-Cult 34:49–54. doi:10.1577/1548-8640(1972)34[49:OOIWE]2.0.CO;2
Jensen AL (1998) Simulation of relations among fish life history parameters with a bioenergetics-based population model. Can J Fish Aquat Sci 55:353–357. doi:10.1139/cjfas-55-2-353
Jones JR, Hoyer MV (1982) Sportfish harvest predicted by summer chlorophyll-a concentration in midwestern lakes and reservoirs. Trans Am Fish Soc 111:176–179. doi:10.1577/1548-8659(1982)111<176:SHPBSC>2.0.CO;2
Jones ML, Netto JK, Stockwell JD, Mion JB (2003) Does the value of newly accessible spawning habitat for walleye (Stizostedion vitreum) depend on its location relative to nursery habitats? Can J Fish Aquat Sci 60:1527–1538. doi:10.1139/f03-130
Juanes F, Letcher BH, Gries G (2000) Ecology of stream fish: insights gained from an individual-based approach to juvenile Atlantic salmon. Ecol Freshw Fish 9:65–73. doi:10.1034/j.1600-0633.2000.90107.x
Kelso JRM (ed) (1996) Proceedings of a workshop on the science and management for habitat conservation and restoration strategies (HabCARES) in the Great Lakes. Can J Fish Aquat Sci 53 (Supplement 1)
Kitchell JF, Stewart DJ, Weininger D (1977) Applications of a bioenergetics model to yellow perch (Perca flavescens) and walleye (Stizostedion vitreum vitreum). J. Fish. Res. Bd. Can. 34:1922–1935
Kratzer JF, Hayes DB, Thompson BE (2006) Methods for interpolating stream width, depth, and current velocity. Ecol Modell 196:256–264. doi:10.1016/j.ecolmodel.2006.02.004
Lomnicki A (1999) Individual-based models and the individual-based approach to population ecology. Ecol Modell 115:191–198. doi:10.1016/S0304-3800(98)00192-6
Magnuson JJ, Crowder LB, Medvick PA (1979) Temperature as an ecological resource. Am Zool 19:331–343
Marschall EA, Crowder LB (1996) Assessing population responses to multiple anthropogenic effects: a case study with brook trout. Ecol Appl 6:152–167. doi:10.2307/2269561
Mason DM, Goyke AP, Brandt SB (1995) A spatially-explicit bioenergetics measure of habitat quality for adult salmonines: comparison between Lakes Michigan and Ontario. Can J Fish Aquat Sci 52:1572–1583. doi:10.1139/f95-750
McDermot D, Rose KA (2000) An individual-based model of lake communities: application to piscivore stocking in Lake Mendota. Ecol Modell 125:67–102. doi:10.1016/S0304-3800(99)00172-6
Miller TJ (2007) Contribution of individual-based coupled physical-biological models to understanding recruitment in marine fish populations. Mar Ecol Prog Ser 347:127–138. doi:10.3354/meps06973
Minns CK, Wichert GA (2005) A framework for defining fish habitat domains in Lake Ontario and its drainage. J Great Lakes Res 31:6–27
Minns CK, Randall RG, Moore JE, Cairns VW (1996) A model simulating the impact of habitat supply limits on northern pike, Esox lucius, in Hamilton Harbour, Lake Ontario. Can J Fish Aquat Sci 53(Suppl. 1):20–34. doi:10.1139/cjfas-53-S1-20
Mion JB, Stein RA, Marschall EA (1998) River discharge drives survival of larval walleye. Ecol Appl 8:88–103. doi:10.1890/1051-0761(1998)008[0088:RDDSOL]2.0.CO;2
Mittelbach G (1981) Foraging efficiency and body size: a study of optimal diet and habitat use by bluegills. Ecology 62:1370–1386. doi:10.2307/1937300
Netto J (2006) A model to link habitat supply to population dynamics for lake trout (Salvelinus namaycush) populations in western Lake Superior. M.S. thesis, Michigan State University, East Lansing, MI
Nibbelink NP, Carpenter SR (1998) Interlake variation in growth and size structure of bluegill (Lepomis macrochirus): inverse analysis of an individual-based model. Can J Fish Aquat Sci 55:387–396. doi:10.1139/cjfas-55-2-387
Oglesby RT (1977) Relationships of fish yield to lake phytoplankton standing crop, production and morphoedaphic factors. J Fish Res Bd Can 34:2271–2279
Olson FC (1950) The currents of western Lake Erie. PhD Dissertation, Ohio State University, Columbus, OH
Parker RL (1977) Understanding inverse theory. Annu Rev Earth Planet Sci 5:35–64. doi:10.1146/annurev.ea.05.050177.000343
Press WH, Teukolsky SA, Vetterling WT, Flannery BP (1992) Numerical recipes in C: the art of scientific computing, second edition edn. Cambridge University Press, New York
Quinlan JA, Blanton BO, Miller TJ, Werner FE (1999) From spawning grounds to the estuary: using linked individual-based and hydrodynamic models to interpret patterns and processes in the oceanic phase of Atlantic menhaden Brevoortia tyrannus life history. Fish Oceanogr 8(Suppl. 2):224–246. doi:10.1046/j.1365-2419.1999.00033.x
Railsback SF, Harvey BC (2002) Analysis of habitat-selection rules using an individual-based model. Ecology 83:1817–1830
Raleigh RF (1982) Habitat suitability index models: brook trout. U.S. Fish and Wildlife Service FWS/OBS-82/10.24
Robillard SR, Marsden JE (2001) Spawning substrate preferences of yellow perch along a sand-cobble shoreline in southwestern Lake Michigan. N Am J Fish Manag 21:208–215. doi:10.1577/1548-8675(2001)021<0208:SSPOYP>2.0.CO;2
Roloff GJ, Kernohan BJ (1999) Evaluating reliability of habitat suitability index models. Wildl Soc Bull 27:973–985
Rose KA (2000) Why are quantitative relationships between environmental quality and fish populations so elusive? Ecol Appl 10:367–385. doi:10.1890/1051-0761(2000)010[0367:WAQRBE]2.0.CO;2
Rose KA, Rutherford ES, McDermott D, Forney JL, Mills EL (1999) Individual-based models of yellow perch and walleye populations in Oneida Lake. Ecol Monogr 69:127–154
Roseman EF (2000) Physical and biological processes influencing walleye early life history in western Lake Erie. Ph.D. Dissertation, Michigan State University, East Lansing, MI
Roseman EF, Taylor WW, Hayes DB, Knight RL, Haas RC (2001) Removal of walleye eggs from reefs in western Lake Erie by a catastrophic storm. Trans Am Fish Soc 130:341–346. doi:10.1577/1548-8659(2001)130<0341:ROWEFR>2.0.CO;2
Ross J, Powles PM, Berrill M (1977) Thermal selection and related behavior in larval yellow perch (Perca flavescens). Can Field-Natur 91:406–410
Roussel J-M, Bardonnet A, Claude A (1999) Microhabitats of brown trout when feeding on drift and when resting in a lowland salmonid brook: effects on weighted usable area. Arch Hydrobiol 146:413–429
Royer L, Hamblin PF, Boyce FM (1987) A comparison of drogues, current meters, winds, and a vertical profiler in Lake Erie. J Gt Lakes Res 13:578–586
Ryder RA, Kerr SR, Loftus KH, Regier HA (1974) The morhpoedaphic index, a fish yield estimator—review and evaluation. J. Fish. Res. Bd. Can. 31:663–688
Saylor JH, Miller GS (1987) Studies of large-scale currents in Lake Erie, 1979–80. J Gt Lakes Res 13:487–514
Schindler DE, Scheuerell MD (2002) Habitat coupling in lake ecosystems. Oikos 98:177–189. doi:10.1034/j.1600-0706.2002.980201.x
Schwab DJ, Bennett JR (1987) Lagrangian comparison of objectively analyzed and dynamically modeled circulation patterns in Lake Erie. J Gt Lakes Res 13:515–529
Shuter BJ (1990) Population level indicators of stress. Am Fish Soc Symp 8:145–166
Shuter BJ, Jones ML, Korver RM, Lester NP (1998) A general, life history based model for regional management of fish stocks: the inland lake trout (Salvelinus namaycush) fisheries of Ontario. Can J Fish Aquat Sci 55:2161–2177. doi:10.1139/cjfas-55-9-2161
Stalnaker C, Lamb BL, Henriksen J, Bovee K, Bartholow J (1995) The instream flow incremental methodology. A primer for IFIM. Biological report no. 29. National Biological Service, Fort Collins 45 pp
Stoneman CL, Jones ML (2000) The influence of habitat features on the biomass and distribution of three species of southern Ontario stream Salmonids. Trans Am Fish Soc 129:639–657. doi:10.1577/1548-8659(2000)129<0639:TIOHFO>2.3.CO;2
Thompson BE (2004) Inverse modeling growth and movement of age-1 steelhead in the Pine River, Michigan. Ph.D. Dissertation, Michigan State University, East Lansing, MI, 137 p
Thorpe JE (1977) Morphology, physiology, behavior, and ecology of Perca fluviatilis L. and P. flavescens Mitchill. J Fish Res Board Can 34:1504–1514
Tyler JA, Hargrove WW (1997) Predicting spatial distribution of foragers over large resource landscapes: a modeling analysis of the ideal free distribution. Oikos 79:376–386. doi:10.2307/3546022
Vilizzi L, Copp GH, Roussel JM (2004) Assessing variation in suitability curves and electivity profiles in temporal studies of fish habitat use. River Res Appl 20:605–618. doi:10.1002/rra.767
Wagner CM, Austin HM (1999) Correspondence between environmental gradients and summer littoral fish assemblages in low salinity reaches of the Chesapeake Bay, USA. Mar Ecol Prog Ser 177:197–212. doi:10.3354/meps177197
Weber JJ, Les BL (1982) Spawning and early life history of yellow perch in the Lake Winnebago system. Technical bulletin no. 130. Department of Natural Resources, Madison
Wiley MJ, Kohler SL, Seelbach PW (1997) Reconciling landscape and local views of aquatic communities: lessons from Michigan trout streams. Freshw Biol 37:133–148. doi:10.1046/j.1365-2427.1997.00152.x
Williams JG, Speed TP, Forrest WF (1999) Comment: transferability of habitat suitability criteria. N Am J Fish Manag 19:623–625. doi:10.1577/1548-8675(1999)019<0623:CTOHSC>2.0.CO;2
Zhao Y, Jones ML, Shuter BJ, Roseman EF (2009) A biophysical model of Lake Erie walleye (Sander vitreus) explains inter-annual variations in recruitment. Can J Fish Aquat Sci (in press)
Acknowledgments
The authors thank the Great Lakes Fishery Commission for providing principal support for this project, and extend their thanks to the Michigan Department of Natural Resources, the Department of Fisheries and Oceans, and the Ontario Ministry of Natural Resources for supplemental funding for this research. The authors also thank the Great Lakes Fishery Commission’s Board of Technical Experts for their constructive comments on this project since its inception. This is contribution number 2009-05 of the Quantitative Fisheries Center at Michigan State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayes, D., Jones, M., Lester, N. et al. Linking fish population dynamics to habitat conditions: insights from the application of a process-oriented approach to several Great Lakes species. Rev Fish Biol Fisheries 19, 295–312 (2009). https://doi.org/10.1007/s11160-009-9103-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-009-9103-8