Abstract
Environmental management is crucial for sustainable growth and development. The use of microorganisms to clean up contaminated environment provides cheap alternative method to the conventional treatment methods. But the choice of easily grown, viable and effective natural occurring microorganism to do the cleaning is a major challenge. In this article we presented and reviewed the application of photosynthetic bacteria in bioremediation due to their utilisation of various kinds of pollutants, minimum nutrients requirement and the possibility of generating valuable products concomitantly cleaning the contaminated environment. Pollutants such as pesticides, heavy metals, dyes, crude oil and odour with the specific photosynthetic bacteria capable of degrading the pollutants were identified and discussed in this article. The possible value added products to be generated as well as the mechanism of degradation are also highlighted and discussed in the article. The utilisation of carbon dioxide and the generation of value added products while cleaning up polluted environment are the major advantages of using these bacteria in bioremediation and have both environmental and economic benefits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Increase in human activities such as in agriculture, industries, urbanisation and population produce pollutants that are harmful to the environment. Industries like biorefineries are constantly faced with the challenge of finding an economical and efficient treatment process before discharging their effluent (ElMekawy et al. 2013). This effluent may include heavy metals, xenobiotic compounds, phenolic compounds, nitroaromatic compounds, volatile organic compounds, polycyclic aromatic compounds, polychlorinated biphenyls and pesticides. When these pollutants find their way into the environment, they do not only affect the environment but also the life of living organisms in the environment. For example, a study conducted by Rawson (1985) revealed that prolong exposure to multiple pesticides has a great effect on both kidney and liver. Heavy metals and some volatile organic compounds are non-degradable and are very difficult to remove from the environment as such they pose a great health risk (Radway et al. 1999). Chemical and biological treatment methods are the main techniques employed in removing such pollutants from the environment. However, increased in cost and sludge production are among the drawbacks of chemical treatment (Nawaz and Ahsan 2014). Moreover, chemical treatment were found to promote filamentous algae growth that may clog water treatment plant filters and result in reduced backwashing, thus reducing the efficiency of chemical treatment (Akpor and Muchie 2010). Biological method therefore offers greater advantages in that it can be done on site, so eliminate liabilities and transportation cost (Juwarkar et al. 2010) and can also be incorporated with other treatment methods, thereby treating complex and mixed waste (Goel et al. 2010; McMahon et al. 2008; Yergeau et al. 2009). The efficiency of this method can be improved by the choice of microorganism with the potential of removing complex contaminants.
One of such groups of potential microorganisms is the photosynthetic bacteria. Photosynthetic bacteria are prokaryotes that are capable of carrying out photosynthesis. They are widely distributed occupying several habitats like soil, lakes, paddy fields, oceans, rivers, and activated sludge (Koblížek et al. 2006; Okubo et al. 2006). They are broadly categorised into two major groups; (1) oxygenic, which are able to produce oxygen (O2) during photosynthesis due to their ability to use water as electron donor such as cyanobacteria and prochlorophytes; (2) anoxygenic, which are unable to produce O2 during photosynthesis due to their inability to use water as electron donor. Instead, they are capable of assimilating nitrogen, carbon dioxide (CO2) and other organic or inorganic compounds such as Rhodobacter and Rhodopseudomonas. The main classification of photosynthetic bacteria is represented in Fig. 1.
Photosynthetic bacteria offer various advantages in bioremediation due to their ability of utilising different kinds of organic substances as a substrates resulting in high growth rate. Some species are involved in denitrification process; they also contained various important substances like ubiquinone, carotenoids and vitamin B12 in their cell. The major advantages and disadvantages of using photosynthetic bacteria in bioremediation are highlighted in Table 1 below.
2 Application of oxygenic photosynthetic bacteria (cyanobacteria) in bioremediation
Cyanobacteria are classified under the kingdom eubacteria and the division cyanophyta. They are gram-negative, planktonic and non-motile organisms. They are capable of consuming CO2, a greenhouse gas which contributes to global warming and release O2 which is vital for cellular respiration. The key enzyme in CO2 utilisation is carbonic anhydrase (CA) and the mechanism involved in cyanobacterial CO2 metabolism is described by Badger and Price (2003) in Fig. 2.
General mechanism for cyanobacterial CO2 metabolism describing the roles of carbonic anhydrase and Rubisco in CO2 uptake. Adapted from (Badger and Price 2003)
Their ability to use inorganic acid and phosphorus makes them grow well in wastewater. This indicates their potential ability in wastewater treatment. In addition, they possess certain characteristics that make them suitable for the treatment process (Subramaniyan 2012a), these are: (1) The ability to be cultivated easily with minimal nutrient requirement of energy rich compounds. (2) The ability to grow where sunlight and moisture are available as such they produce high amount of biomass that can be used as a source of food for animals. (3) Some species have the ability of combining nitrogen fixation and photosynthesis. (4) They can also be easily separated from other biomass because of their size and cause no harm to other biomass therefore environmentally friendly. Because of their fast nutrients uptake and storage ability, they mostly dominate algal community forming a mat of cells called “bloom”. Today the approach to utilise cyanobacteria in bioremediation has been widely broadened to include wastewater treatment, heavy metal removal, crude oil, pesticides, and dye degradations as discussed below.
-
(a)
Wastewater treatment
Groundwater is the major source of drinking water in most part of the world. However due to the human activities this water is highly contaminated with different kinds of pollutant. For example livestock farming and application of fertiliser increase the amount of nitrate concentration to a level that is harmful to living organisms. Since cyanobacteria require nitrogen for metabolism, the removal of nitrate by these organisms is usually very effective. Three different cyanobacterial species were used for the removal of nitrate from groundwater by Hu et al. (2000). All the species effectively removed nitrate but Synechococcus sp. strain PCC7942 showed the highest removal compared to strain PCC6803 and Synechocystis minima CCAP148014. The strain Phormodium tenue and Phormodium bohneri were also found to effectively removed nitrogen and phosphorus (Chevalier et al. 2000).
Furthermore, mixtures of industrial and domestic wastewater were also treated with the cyanobacteria strains, Anabaena variabilis, Anabaena oryzae and Tolypothrix ceytonica for a week in a batch system (El-Bestawy 2008). For removal of organic matter, the highest removal efficiency of BOD5 and COD were 89.29 and 73.68 % recorded by A. variabilis and A. oryzae respectively. For solid removal, the highest removal efficiency for TSS and TDS were 64.37 and 38.84 % recorded by T. ceytonica and A. variabilis respectively. For heavy metal removal, the highest removal efficiency was recorded by T. certonic for both Zn and Cu at 86.12 and 94.63 % respectively. T. certonica also has the highest removal efficiency of fats, oil and grease of 93.75 %. These results indicate that the cyanobacteria are very good candidates in treatment of the wastewater.
One of the major problems facing cyanobacterial wastewater treatment is the separation of the cyanobacteria from the wastewater. The use of immobilised technique does not only solve this problem but also shown to have higher pollutants uptake than using free cells. The cyanobacteria species Chlorella vulgaris and Anabaena doliolum were used in comparison between free cells and alginate immobilised cells in heavy metal removal. The immobilised cells showed higher Cu and Fe removal compared to the free cell in all the two species (Rai and Mallick 1992). Another cyanobacteria species Nostoc calcicola was also used in the uptake of Cu. Under the same environmental condition, the immobilised cells showed higher uptake of Cu (up to two and half time) more than the free cell. The immobilised cells showed higher positive correlation between copper adsorption and uptake (Singh et al. 1989). Immobilised cells also offer easy separation and regeneration of cells, high loading and reuse of biomass as well as minimal clogging (Prasanna et al. 2008).
-
(b)
Heavy metal removal
Unlike organic pollutants, heavy metals are resistance to biodegradation into less toxic or non-toxic substances by microorganisms due to their chemical characteristics. They accumulate in the food chain usually by changing from one chemical state to another. Due to their ability to utilise most heavy metals, Fiore and Trevors (1994) conclude that cyanobacteria are relatively more tolerant to heavy metals compared to other photoautotrophs. Several studies proved this assertion. The cyanobacterial culture of Anabaena subcylindrica and Nostoc muscorum were found to removed copper, cobalt manganese and lead from sewage wastewater. When sterilised sewage wastewater and the standard medium were compared, the protein, carotenoids and chlorophyll a contents of the former was higher than the later. Single cell cultures were also found to be more effective in these metals removal than mixed culture of these cyanobacteria which was attributed to competition of nutrients in mixed culture (El-Sheekh et al. 2005). When exopolysaccharides producing cyanobacteria, Nostoc PCC 7936 and Cyanospira capsulate were used in removal of Copper(II), the species C. capsulate removed higher amount of copper compared to Nostoc PCC 7936. In single and multi-metal systems, both species removed Ni(II) and Zn(II) with Cu (De Philippis et al. 2007).
Generally, microorganism developed several mechanisms to response to the toxicity effect of heavy metals (Ibrahim et al. 2001). As such, ion exchange was suggested as the mechanism for removal of zinc and copper using Oscillatoria anguistissima because of the liberation of magnesium ion that accompanied the metal uptake (Ahuja et al. 1999). Similar suggestion was made in the uptake of Cr, Cd, Cu, Pb and Ni by Greene et al. (1987). The uptake of the above mentioned metals was accompanied by the release proton using Spirulina plantensis. But when Spirulina maxima and Tetraselmis chuii were used in cadmium uptake, the two cyanobacteria showed different mechanisms of cadmium uptake. Sorption of cadmium by T. chuii was mainly restricted on the surface of the cyanobacterium while accumulation of cadmium by S. maxima was found on different surface layers of the cyanobacterium (da Costa and de Franca 2003). Several other mechanisms depending on the species and the types of pollutant were also suggested. For metal uptake, the mechanisms used according to Pandi et al. (2009) are bioaccumulation and bioadsorption or both while in some species bioflocculation is the main mechanism of metal uptake. In bioaccumulation, the process requires energy and involves the concentration and retention of metal ion by cyanobacteria. During the retention time, the metal ion diffuses into the cell through the cell membrane, forming immobilised metal ion by ion-binding process. Bioadsorption is a passive process which requires no energy. It may involve ion-exchange, chelating, microprecipitation and physical adsorption followed by chemical bondage. The metal ions bind to the cell surface which has high chemical affinity for the metal. Bioflocculation may occur in some species of cyanobacteria because they produced substances like protein, polyhydroxylbutrate and lipopolysaccharides. Bioflocculation occurs when the cationic side of organic matter electrostatically forms a complex with the reactive group of the produced substances such as half ester sulphate (OSO−3) or carboxyl group (COO−).
-
(c)
Crude oil degradation
Oil spill cause serious environmental pollution that makes the soil unsuitable for plant growth and water bodies difficult for the survival of aquatic organisms. Furthermore, due to health related problems associated with oil spill, studies are directed toward finding a suitable solution to this problem. Bioremediation offers a better solution to treat oil spills due to its cost effective nature. Cyanobacteria are suitable candidates in bioremediation of crude oil due to their ability of growing under various conditions and forming bloom with other organisms. Naphthalene, a main constituent of water-soluble part of crude oil was reported to have been degraded by the cyanobacteria Oscillatoria sp. strain JCM (Narro et al. 1992). The cyanobacteria species Oscillatoria salina, Aphanocapsu sp. and Plectonema terenbans were also found to degrade crude oil (Raghukumar et al. 2001). Within 10 days 50–65 % of pure hexadecane, 45–55 % of total crude oil fractions and 20–90 % of aromatic compound were removed. After forming a cyanobacterial mat by the mixed culture of the three cyanobacterial species about 40 % of the crude oil was removed. Phormidium corium and Microleus chthonoplastes species of cyanobacteria also degraded crude oil by consuming the individual n-alkanes (Al Hasan et al. 1994). When the growth were measured in terms of biliprotein content and dry biomass, the two species grew better in crude oil suggesting the utilisation of these hydrocarbon.
Contrary to the above studies, other investigations revealed that cyanobacteria are not directly involve in the degradation of petroleum compound but rather play a major role by enhancing the activity and growth of the actual degraders. For example Abed and Köster (2005) concluded that the aerobic heterotrophic bacteria associated with the cyanobacteria were responsible for the degradation of oil compounds and not the cyanobacteria. Oil degrading consortium was also analysed using 16S rRNA (Sánchez et al. 2005). The organisms obtained were found to be related to agrobacterium and rhizobium nitrogen fixing bacteria. The result indicates that the heterotrophic bacteria associated with cyanobacteria are the ones responsible for degradation of the hydrocarbon while the cyanobacteria provide oxygen, organic matter and habitat for the heterotrophic bacteria. The same conclusion was made by Radwan et al. (2002) and Sánchez et al. (2005). While the actual degraders degrade the hydrocarbon, the cyanobacteria provide fixed nitrogen and simple organic compound and oxygen for the breakdown of aromatic rings (Abed and Köster 2005) which are also necessary for the growth and the activity of the actual degraders. The evidences for degrading and not degrading hydrocarbon by cyanobacteria are tentative, however Radwan and Al-Hasan (2002) concluded that some species/strain are capable of utilising aliphatic and aromatic compounds while others that occur together with other natural hydrocarbon degrading bacteria forming cyanobacterial mat only enhance the growth and activities of the degrading bacteria. The association between the two appeared to be significant and crucial in remediation of crude oil.
-
(d)
Pesticides degradation
In effort to enhance food production to meet the growing human population, the use of pesticide in agriculture is on increased nowadays. The use of this pesticide released toxic substances that are hazardous to man. Pesticides can also bioaccumulate or biomagnify and cause danger to the environment (Ragini and Bisen 2011). Several cyanbacterial species were revealed to be capable of reducing the toxicity of this hazardous substance. Lindane, a highly chlorinated aliphatic pesticide was found to be degraded by two filamentous cyanobacteria Anabaena sp. PCC 7120 and Nostoc ellipsosporum strain B1453-7 (Kuritz and Wolk 1995). The same species was also found by Kuritz (1998) to degrade the same pollutant, first to pentachlorocyclohexane and then to trichlorobenzenes. Because the degradation occurred in the presence of nitrate and the process is inhibited by both darkness and ammonia, the mechanism of degradation was proposed to be nitrate-reduction system of cyanobacteria. Fenamiphos, an organophosphorus pesticide was also found to be degraded by five different cyanobacterial species (Cáceres et al. 2008). These species are: Anabaena sp., Nostoc muscorum, Nostoc sp. MMI, Nostoc sp. MM2 and Nostoc sp. MM3. The degradation of this pollutant was found to be through hydrolysis and oxidation. The products of oxidation were toxic while hydrolysis was referred to be detoxification.
Pesticide degradation process involves three phases. Phase I involves hydrolysis, oxidation or reduction. This phase converts the pesticides into less toxic and water soluble substances. Phase II involves the binding of pesticides metabolites or pesticides to the sugar, glutathione or amino acids. Toxicity is reduced and solubility is increased in this phase compared to Phase I. Phase III involves the conversion of metabolites of phase II to form non-toxic substances (Hatzios 1991; Shimabukuro 1985). Because of the complexity and toxicity of pesticides, organisms must possess certain features to be able to degrade them to non-toxic substances. According to Ragini and Bisen (2011) organisms can possess the ability to degrade pesticide via adaptive enzymes that can be induced in the presence of pesticides. The constitutive enzymes may be induced by the adapted organisms in the polluted environment before degrading pesticide via random mutation.
-
(e)
Dye degradation
Textile industries release large amount of dye which find their way into water bodies and cause serious environmental problems. They consist of many substances that are highly toxic to aquatic organisms (Mondal and Chauhan 2012). They also limit light penetration in the water body thus reduce the photosynthetic activity and cause a serious health and environmental problems (Forgacs et al. 2004). The cyanobacterium Oscillatoria formosa NTDM 02 was found to be very effective in removal of dye from textile industry (Ali et al. 2011). Oscillatoria brevis and Westiellopsis prolific were also found to be effective in the treatment of dye (Subramaniyan 2012b). Apart from organic and inorganic chemical removal, the colour intensity of the effluent was also reduced. More than 75 % of the colour and 95 % of both COD and BOD were removed within 30 days. The result also showed an increase in DO and complete removal of ammonia, nitrates and phosphate. Another cyanobacterium, P. tenue KDM33 has also shown to be an effective candidate for colour removal in paper mill effluent. Using this bacterium decrease in physiochemical parameters such as BOD, COD, salinity, alkalinity, calcium, magnesium, inorganic sulphite and increase in DO, electricity conductivity and pH were observed after 20 days of incubation (Nagasathya and Thajuddin 2008).
Many different mechanisms have been proposed in dye degradation using cyanobacteria. Adsorption which results in physical removal of dyes is a rapid process whereas enzyme mediated degradation results in slower degradation is the two major mechanisms for dye degradation using cyanobacteria (Shah et al. 2001). Generally, longer time is needed for the degradation process via enzymatic reactions which may result in eventual mineralisation of the dye molecules. When Shah et al. (2001) used the cyanobacterium Phormidium valderianum more than 90 % of dye was removed instantly indicating adsorption as the mechanisms for dye removal using this bacterium. Different mechanism was postulated when Oscillatoria boryana BDU 92181 was used in melanoidin treatment. After about 65 % decolourization of pure melanoidin pigment and 60 % of crude pigment, Kalavathi et al. (2001) postulated that the mechanism of colour removal was due to the production of molecular oxygen, hydroxyl anion and hydrogen peroxide during cyanobacterial photosynthesis.
3 Application of anoxygenic photosynthetic bacteria in bioremediation
Anoxygenic photosynthetic, example purple phototrophic bacteria (PPB) are gram-negative bacteria that possessed versatile metabolic activities such as photoheterotrophic and photoautotrophic growth under aerobic-dark and anaerobic-light conditions (Holt 1994). Because of this diverse metabolic activities they are widely distributed occurring in various habitats and can be grown in both natural and synthetic substrate. The diversity in metabolic activities and the production of valuable products such as enzymes, proteins, antiviral (Hirotani et al. 1991) are the major advantages of this group of bacteria in biotechnology. The two most popular groups of anoxygenic photosynthetic bacteria are: purple sulphur bacteria (PSB) and purple non sulphur bacteria (PNSB). PSB tolerate small amount of sulphide, oxidised it to sulphur and store intracellularly. They are strongly photoautotrophs with limited capability to photoheterotrophy and poorly grown in dark. In contrast, PNSB may tolerate much smaller amount of sulphide and store extracellularly. They are photoheterotroph capable of photoautotrophic nutrition and can be grown effectively in the dark (Madigan and Jung 2008).
-
(a)
Wastewater treatment
Wastewater consists of complex organic and inorganic pollutants that are discharged into the water bodies from different sources. For example distillery wastewater is considered as one of most contaminated effluent (Pant and Adholeya 2010). It has been shown to contain high amount of organic and solid content as well as dark brown colouration (Pant and Adholeya 2009). Most of these pollutants are harmful to living organisms; they increase the susceptibility to disease and contaminate the groundwater as well as the whole ecosystem. Because of their aquatic and versatile metabolic nature, PPB has high efficiency in wastewater treatment. The PPB, Rhodobacter sphaeroides Z08 was applied during pharmaceutical wastewater treatment. After 1:4 dilutions, reduction of 50 % in COD was observed, biomass yield of 780 mg/L and specific growth of 0.015/h were observed (Madukasi et al. 2011). Using the same R. sphaeroides Z08 by the same authors, soybeans wastewater was treated. The results indicate reduction in 80 % COD, 0.025/h specific growth rate and 1,540 mg/L of biomass yield. When Rhodovulum sulfidophilium was used in the treatment of sardine processing wastewater, the COD removal was 65 % after 96 h. Increased in total biomass yield, total carotenoids and soluble protein was also observed (Azad et al. 2001). R. sphaeroides IL106 also removed phosphorus compound from an Oyster farm under anaerobic condition (Takeno et al. 1999). 10 and 82 % of the phosphorus and COD were respectively removed after 7 days of incubation. The immobilisation of R. sphaeroides S, R. sphaeroides NR-3 and Rhodopseudomonas palustris using porous ceramic simultaneously removed COD, nitrate, phosphate and H2S from synthetic sewage wastewater. The result obtained indicates 89 % COD removal, 77 % phosphate removal, 99 % nitrate removal and 99.8 % H2S removal after 48 h in batch treatment (Nagadomi et al. 2000). The strain of Rubrivivas gelatinous designated as SS51 and SY40 degraded the organic pollutants from latex processing wastewater. The COD removal efficiency revealed to be up to 57 % (Choorit et al. 2002).
The most significant parameter used in determining the amount of pollutants in wastewater is the oxygen demand. This is measured in terms of COD, BOD and TOC. BOD and COD measure oxygen demand directly while TOC indirectly measures oxygen demand (Boyles 1997). Reduction of these parameters is used to determine the effectiveness of the remediation process. BOD measures the amount of dissolved oxygen needed for oxidation by microorganisms and its level in water is proportional to the concentration of organic matter in the water. High amount of BOD results in severe depletion of dissolved oxygen and cause problems to some aquatic organisms (Penn et al. 2006). COD measures oxygen demand by determining the amount of oxygen required for oxidation of organic matter to carbon dioxide. This method indirectly measures the amount of organic matter in water. Its measurement reflects the level of pollution by reductive substance in water (Huang et al. 2013). TOC measures oxygen demand by determining the amount of carbon dioxide produced in water (Boyles 1997).
-
(b)
Heavy metal removal
Industrial and human activities increase the release of heavy metals into the environment. These metals are non-degradable and tend to bioaccumulate eventually causing health related problems. PNSB are group of microorganisms that are known to remove such pollutants. The strains R. sphaeroides KMS24 and Rhodobium marium NW16 were found to remove heavy metals in contaminated shrimp pond under aerobic-dark and microaerobic-light conditions (Panwichian et al. 2011). The metals removal was in the order of Cu2+> Zn2+>Cd2+. The two strains produced exopolymeric substances which played a significant role in the accumulation of these metals and they grew better in medium containing higher heavy metals concentration than that of contaminated shrimp pond. Under dark aerobiosis condition, the organophosphorus compound tributyl phosphate (TBP) was degraded by R. palustris from 2 to 0.6 mM within 3 weeks (Berne et al. 2005). R. capsulatus also absorb the metal Trivalen Aurium (Feng et al. 2007). In separate study, Rhodovulum sp. PS88 and R. spaheroides S were also found to remove the metal cadmium in batch culture system. Under both heterotrophic (aerobic-dark) and photoheterotrophic (anaerobic-light), the strain PS88 showed higher empirical constant value and hence higher cadmium uptake compared to R. sphaeroide S (Watanabe et al. 2003).
The major mechanisms used by PPB for the removal of metal ions are biosorption and bioaccumulation process. The difference between the two is in terms of energy requirement. The former does not require energy while the later require energy. Some PPB may use more than one mechanism for the removal of metal ions and hence are likely to be more effective when compared to the ones that use only one mechanism. The type of mechanism to be used by the organism is dependent on the properties of the metal ion that it is exposed to. For example, when Saijai et al. (2010) used PPB strain NW16 and KMS24 in bioremediation of heavy metal and sodium from contaminated shrimp pond, both strains were found to remove metal ions but strain NW16 has higher percentage of removal than strain KMS24. Under the same incubating condition, both strains showed differences in the removal efficiency that were not related to their initial concentration in the order Pb > Cu > Zn > Cd which suggested different mechanisms of removal.
-
(c)
Pesticides degradation
Pesticide involves large number of chemicals most of which are ubiquitous and toxic to the environment. Its constituent mostly involves the derivatives of benzene consisting of negative resonance energy and stable derivatives group of compounds. Many groups of PPB are capable of utilising this aromatic compound as a sole source of carbon for metabolism and require no energy from its catabolism unlike heterotrophic organisms. These features make them unique and suitable in pesticide degradation. PPB degrades most of these toxic substances under light and anaerobic conditions (Sikdar and Irvine 1998; van der Woude et al. 1994). A mixed phototrophic bacterial culture in which R. palustris WS17 is the dominant strain was found to completely degrade 3-chlorobenzoate to CO2 and biomass in the presence of light and benzoate and in the absence of oxygen (Kamal and Wyndham 1990). The same pollutant was degraded by different strain, R. palustris DCP3 under phototrophic and low oxygen condition (Krooneman et al. 1999). The degradation followed the dechlorination pathway in which 3-chlorobenzoate was dechlorinated to benzoate. In another study three species of PNSB, Rhodospirullum photometrican, R. palustris and Rhodospirullum rubum were found to grow on two and three carbon halocarboxylic acid in the presence of CO2 (McGrath and Harfoot 1997). The process occurred by reductive dehalogenation and subsequent assimilation of the resulting acids. The utilisation of halocarboxylic acid by these organisms indicates their ability to degrade such pollutant.
The degradation of these compounds is dependent on the catabolic ability of the bacteria, the chemical structure of pesticides, physiochemical conditions of the environment in addition to anaerobic and light conditions. Anaerobic condition provides favourable environment for the proliferation of these bacteria and it is beneficial in areas where there is limited oxygen. The light energy is transformed through photosynthetic membrane into electrochemical gradient of proton and use in generation of ATP, motility, active transport and other processes that require energy (Kushalatha 2010).
-
(d)
Odour removal
In removing odour, the identification of compound responsible for the emission of the odour is very important. In most cases hydrogen sulphite and volatile organic acids are the main compounds responsible for odour emission. In the case of volatile organic acid the PNSB are mostly apply due to their ability to utilised different organic acids as a substrate while PSB are well known in reducing odour resulting from hydrogen sulphite due to their ability to effectively use sulphur. When Byler et al. (2004) compared the emission of odour, H2S, and NH3 from non-phototrophic and phototrophic swine lagoons, higher emission of these gases were found in non-phototrophic swine lagoon than phototrophic one. This was attributed to the presence of phototrophic organisms like PSB which normally have high affinity in utilising H2S during its metabolism and is highly connected to odour. In the same view, eight swine lagoons were also studied base on their colour appearance purple and non-purple. The purple colour lagoon is expected to emit lower odour due to the presence of phototrophic bacteria. When characteristics of the two groups of lagoons were analysed, bacteriochorophyll a was found to be more abundant in purple lagoon which indicates the presence of phototrophic organisms. Temperature and other conditions in the purple lagoon were favourable to phototrophism (Chen et al. 2003). In separate study, PNSB R. palustris was found to remove odorous organic compounds when cultured in odorous swine wastewater for seven days. COD and phosphate were also effectively removed (Kim et al. 2004). Rhodobacter sp. strain PS9 was also found to grow on different organic compounds including VOC (Do et al. 2003). Decrease in VOC concentrations of 80–93 % was observed during the photosynthetic bloom, which indicates the strain ability to degrade VOA associated with odour. The result further revealed that population changes which leads to the reduction of VOA in the swine lagoon occur naturally. Maintenance of temperature above 20 °C and organic loads of less than 4 g (dry weight) per litre were suggested by the researchers as a strategy for increasing the photosynthetic bloom.
-
(e)
Dye degradation
Textile wastewater is considered as one of the most polluted wastewater that increased colour, COD and toxicity of wastewater due to large quantity of chemical and dye (Ibrahim et al. 2010). In degrading or removal of these dye, investigating the microorganisms that possess the azoreductase enzyme is essential in developing a practical bioprocess for degradation or decolourisation of azo dye. The azoreductase is the necessary enzyme responsible for catalysing the reductive cleavage of azo bond making it less or non-toxic. The PNSB, R. palustris ASI.2353 has been shown by Liu et al. (2006) to possess the enzyme azoreductase which is responsible for decolourisation of azo dye. Anaerobic condition was found to be necessary for the decolourisation of this dye and the optimum temperature and pH were 30–35 °C and 8 respectively. Another strain R. palustris 51ATA effectively mineralised and degraded reactive red dye (RR195) under anaerobic condition. The result shows that bacterial growth, decolourisation and degradation were increased in the presence of this dye and co-substrate (Çelik et al. 2012). In a separate study, five different species of PNSB were also found to be capable of decolourising azo dye. These species are; R. blasticus, R. adriaticus, Rhodopseudomonas capsulatus, R. palustris and Rhodovulum strictrum. About 96 % of dye were decolourised by these bacteria within 2 days under anaerobic and light conditions (Tran et al. 2012). The study further showed that mixed culture of these bacteria was more effective in decolourisation than pure culture. The degradation of azo dye by the above mentioned bacteria indicates the possession of azo reductase enzyme by these bacteria. The reductase enzyme is the common enzyme reported to be responsible for dye decolourisation but in some cases oxidase enzyme has also been reported. Goszczynski et al. (1994) and Molina-Guijarro et al. (2010) reported the role of oxidase in the degradation of dye. Also reported are laccase in decolourisation of remazol brilliant blue (Niladevi and Prema 2008) and phenol oxidase combined with NADH–DCIP reductase in the decolourisation of reactive dye (Telke et al. 2009).
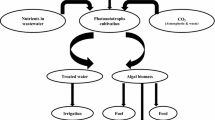
4 Generation of value added products during bioremediation with photosynthetic bacteria
As stated earlier, photosynthetic bacteria have the potential of degrading pollutant yielding a product of high value. Most of these products can be used as food supplement for animals. They produced biomass rich in carotenoids, vitamin, protein that can be used as animal feed (Carlozzi and Sacchi 2001; Ponsano et al. 2003). This biomass can be obtained by drying and cell breakage for easy digestion (Benemann 2013). Some species possess hydrogenase enzyme as such they are good candidates for biohydrogen production. The Table 2 below gives some valuable products, the pollutants, and the species involved during bioremediation.
5 Conclusion
Human activities have resulted in the released of various pollutants that have negative impacts on the environment. Bioremediation as a green process offers a better solution to the conventional method in treatment of such pollutants. Most of these pollutants can be effectively remove from the environment by the use of photosynthetic bacteria. The use of photosynthetic bacteria in the process further reduces the environmental impact due to their ability to use CO2. While bioremediation using some microorganisms produces substances that sometimes are more toxic than the original pollutants, photosynthetic bacteria mostly produce highly valuable substances. The application of this group of bacteria is therefore much more economical compared to the use of other groups of microorganism. Hence for economic and environmental benefits, this group of bacteria is highly recommended as an alternative to other groups of bacteria in bioremediation.
References
Abed R, Köster J (2005) The direct role of aerobic heterotrophic bacteria associated with cyanobacteria in the degradation of oil compounds. Int Biodeterior Biodegrad 55(1):29–37. doi:10.1016/j.ibiod.2004.07.001
Ahuja P, Gupta R, Saxena RK (1999) Zn2+ biosorption by Oscillatoria anguistissima. Process Biochem 34:77–85. doi:10.1016/S0032-9592(98)00072-7
Akpor O, Muchie M (2010) Bioremediation of polluted wastewater influent: phosphorus and nitrogen removal. Sci Res Essays 5:3222–3230
Al Hasan R, Sorkhoh N, Al Bader D, Radwan S (1994) Utilization of hydrocarbons by cyanobacteria from microbial mats on oily coasts of the Gulf. Appl Microbiol Biotechnol 41(5):615–619. doi:10.1007/BF00178499
Ali DM, Suresh A, Kumar RP, Gunasekaran M, Thajuddin N (2011) Efficiency of textile dye decolorization by marine cyanobacterium, Oscillatoria formosa NTDM02. Afr J Basic Appl Sci 3(1):09–13
Azad S, Vikineswary S, Ramachandran K, Chong V (2001) Growth and production of biomass of Rhodovulum sulfidophilum in sardine processing wastewater. Lett Appl Microbiol 33:264–268. doi:10.1046/j.1472-765X.2001.00993.x
Azad S, Vikineswary S, Chong V, Ramachandran K (2004) Rhodovulum sulfidophilum in the treatment and utilization of sardine processing wastewater. Lett Appl Microbiol 38:13–18
Badger MR, Price GD (2003) CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J Exp Bot 54:609–622
Bartram J, Chorus I (1999) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. Taylor & Francis, London
Benemann J (2013) Microalgae for biofuels and animal feeds. Energies 6:5869–5886. doi:10.3390/en6115869
Berne C, Allainmat B, Garcia D (2005) Tributyl phosphate degradation by Rhodopseudomonas palustris and other photosynthetic bacteria. Biotechnol Lett 27:561–566. doi:10.1007/s10529-005-2882-7
Boyles W (1997) The science of chemical oxygen demand. Hach Lange, Colorado
Burgess JG, Kawaguchi R, Yamada A, Matsunaga T (1994) Rhodobacter marinus sp. nov.: a new marine hydrogen producing photosynthetic bacterium which is sensitive to oxygen and sulphide. Microbiology 140:965–970
Byler J, Schulte DD, Koelsch RK (2004) Odor, H2S and NH3 emissions from phototrophic and non-phototrophic anaerobic swine lagoons. In: Conference presentations and white papers. Biological Systems Engineering, p 18
Cáceres T, Megharaj M, Naidu R (2008) Biodegradation of the pesticide fenamiphos by ten different species of green algae and cyanobacteria. Curr Microbiol 57:643–646. doi:10.1007/s00284-008-9293-7
Carlozzi P, Sacchi A (2001) Biomass production and studies on Rhodopseudomonas palustris grown in an outdoor, temperature controlled, underwater tubular photobioreactor. J Biotechnol 88:239–249. doi:10.1016/S0168-1656(01)00280-2
Çelik L, Öztürk A, Abdullah MI (2012) Biodegradation of reactive red 195 azo dye by the bacterium Rhodopseudomonas palustris 51ATA. Afr J Microbiol Res 6(1):120–126. doi:10.5897/AJMR11.1059
Chen T, Schulte DD, Koelsch RK, Parkhurst AM (2003) Characteristics of phototrophic and non-phototrophic lagoons for swine manure. Biological Systems Engineering, Papers and Publications, p 11
Chevalier P, Proulx D, Lessard P, Vincent W, De la Noüe J (2000) Nitrogen and phosphorus removal by high latitude mat-forming cyanobacteria for potential use in tertiary wastewater treatment. J Appl Phycol 12(2):105–112. doi:10.1023/A:1008168128654
Choorit W, Thanakoset P, Thongpradistha J, Sasaki K, Noparatnaraporn N (2002) Identification and cultivation of photosynthetic bacteria in wastewater from a concentrated latex processing factory. Biotechnol Lett 24(13):1055–1058. doi:10.1023/A:1016026412361
da Costa ACA, de Franca FP (2003) Cadmium interaction with microalgal cells, cyanobacterial cells, and seaweeds; toxicology and biotechnological potential for wastewater treatment. Mar Biotechnol 5(2):149–156. doi:10.1007/s10126-002-0109-7
de Lima LKF, Ponsano EHG, Pinto MF (2011) Cultivation of Rubrivivax gelatinosus in fish industry effluent for depollution and biomass production. World J Microbiol Biotechnol 27:2553–2558
De Philippis R, Paperi R, Sili C (2007) Heavy metal sorption by released polysaccharides and whole cultures of two exopolysaccharide-producing cyanobacteria. Biodegradation 18(2):181–187. doi:10.1007/s10532-006-9053-y
Deng X, Jia P (2011) Construction and characterization of a photosynthetic bacterium genetically engineered for Hg2+ uptake. Bioresour Technol 102:3083–3088
Do YS, Schmidt TM, Zahn JA, Boyd ES, de la Mora A, DiSpirito AA (2003) Role of Rhodobacter sp. strain PS9, a purple non-sulfur photosynthetic bacterium isolated from an anaerobic swine waste lagoon, in odor remediation. Appl Environ Microbiol 69(3):1710–1720. doi:10.1128/AEM.69.3.1710-1720.2003
El-Bestawy E (2008) Treatment of mixed domestic–industrial wastewater using cyanobacteria. J Ind Microbiol Biotechnol 35(11):1503–1516. doi:10.1007/s10295-008-0452-4
ElMekawy A, Diels L, De Wever H, Pant D (2013) Valorization of cereal based biorefinery byproducts: reality and expectations. Environ Sci Technol 47:9014–9027
El-Sheekh MM, El-Shouny WA, Osman ME, El-Gammal EW (2005) Growth and heavy metals removal efficiency of Nostoc muscorum and Anabaena subcylindrica in sewage and industrial wastewater effluents. Environ Toxicol Pharmacol 19(2):357–365. doi:10.1016/j.etap.2004.09.005
Eroğlu E, Gündüz U, Yücel M, Türker L, Eroğlu I (2004) Photobiological hydrogen production by using olive mill wastewater as a sole substrate source. Int J Hydrog Energy 29:163–171
Feng Y, Yu Y, Wang Y, Lin X (2007) Biosorption and bioreduction of trivalent aurum by photosynthetic bacteria Rhodobacter capsulatus. Curr Microbiol 55(5):402–408. doi:10.1007/s00284-007-9007-6
Fiore MF, Trevors JT (1994) Cell composition and metal tolerance in cyanobacteria. Biometals 7:83–103. doi:10.1007/BF00140478
Forgacs E, Cserháti T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30(7):953–971. doi:10.1016/j.envint.2004.02.001
Garrity G (2006) Bergey’s manual® of systematic bacteriology, vol 2. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT (eds) The proteobacteria, part A introductory essays. Springer
Goel M, Chovelon J-M, Ferronato C, Bayard R, Sreekrishnan T (2010) The remediation of wastewater containing 4-chlorophenol using integrated photocatalytic and biological treatment. J Photochem Photobiol B: Biol 98(1):1–6. doi:10.1016/j.jphotobiol.2009.09.006
Goszczynski S, Paszczynski A, Pasti-Grigsby M, Crawford R, Crawford D (1994) New pathway for degradation of sulfonated azo dyes by microbial peroxidases of Phanerochaete chrysosporium and Streptomyces chromofuscus. J Bacteriol 176(5):1339–1347
Greene B, McPherson R, Darnall D (1987) Algal sorbents for selective metal ion recovery. Metals speciation, separation and recovery. Lewis, Michigan
Hatzios KK (1991) Biotransformation of herbicides in higher plants. In: Cessna AJ, Grover R (eds) Environmental chemistry of herbicides. CRC Press, Boca Raton
He J, Zhang G, Lu H (2010) Treatment of soybean wastewater by a wild strain Rhodobacter sphaeroides and to produce protein under natural conditions. Front Environ Sci Eng China 4:334–339. doi:10.1007/s11783-010-0239-5
Hirotani H, Ohigashi H, Kobayashi M, Koshimizu K, Takahashi E (1991) Inactivation of T5 phage by cis -vaccenic acid, an antivirus substance from Rhodopseudomonas capsulata, and by unsaturated fatty acids and related alcohols. FEMS Microbiol Lett. doi:10.1111/j.1574-6968.1991.tb04314.x
Holt JG (1994) Bergey’s manual of determinative bacteriology. Williams & Wilkins, Baltimore
Hu Q, Westerhoff P, Vermaas W (2000) Removal of nitrate from groundwater by cyanobacteria: quantitative assessment of factors influencing nitrate uptake. Appl Environ Microbiol 66(1):133–139. doi:10.1128/AEM.66.1.133-139.2000
Huang PJ, Hou DB, Zhang GX, Li JC (2013) Estimation of chemical oxygen demand by ultraviolet spectroscopic profiling and physical parameters using IPW-PLS algorithm. Appl Mech Mater 316:606–609. doi:10.4028/www.scientific.net/AMM.316-317.606
Ibrahim Z, Ahmad WA, Baba AB (2001) Bioaccumulation of silver and the isolation of metal-binding protein from P. diminuta. Braz Arch Biol Technol 44:223–225
Ibrahim Z, Amin MF, Yahya A, Aris A, Muda K (2010) Characteristics of developed granules containing selected decolourising bacteria for the degradation of textile wastewater. Water Sci Technol 61:1279–1288. doi:10.2166/wst.2010.021
Jacob-Lopes E, Lacerda LMCF, Franco TT (2008) Biomass production and carbon dioxide fixation by Aphanothece microscopica Nägeli in a bubble column photobioreactor. Biochem Eng J 40:27–34
Jamil Z, Mohamad Annuar MS, Ibrahim S, Vikineswary S (2009) Optimization of phototrophic hydrogen production by Rhodopseudomonas palustris PBUM001 via statistical experimental design. Int J Hydrog Energy 34:7502–7512. doi:10.1016/j.ijhydene.2009.05.116
Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Rev Environ Sci Bio/Technol 9(3):215–288. doi:10.1007/s11157-010-9215-6
Kalavathi DF, Uma L, Subramanian G (2001) Degradation and metabolization of the pigment—melanoidin in distillery effluent by the marine cyanobacterium Oscillatoria boryana BDU 92181. Enzyme Microb Technol 29(5):246–251. doi:10.1016/S0141-0229(01)00383-0
Kamal VS, Wyndham RC (1990) Anaerobic phototrophic metabolism of 3-chlorobenzoate by Rhodopseudomonas palustris WS17. Appl Environ Microbiol 56(12):3871–3873
Kantachote D, Torpee S, Umsakul K (2005) The potential use of anoxygenic phototrophic bacteria for treating latex rubber sheet wastewater. Electron J Biotechnol 8:314–323
Kaushik A, Mona S, Kaushik C (2011) Integrating photobiological hydrogen production with dye–metal bioremoval from simulated textile wastewater. Bioresour Technol 102:9957–9964
Kim M, Choi K-M, Yin C-R, Lee K-Y, Im W-T, Lim J, Lee S-T (2004) Odorous swine wastewater treatment by purple non-sulfur bacteria, Rhodopseudomonas palustris, isolated from eutrophicated ponds. Biotechnol Lett 26(10):819–822. doi:10.1023/B:BILE.0000025884.50198.67
Koblížek M, Falkowski PG, Kolber ZS (2006) Diversity and distribution of photosynthetic bacteria in the Black Sea. Deep Sea Res Part II 53(17):1934–1944
Krooneman J, van den Akker S, Gomes TMP, Forney LJ, Gottschal JC (1999) Degradation of 3-chlorobenzoate under low-oxygen conditions in pure and mixed cultures of the anoxygenic photoheterotroph Rhodopseudomonas palustris DCP3 and an aerobic Alcaligenes species. Appl Environ Microbiol 65(1):131–137
Kuritz T (1998) Cyanobacteria as agents for the control of pollution by pesticides and chlorinated organic compounds. J Appl Microbiol 85:186S–192S. doi:10.1111/j.1365-2672.1998.tb05298.x
Kuritz T, Wolk CP (1995) Use of filamentous cyanobacteria for biodegradation of organic pollutants. Appl Environ Microbiol 61(1):234–238
Kushalatha M (2010) Photobiodegradation of halogenated aromatic pollutants. Adv Biosci Biotechnol 1:238–240. doi:10.4236/abb.2010.13033
Liu G-F, Zhou J-T, Wang J, Song Z-y, Qv Y-y (2006) Bacterial decolorization of azo dyes by Rhodopseudomonas palustris. World J Microbiol Biotechnol 22(10):1069–1074. doi:10.1007/s11274-005-4857-1
Lu H, Zhang G, Wan T, Lu Y (2011) Influences of light and oxygen conditions on photosynthetic bacteria macromolecule degradation: different metabolic pathways. Bioresour Technol 102:9503–9508
Madigan MT, Jung DO (2008) An overview of purple bacteria: systematics, physiology, and habitats. In: The purple phototrophic bacteria. Adv Photosynth Respir 28:1–15. doi:10.1007/978-1-4020-8815-5_1
Madukasi E, Dai X, He C, Zhou J (2010) Potentials of phototrophic bacteria in treating pharmaceutical wastewater. Int J Environ Sci Technol 7:165–174
Madukasi EI, Chunhua H, Zhang G (2011) Isolation and application of a wild strain photosynthetic bacterium to environmental waste management. Int J Environ Sci Technol 8(3):513–522. doi:10.1007/BF03326237
Mandal S, Mallick N (2012) Biodiesel production by the green microalga Scenedesmus obliquus in a recirculatory aquaculture system. Appl Environ Microbiol 78:5929–5934
McGrath JE, Harfoot CG (1997) Reductive dehalogenation of halocarboxylic acids by the phototrophic genera Rhodospirillum and Rhodopseudomonas. Appl Environ Microbiol 63(8):3333–3335
McMahon V, Garg A, Aldred D, Hobbs G, Smith R, Tothill I (2008) Composting and bioremediation process evaluation of wood waste materials generated from the construction and demolition industry. Chemosphere 71(9):1617–1628. doi:10.1016/j.chemosphere.2008.01.031
Molina-Guijarro JM, Pérez J, Muñoz-Dorado J, Guillén F, Moya R, Hernández M, Arias ME (2010) Detoxification of azo dyes by a novel pH-versatile, salt-resistant laccase from Streptomyces ipomoea. Int Microbiol 12(1):13–21
Mondal P, Chauhan B (2012) Biodegradation of azo dyes from wastewater. In: Lichtfouse E et al (eds) Environmental chemistry for a sustainable world. Springer, Netherlands, pp 255–275
Nagadomi H, Kitamura T, Watanabe M, Sasaki K (2000) Simultaneous removal of chemical oxygen demand (COD), phosphate, nitrate and H2S in the synthetic sewage wastewater using porous ceramic immobilised photosynthetic bacteria. Biotechnol Lett 22(17):1369–1374. doi:10.1023/A:1005688229783
Nagasathya A, Thajuddin N (2008) Decolourization of paper mill effluent using hypersaline cyanobacterium. Res J Environ Sci 2(5):408–414. doi:10.3923/rjes.2008.408.414
Narro M, Cerniglia C, Van Baalen C, Gibson D (1992) Evidence for an NIH shift in oxidation of naphthalene by the marine cyanobacterium Oscillatoria sp. strain JCM. Appl Environ Microbiol 58(4):1360–1363
Nawaz MS, Ahsan M (2014) Comparison of physico-chemical, advanced oxidation and biological techniques for the textile wastewater treatment. Alex Eng J. doi:10.1016/j.aej.2014.06.007
Niladevi K, Prema P (2008) Effect of inducers and process parameters on laccase production by Streptomyces psammoticus and its application in dye decolourization. Bioresour Technol 99(11):4583–4589
Noparatnaraporn N, Wongkornchawalit W, Kantachote D, Nagai S (1986) SCP production of Rhodopseudomonas sphaeroides on pineapple wastes. J Ferment Technol 64:137–143. doi:10.1016/0385-6380(86)90008-7
Noparatnaraporn N, Trakulnaleumsai S, Silveira RG, Nishizawa Y, Nagai S (1987) SCP production by mixed culture of Rhodocyclus gelatinosus and Rhodobacter sphaeroides from Cassava Waste. J Ferment Technol 65:11–16. doi:10.1016/0385-6380(87)90059-8
Okubo Y, Futamata H, Hiraishi A (2006) Characterization of phototrophic purple nonsulfur bacteria forming colored microbial mats in a swine wastewater ditch. Appl Environ Microbiol 72(9):6225–6233. doi:10.1128/AEM.00796-06
Pandi M, Shashirekha V, Swamy M (2009) Bioabsorption of chromium from retan chrome liquor by cyanobacteria. Microbiol Res 164:420–428. doi:10.1016/j.micres.2007.02.009
Pant D, Adholeya A (2009) Concentration of fungal ligninolytic enzymes by ultrafiltration and their use in distillery effluent decolorization. World J Microbiol Biotechnol 25:1793–1800. doi:10.1007/s11274-009-0079-2
Pant D, Adholeya A (2010) Development of a novel fungal consortium for the treatment of molasses distillery wastewater. Environmentalist 30:178–182. doi:10.1007/s10669-010-9255-z
Panwichian S, Kantachote D, Wittayaweerasak B, Mallavarapu M (2011) Removal of heavy metals by exopolymeric substances produced by resistant purple nonsulfur bacteria isolated from contaminated shrimp ponds. Electron J Biotechnol 14(4):1–13. doi:10.2225/vol14-issue4-fulltext-2
Pattanamanee W, Choorit W, Deesan C, Sirisansaneeyakul S, Chisti Y (2012) Photofermentive production of biohydrogen from oil palm waste hydrolysate. Int J Hydrog Energy 37:4077–4087
Penn MR, Pauer JJ, Mihelcic JR (2006) Biochemical oxygen demand. In: Sabljic A (ed) Environmental and ecological chemistry. Encyclopedia of Life Support Systems (EOLSS), Eolss Publishers, Oxford
Ponsano EHG, Lacava PM, Pinto MF (2003) Chemical composition of Rhodocyclus gelatinosus biomass produced in poultry slaughterhouse wastewater. Braz Arch Biol Technol 46(2):143–147. doi:10.1590/S1516-89132003000200001
Ponsano EH, Paulino CZ, Pinto MF (2008) Phototrophic growth of Rubrivivax gelatinosus in poultry slaughterhouse wastewater. Bioresour Technol 99:3836–3842
Prasanna R, Jaiswal P, Kaushik B (2008) Cyanobacteria as potential options for environmental sustainability—promises and challenges. Indian J Microbiol 48(1):89–94. doi:10.1007/s12088-008-0009-2
Prasertsan P, Jaturapornpipat M, Siripatana C (1997) Utilization and treatment of tuna condensate by photosynthetic bacteria. Pure Appl Chem 69(11):2439–2446. doi:10.1351/pac199769112439
Radwan S, Al-Hasan R (2002) Oil pollution and cyanobacteria. In: Whitton B, Potts M (eds) The ecology of cyanobacteria. Springer, Netherlands, pp 307–319
Radwan S, Al-Hasan R, Salamah S, Al-Dabbous S (2002) Bioremediation of oily sea water by bacteria immobilized in biofilms coating macroalgae. Int Biodeterior Biodegrad 50(1):55–59. doi:10.1016/S0964-8305(02)00067-7
Radway JC, Yozua BA, Benemann JR, Chini Zitelli G, Malda J, Babcock RWJR, Tredici MR (1999) Evaluation of near-horizontal tubular photobioreactor system in Hawaii. In: 8th international conference on applied algology, Montecassini, Italy
Raghukumar C, Vipparty V, David J, Chandramohan D (2001) Degradation of crude oil by marine cyanobacteria. Appl Microbiol Biotechnol 57:433–436. doi:10.1007/s002530100784
Ragini G, Bisen PS (2011) Bioremediation. In: Pimentel D (ed) Encyclopedia of biotechnology in agriculture and food. Taylor and Francis, New York
Rai L, Mallick N (1992) Removal and assessment of toxicity of Cu and Fe to Anabaena doliolum and Chlorella vulgaris using free and immobilized cells. World J Microbiol Biotechnol 8(2):110–114. doi:10.1007/BF01195827
Rawson D (1985) The effects of exogenous amino acids on growth and nitrogenase activity in the cyanobacterium Anabaena cylindrica PCC 7122. J Gen Microbiol 134:2549–2554. doi:10.1099/00221287-131-10-2549
Saijai P, Duangporn K, Banjong W, Megharaj M (2010) Isolation of purple nonsulfur bacteria for the removal of heavy metals and sodium from contaminated shrimp ponds. Electron J Biotechnol 13(4). doi:10.2225/vol13-issue4-fulltext-8
Samantaray S, Nayak JK, Mallick N (2011) Wastewater utilization for poly-β-hydroxybutyrate production by the cyanobacterium Aulosira fertilissima in a recirculatory aquaculture system. Appl Environ Microbiol 77:8735–8743
Sánchez O, Diestra E, Esteve I, Mas J (2005) Molecular characterization of an oil-degrading cyanobacterial consortium. Microb Ecol 50(4):580–588. doi:10.1007/s00248-005-5061-4
Sasaki K (1999) Hydrogen and 5-aminolevulinic acid production by photosynthetic bacteria. In: Zaborsky O, Benemann J, Matsunaga T, Miyake J, San Pietro A (eds) Biohydrogen. Springer, pp 133–142. doi:10.1007/978-0-585-35132-2_17
Sasaki K, Tanaka T, Nishizawa Y, Hayashi M (1990) Production of a herbicide, 5-aminolevulinic acid, by Rhodobacter sphaeroides using the effluent of swine waste from an anaerobic digestor. Appl Microbiol Biotechnol 32:727–731. doi:10.1007/BF00164749
Sasaki K, Watanabe M, Suda Y, Ishizuka A, Noparatnaraporn N (2005) Applications of photosynthetic bacteria for medical fields. J Biosci Bioeng 100:481–488
Shah V, Garg N, Madamwar D (2001) An integrated process of textile dye removal and hydrogen evolution using cyanobacterium, Phormidium valderianum. World J Microbiol Biotechnol 17(5):499–504. doi:10.1023/A:1011994215307
Shimabukuro RH (1985) Detoxification of herbicides. In: Duke SO (ed) Weed physiology, vol 2. CRC Press, Boca Raton
Shipman RH, Kao IC, Fan LT (1975) Single-cell protein production by photosynthetic bacteria cultivation in agricultural by-products. Biotechnol Bioeng 17:1561–1570. doi:10.1002/bit.260171102
Sikdar SK, Irvine RL (1998) Bioremediation: biodegradation technology developments, vol 2. CRC Press, Boca Raton
Singh SP, Verma SK, Singh RK, Pandey PK (1989) Copper uptake by free and immobilized cyanobacterium. FEMS Microbiol Lett 60(2):193–196. doi:10.1111/j.1574-6968.1989.tb03444.x
Subramaniyan V (2012a) Potential applications of cyanobacteria in industrial effluents—a review. J Bioremed Biodegrad 3(6):154–158. doi:10.4172/2155-6199.1000154
Subramaniyan V (2012b) Treatment of dye industry effluent using free and immobilized cyanobacteria. J Bioremed Biodegrad 3(10):165–170. doi:10.4172/2155-6199.1000165
Takeno K, Sasaki K, Watanabe M, Kaneyasu T, Nishio N (1999) Removal of phosphorus from oyster farm mud sediment using a photosynthetic bacterium, Rhodobacter sphaeroides IL106. J Biosci Bioeng 88(4):410–415. doi:10.1016/S1389-1723(99)80218-7
Telke AA, Kalyani DC, Dawkar VV, Govindwar SP (2009) Influence of organic and inorganic compounds on oxidoreductive decolorization of sulfonated azo dye CI Reactive Orange 16. J Hazard Mater 172(1):298–309. doi:10.1016/j.jhazmat.2009.07.008
Tran THK, Le TTA, Tran LT, Ooi T, Kinoshita S (2012) Decolorization of azo dyes by purple non-sulfur bacteria. Annual report of FY 2002, The Core University Program between Japan Society for the Promotion of Science (JSPS) and National Centre for Natural Science and Technology (NCST), pp 112–118
van der Woude BJ, de Boer M, van der Put NMJ, van der Geld FM, Prins RA, Gottschal JC (1994) Anaerobic degradation of halogenated benzoic acids by photoheterotrophic bacteria. FEMS Microbiol Lett 119(1–2):199–207. doi:10.1111/j.1574-6968.1994.tb06889.x
Vincenzini M, Materassi R, Tredici MR, Florenzano G (1982) Hydrogen production by immobilized cells—II. H2-photoevolution and waste-water treatment by agar-entrapped cells of Rhodopseudomonas palustris and Rhodospirillum molischianum. Int J Hydrog Energy 7:725–728. doi:10.1016/0360-3199(82)90021-0
Vrati S (1984) Single cell protein production by photosynthetic bacteria grown on the clarified effluents of biogas plant. Appl Microbiol Biotechnol 19:199–202. doi:10.1007/BF00256454
Watanabe M, Kawahara K, Sasaki K, Noparatnaraporn N (2003) Biosorption of cadmium ions using a photosynthetic bacterium, Rhodobacter sphaeroides S and a marine photosynthetic bacterium, Rhodovulum sp. and their biosorption kinetics. J Biosci Bioeng 95:374–378
Yergeau E, Arbour M, Brousseau R, Juck D, Lawrence JR, Masson L, Whyte LG, Greer CW (2009) Microarray and real-time PCR analyses of the responses of high-arctic soil bacteria to hydrocarbon pollution and bioremediation treatments. Appl Environ Microbiol 75:6258–6267
Yetis M, Gündüz U, Eroglu I, Yücel M, Türker L (2000) Photoproduction of hydrogen from sugar refinery wastewater by Rhodobacter sphaeroides OU 001. Int J Hydrog Energy 25:1035–1041
Yiğit DÖ, Gündüz U, Türker L, Yücel M, Eroğlu N (1999) Identification of by-products in hydrogen producing bacteria; Rhodobacter sphaeroides O.U. 001 grown in the waste water of a sugar refinery. In: Osinga R, Tramper J, Burgess JG, Wijffels RH (eds) Prog Ind Microbiol 35:125–131. doi:10.1016/S0079-6352(99)80106-4
Zhu H, Suzuki T, Tsygankov AA, Asada Y, Miyake J (1999) Hydrogen production from tofu wastewater by Rhodobacter sphaeroides immobilized in agar gels. Int J Hydrog Energy 24:305–310. doi:10.1016/S0360-3199(98)00081-0
Acknowledgments
The authors would like to thank the Ministry of Education Malaysia (MOE) and Universiti Teknologi Malaysia (UTM) for funding this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Idi, A., Md Nor, M.H., Abdul Wahab, M.F. et al. Photosynthetic bacteria: an eco-friendly and cheap tool for bioremediation. Rev Environ Sci Biotechnol 14, 271–285 (2015). https://doi.org/10.1007/s11157-014-9355-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-014-9355-1