Abstract
Increasing quantities of evidence-based data incriminate a large number of environmental pollutants for toxic effects on the thyroid. Among the many chemical contaminants, halogenated organochlorines and pesticides variably affect the hypothalamic-pituitary-thyroid axis and disrupt thyroid function. PCBs and their metabolites and PBDEs bind to thyroid transport proteins, such as transthyretin, displace thyroxine, and disrupt thyroid function. Meanwhile, at the molecular level, PCB congeners may activate phosphorylation of Akt, p-Akt, and forkhead box O3a (FoxO3a) protein resulting in inhibition of the natrium/iodide symporter. Given therefore the growing concern developing around these multiple toxic chemicals today invading numerous environments and their long-term deleterious effects not only on the thyroid but also on general health, we strongly advocate their strict regulation and, moreover, their gradual reduction. A good degree of “lateral thinking”, we feel, will lead to a use of chemicals that will enhance life while concurrently carefully protecting the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The countless technological advances made over the last 100 years have greatly improved our life by providing technical means in all spheres. On the other hand, present-day technological progress has also generated, inter alia, thousands of new toxic chemicals and pollutants which, inevitably, enter the body through air, water, food and skin and, as they cannot be eliminated, accumulate in bodily tissues creating a perpetual condition of contamination and disease [1]. Among these, the negative health impact of endocrine-disrupting chemicals (EDCs) on humans is of particular concern. Starting even from infancy, babies in America are now born with an average of 287 chemicals in their blood and pediatric endocrinologists are advised to check for EDCs when evaluating complex endocrine disorders [2, 3]. Regarding adulthood, the Fourth National Report on Human Exposure to Environmental Chemicals produced by the Center for Control Disease (CDC) tested 2,400 people and detected 215 toxic compounds in their blood and urine, while it has also been documented that as many as 40 % of Americans have toxic levels of lead in their bodies [4, 5].
The Endocrine Society has recently released its Second Scientific Statement on environmental EDCs: this consists of a comprehensive review of the literature on seven topics for which there is strong evidence of endocrine disruption, specifically, obesity and diabetes, female reproduction, male reproduction, hormone-sensitive cancers in females, prostate cancer, thyroid, neurodevelopment and neuroendocrine systems [6]. Emphasis is placed on such EDCs as persistent organic pollutants including polychlorinated biphenyls, (PCBs) polybrominated diethyl ethers, (PBDEs) and dioxins, bisphenol A, phthalates, and pesticides, since they are accompanied by a large amount of solid information. Also stated is the fact that the tracking of xenobiotics, which are likely to stimulate signaling pathways, as well as of genetic mutation and DNA methylation are some of the methods that will help in identifying the impact of xenobiotic action on the endocrine system [7].
It is thus the aim of this review to focus on the various mechanisms by which synthetic chemicals and their by-products exert complex and detrimental effects on thyroid function. Against the background of several reports on the disruptive effects of chemicals and their mechanisms on the thyroid [8–10], the latest data will be examined concerning the chemical contamination of the thyroid gland by persistent organic pollutants: mainly by halogenated organochlorines and pesticides.
2 Organochlorines and thyroid function
PCBs, some of the most persistent of the environmental pollutants, impair host resistance and are highly lipophilic, while their bioaccumulation by aquatic and terrestrial organisms facilitates their entrance into the food chain [11]. The susceptibility of PCBs to degradation and bioaccumulation is congener-specific, thus the composition in the environment differs substantially from the original mixtures released into the environment. Specifically concerning the thyroid, thyroid function is variably affected by occupational and other exposure to industrial chemicals and byproducts including PCBs, polybrominated diphenyl ethers (PBDEs), dioxins, and halogenated organochlorines (Table 1). PCBs appear able to interfere with the production, transportation, and metabolism of thyroid hormones, while some possessing structural similarity to thyroid hormone have been shown to bind to thyroid receptors with both agonist and antagonist effects on thyroid hormone signaling [12]. PCBs exert deleterious effects on the neurodevelopment of the fetus and the behavior of the young child, while in adults they have been associated with tumorigenesis/goitergenesis and thyroid autoimmunity [12]. The effects are dependent on age at exposure, the iodine and possibly selenium status of the individual, detoxification enzymes, and genetic variants.
PCBs and PBDEs are metabolized into hydroxylated metabolites (OH-PCBs/PBDEs), which they bind to thyroid transport proteins, such as transthyretin (TTR), displace thyroxine (T4), and disrupt thyroid hormone homeostasis [13, 14]. In addition, conjugated PCB metabolites such as PCB sulfates can also alter serum thyroid hormone levels in humans by binding to TTR, to which they show a high affinity, in contrast to the low-binding ability they exhibit to the thyroid hormone receptor [15]. The latter is an important mechanism of thyroid toxicity that characterizes PCBS. Although the competitive thyroxine displacement potency of metabolized PCBs fluctuates depending on the various metabolites, the risk factors implicated in this toxic potential should be carefully borne in mind.
Recently, experimental studies have focused on the molecular mechanisms by which PCBs may disrupt the thyroid. More specifically, to assess the molecular mechanisms underlying 2,3′,4,4′,5-pentachlorobiphenyl (PCB118)-induced thyroid dysfunction, Fischer rat thyroid cell line-5 (FRTL-5) cells were treated with different concentrations of PCB118 [16]. Quantitative real-time polymerase chain reaction was applied to quantify protein kinase B (Akt), forkhead box protein O3a (FoxO3a), and natrium/iodide symporter (NIS) mRNA expression levels. It was shown that relatively higher PCB118 concentrations can inhibit cell viability in a concentration- and time-dependent manner. Akt, p-Akt, and p-FoxO3a protein levels increased significantly in PCB118-treated groups, while NIS protein and mRNA levels decreased compared with the control groups [16]. Most importantly, FoxO3a promoter activity increased, whereas NIS promoter activity, NIS protein, and mRNA levels significantly decreased compared with the control group. In a study where thyroid epithelial cells were incubated with PCB118, there was some evidence that relatively low concentrations decreased thyroglobulin (Tg) and T4, while high concentrations resulted in loss of cell viability [17]. Moreover, a significant increase of the protein levels of p–AKT and p-FoxO3a and concomitantly a decrease of mRNA and protein expression levels of NIS were observed [17].
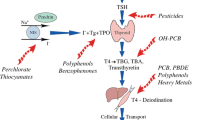
Another mechanism of thyroid disruption is the induction of inflammation. In a recent study, Wistar rats were administered intraperitoneally various doses of PCB118 for 5 days a week over 13 weeks, while thyroid FRTL-5 cells were treated with PCB118 for a period of time. PCB induced the generation of interleukin-6 (IL-6), tumor necrosis factor-α (ΤΝF-α), and intercellular adhesion molecule-1 (ICAM-1) in a time- and dose-dependent manner resulting in inhibition of NIS [18]. In FRTL-5 treated cells, PCB118 upregulated the aryl hydrocarbon receptor [Ahr)-responsive gene cytochrome P450 1A1 which activates the c-Jun N-terminal kinase (JNK). Thus, it was suggested that PCB 118 induces an inflammatory response by activating AhR and JNK that stimulate the production of cytokines, leading to suppression of NIS expression and subsequently to thyroid disruption (Fig 1).
PCB congener 118 can in a concentration- and time-dependent manner activates the PI3K/Akt signaling pathway, enhances Akt phosphorylation, and subsequently phosphorylation of FoxO3a gene, leading to inhibition of NIS, and increase of apoptosis. Concomitantly, PCB118 stimulates the secretion of cytokines, IL-6, and TNF-α, promoting an inflammatory response and that further inhibits NIS. PI3K/Akt = phosphatidylinositol 3- kinase; FoxO3a = forkhead box O3a (FoxO3a) transcription factor; Ahr = aryl hydrocarbon receptor; IL-6 = Interleukin-6; TNF-α: tumor necrotic factor-α; NIS = natrium iodine symporter
Furthermore, studies in Sprague–Dawley rats revealed that following exposure to PCB153 and p,p’-DDE, activated PI3K/Akt and ERK pathways disrupt the hypothalamic-pituitary-thyroid axis via TRb1 and TRH receptors resulting in a decrease of thyroid stimulating hormone (TSH), T4 and free-T4 (FT4). This would appear to constitute a plausible mechanism of thyroid disruption by PCB153 and p,p’-DDE [19].
Studies conducted in areas of eastern Slovakia that are highly contaminated by a mixture of PCBs, DDE, and hexachlorobenzene (HCZ) revealed an increased prevalence of goiter and metabolic disorders in adults, this pointing to a possible link between the high PCBs levels and signs of thyroid disease [20]. Moreover, field surveys in the same country focused on the thyroid have been performed in areas severely polluted by nitrates and in a large area contaminated mainly by organochlorinated (OC) toxicants, respectively [21]. A higher thyroid volume that was estimated by ultrasound was found to be significantly more frequent in children from the high nitrate area (HNA, n = 324) as compared with age-matched children from areas with low nitrate (LNA, n = 764), whereas no difference was found in the level of T4 and free-T3 [21]. However, positive thyroid peroxidase antibodies (TPOAb) (2.2 %) and higher TSH levels > 4.0 mIU/L (4.0 %) were detected in children from the HNA area, while no positive values were obtained in the LNA. Similar findings, associated with goiter, were observed in 2046 adults from an area heavily polluted by an OC cocktail consisting of PCBs, 2,2′-bis(4-chlorophenyl)-1,1-dichloroethylene (p,p'-DDE), hexachlorobenzene (HCB), and dioxins and furans. Increasing PCB levels were significantly associated with the increase of FT4 (p <0.001) and T3 (p <0.05) in blood, while a slight but non-significant negative association of PCBs was observed with the level of TSH. Interestingly, the prevalence of TPOAb was seen to be considerably more elevated among both women and men in the affected area.
During the early stages of life, exposure to endocrine-disrupting chemicals can disrupt the patterns of neurodevelopment, thereby altering brain function and disease susceptibility later in life [6, 22]. A pilot study showed that in an area of borderline iodine deficiency, exposure to PCB118 mitigates the benefit of iodine supplementation and thus has a negative impact on neurocognitive development [23]. It is of importance that among maternal and cord blood (CB) thyroid tests, only CB Tg, the best marker of iodine status, negatively correlated with neurodevelopment scales. Based on this fact, the authors have suggested that exposure to environmental neurotoxicants should be considered when implementing studies on the benefit of iodine supplementation in pregnancy [23].
The potential for the endocrine disruptor bisphenol A [BPA] to affect thyroid function is becoming increasingly a cause of concern. In the HOME Study (2003–2006, Cincinnati, Ohio, USA), measurements were taken of urinary BPA concentrations of pregnant women at 16 and 26 weeks gestation. Specifically, TSH and both free and total T4 and T3 were measured in maternal serum at 16 weeks (n = 181) and cord serum at delivery (n = 249) [24]. It was determined that among iodine-deficient vs iodine-sufficient mothers, the inverse BPA-TSH relation in girls was stronger, though less clear-cut. The conclusion was that prenatal exposure to BPA can result in lower levels of TSH among female neonates, this being especially true when exposure has occurred later in gestation.
BPA and triclosan (TCS) were determined in the urine of Belgian overweight and obese (n = 151) as well as lean (n = 43) individuals. Multiple linear regression analyses, after adjusting for age and weight loss, revealed negative associations between urinary TCS and serum FT4 in female obese individuals and positive associations between urinary BPA and serum TSH in the lean group [25].
Polybrominated diphenyl ether (PBDE) flame retardants are endocrine disruptors and suspected neurodevelopmental toxicants. Prenatal exposure to PBDEs has been found to disrupt thyroid function and to subsequently bring about adverse neurodevelopmental outcomes. The relationship between serum concentrations of lower-brominated PBDEs, higher-brominated PBDEs congeners, and hydroxylated PBDE metabolites (OH-PBDEs) with parameters of thyroid function in pregnant women was investigated in California [26]. While the PBDRs levels were elevated, associations with free and total T4 were observed to be weak and somewhat inconsistent, indicating that the risk of PBDE exposure during pregnancy is not well defined [26].
Although the direct mechanisms of neurodevelopmental toxicity have not to date been fully elucidated, it is conceivable that alterations in thyroid hormone levels in the developing brain may contribute to these effects. Recently, an investigation was carried out on the impact of several PBDEs and hydroxylated metabolites (OH-BDEs) on selenoprotein deiodinase type 2 (DIO2) activity in astrocytes, a specialized glial cell where the generation of more than 50 % of T3 required by the brain occurs [27]. Following exposure to PBDEs and OH-BDEs, primary human astrocytes and H4 glioma cells showed a decrease of up to 50 % and 80 % of DIO2, respectively [27]. According to the authors, multiple mechanisms might be involved in the diminished DIO2 activity, including weakened expression of DIO2 mRNA along with competitive inhibition and/or enhanced post-translational degradation of DIO2.
It is of interest that studies in developing zebra fish to determine the effects of OH-BDE on the expression of genes and to pinpoint the tissues expressing the genes critical to thyroid hormone regulation have clearly revealed localized response at the hypothalamic-pituitary-thyroid axis [28]. Significant increases in mean intensity of DIO1 and DIO3 expression in the periventricular zone of the brain were observed, suggesting another thus far undetermined mechanism impacting neurodevelopment.
3 Perfluoroalkyl substances
Exposure to perfluoroalkyl and polyfluoroalkyl substances (PFASs) is extensive in many regions of the world [29]. The most commonly studied PFASs are perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). A study examining the associations in serum between four different PFASs and thyroid hormones (TH) found that in a sample of 1,525 adults (≥18 years) from the 2007–2008 NHANES study, and specifically in a subgroup with high TPOAB and low-iodine status, all four PFASs were positively associated (p <0.05) with TH and fT3/fT4 ratio and TSH [30]. The authors hypothesized that individuals with high thyroid peroxidase antibodies and low iodine intake would be more susceptible to PFAS-induced thyroid disruption. In another study performed in Northern Norway in pregnant women, the associations were investigated between THs, thyroid binding proteins, and PFAS concentrations [31]. The women were assigned to quartiles according to PFAS concentrations during the second trimester. Although the variation in levels of THs between PFAS quartiles was within normal reference ranges, maybe not significant during pregnancy, and yet subtle individual changes in maternal THs could have a considerable impact on fetal health [31].
4 Pesticides
In 1962, Rachel Carson, already deeply concerned since the 1940s about the widespread use of synthetic pesticides, published her groundbreaking work Silent Spring that alerted the world to the human being’s ever more adverse impact on nature. This was followed by changes in the laws for the protection of the environment, notably forcing the banning of DDT, and contributed substantially to the launching of the environmental movement.
Numerous chemicals are applied in agriculture today in order to protect crops and increase their yield. Some are categorized as herbicides employed for the elimination of unwanted plants such as grasses and weeds, and others as pesticides used for the elimination of animal parasites. The list of the most frequently used herbicides and pesticides is presented in Table 1. Although these chemicals have indeed helped to increase crop yields and to produce significantly larger amounts of food for an ever-growing global population, they have clearly been demonstrated as having major adverse effects on human physiology and to be associated with multiple pathologic states, including cancer.
Alachlor is a herbicide of the chloroatanilide family. Its mode of action is inhibition of elongase and of geranylgeranyl pyrophosphate (GGPP) cyclisation enzymes, part of the gibberellin pathway [32]. While still in use in some countries (e.g., the USA), it has been banned in others (e.g., the European Union). Alachlor has been shown to have inhibitory effects on the function of the thyroid gland. In a recent prospective study [33] from the USA, an exposure response analysis documented increased odds of hypothyroidism in male applicators as well as their spouses [34]. Furthermore, it has been shown to induce several malignancies in animal feeding studies, including thyroid cancer [35], although the concentrations used are appreciably higher than expected human exposure from the application of alachlor in agriculture. Specifically, there seems to be no notably increased mortality (either all-cause or cancer-related) in workers exposed to alachlor [36].
Dicamba is a herbicide which acts by increasing plant growth rate. The plant outgrows its nutrient supplies and dies. Carbamates are a group of similar compounds that are used as pesticides which reversibly inactivate the enzyme acetylcholinesterase (in contrast to the irreversible inhibition of this enzyme by the organophosphates). These compounds have been associated with hypothyroidism in the Agricultural Health Study [33].
Dichlorodiphenyltrichloroethane (DDT) was a widely used pesticide some decades ago. Shown to be effective in reducing malaria-related deaths and subsequently used as an agricultural insecticide, it was however banned in 1972 due to its hazardous effect on the environment, wildlife and human health. It is thought to act on sodium channels in neurons, leading to spasms and eventual death. The effects of DDT on various tissues, including the thyroid, have been extensively studied. When adult rats are exposed to DDT for 6 to 10 weeks, a decrease of the NIS activity and iodine transport into the thyroid cells was noted, while the serum concentration of thyroid peroxidase increased [37]. The effect on the symporter seemed to be transient and recovery was observed in the setting of continued DDT exposure.
Despite the banning of DDT many years ago, it is still used to a limited degree in disease vector control, while it persists in the environment in sufficient amounts to have potentially severe effects. Thus, although it was banned in China in 1983, it has been detected in the blood of mothers and newborn babies and is suspected of disrupting the thyroid axis [38]. Similar results were described in a cross-sectional study from Brazil carried out in an area heavily contaminated by organochlorine pesticides [39] When middle-aged men and women were tested for organic pollutants including DDT [40], significant correlations between thyroid hormone levels and the level of exposure were identified. For example, among women, exposure to DDT (in combination with DDE which is the major metabolite of DDT) resulted in an increase in T4 by 0.34 ug/dL (P = 0.04). Thus, although the actual clinical implication of such changes remains largely unknown, there is some evidence indicating that these compounds are sufficiently present in the environment to have a measurable effect on the thyroid axis of people exposed to them.
In another study on rats, exposure to DDE (dichlorodiphenyldichloroethylene, the main metabolite of DDT) resulted in significantly reduced serum levels of total and free T4 and TTR [41]. Of interest, thyroid hormone receptor mRNA expression was increased in the hypothalamus.
Fipronil is an hexachlorobenzene compound, an insecticide that acts on the g-aminobutyric acid receptor. It has been associated with a significant increase in the incidence of thyroid gland tumors [42]. Of about 240 pesticides screened for carcinogenicity by the U.S. Environmental Protection Agency Office of Pesticide Programs, 10 % (24 compounds) was found to produce thyroid follicular cell tumors in rodents [43]. An association between fipronil and thyroid cancer also in humans has been reported, although another study failed to verify this finding [44, 45]. Fipronil exposure in rats has been associated with increased serum TSH, but in fipronil-exposed workers fipronil sulfone concentrations have been negatively associated with serum TSH concentrations, raising the possibility that fipronil has a central inhibitory effect on TSH secretion in humans [45, 46]. Fipronil has been described as causing perturbation of hepatic thyroid hormone metabolism, while a recent study examined the pathways involved in fipronil-induced liver gene expression regulations [47]. There were indications that androstane receptor (CAR) and pregnane X receptor (PXR) are the modulators of the hepatic gene expression profile following fipronil treatment which, at least in rats, increase thyroid hormone clearance [47].
In sum, the data provided herein suggest that it is of great importance for the protection of public health that studies be carried out to examine the endocrine-disrupting effects of pesticide metabolites, especially those characterized by high toxicity and persistence in the environment [48].
Endosulfan is an organochlorine insecticide which, when used as an agrichemical, ignited much controversy due to its acute toxicity, potential for bioaccumulation, and role as an endocrine disruptor. Because of its threats to human health and the environment, a global ban on the manufacture and use of endosulfan was negotiated under the Stockholm Convention in April 2011 [49].
In a cross-sectional study in Brazil [50] involving children living in an area of heavy exposure, endosulfan exposure was associated with higher T4 levels. As mentioned above, the actual clinical significance of these findings cannot be assessed from these reports.
Heptachlor is an organochlorine and one of the cyclodiene insecticides. Due to its highly stable structure, heptachlor can persist in the environment for decades [51].
Lindane, also known as gamma-hexachlorocyclohexane, (γ-HCH), gammaxene, is an organochlorine chemical that has been used both as an agricultural insecticide and as a pharmaceutical treatment for lice and scabies. It is a neurotoxin that interferes with GABA neurotransmitter function by interacting with the GABAA- receptor-chloride channel complex at the picrotoxin binding site. In humans, lindane affects the nervous system, liver, and kidneys and may be a carcinogen [52], although no data are available specifically regarding thyroid malignancies.
Exposure to either of these two compounds was associated with hypothyroidism in the Agricultural Health Study [33]. Heptachlor has been specifically shown to inhibit TPO activity [53] in an in-vitro assay that revealed a potential molecular target that may explain this association.
Chlorpyrifos is a widely-used organophosphate pesticide. It is used to manage insect pests on crops like fruit and nut trees, vines, vegetables, corn, and soybeans. In a recent review of the available evidence concerning this compound as an endocrine disruptor, it was shown not to have a significant effect on the thyroid axis at dose levels below those that inhibit cholinesterase activity [54]. In an animal study [55], rats were exposed to chlorpyrifos either on gestational days 17–20 or postnatal days 1–4. Prenatal exposure resulted in a small reduction of brain thyroxine levels, while postnatal exposure resulted in a transient elevation in young adulthood. The authors concluded that it remains unproven whether the neurobehavioral abnormalities induced by chlorpyrifos is due to its effects on thyroid function. An association between perinatal exposure to chlorpyrifos and attention deficit hyperactivity disorders in humans was noted recently [56].
Finally, there are indications that environmental contamination by EDCs can exert epigenetic effects via DNA methylation and histone modifications [57]. Exposure to EDCs early in life may program epigenomic changes which will be later expressed as dysfunctions, among others, of metabolism and thyroid [58].
This points to the need for further investigation into the possibility that epigenetic transgenerational inheritance of EDC action can contribute also to the development of thyroid disease.
Taking into consideration all the above, it has become very apparent that exposure to these chemicals has considerable and measurable effects on multiple types of tissues, and, most crucially, the thyroid gland.
5 A well regulated chemical industry will contribute to ‘well regulated’ thyroid function
The reality is that despite their various uses, many chemicals used today are so toxic, persistent, and widespread that their disadvantages could well be outweighing their advantages to society as a whole. Do we not therefore need to profoundly reconsider our aims and procedures? In general, the fact that our ever larger environmental footprint is adversely affecting countless chains of interrelated ecosystems begs the question of whether, instead of improving our world, we are not seriously jeopardizing all life, in the case of chemicals, by incurring, for instance, the loss of vital genetic material, of life-saving medications, and even of safer alternatives to the hazardous chemicals presently being utilized.
Jean-Claude Clamadieu, of General Director of Solvay Rhodia, notably stated at the recent COP21 Climate Summit (COP21: Road to Paris – Cefic): “…We take a holistic approach to sustainability.... We can produce innovative services and solutions for a growing global population, while striving to preserve our planet’s resources and respecting the environment.” The regulatory framework of the European Chemical Industry Council, or Cefic, is dedicated to “the use of chemicals properly, safely and in an environmentally-friendly and healthy manner.” It is to be hoped that Clamadieu’s words and Cefic’s principles can be a rallying call for much greater forethought and prudence in chemical production and use worldwide. This can be brought about by, indeed, “a holistic approach” and a manner of reasoning that is highly creative and “outside the box”, in a word, lateral thinking.
References
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev. 2009;30(4):293–342. doi:10.1210/er.2009-0002.
Body Burden – The pollution in newborns, environmental working group, 2005.
Skakkebaek NE, Toppari J, Söder O, Gordon CM, Divall S, Draznin M. The exposure of fetuses and children to endocrine disrupting chemicals: a european society for paediatric endocrinology (ESPE) and pediatric endocrine society (PES) call to action statement. J Clin Endocrinol Metab. 2011;96(10):3056–8. doi:10.1210/jc.2011-1269.
Fourth National Report on Human Exposure to Environmental Chemicals. U.S. CDC 2009.
Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114(13):1388–94.
Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. Executive summary to EDC-2: the endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:593–602. doi:10.1210/er.2015-1093.
Maqbool F, Mostafalou S, Bahadar H, Abdollahi M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2015. doi:10.1016/j.lfs.2015.10.022.
Brucker-Davis F. Effects of environmental synthetic chemicals on thyroid function. Thyroid. 1998;8:827–55.
Zoeller TR. Environmental chemicals targeting thyroid. Hormones (Athens). 2010;9:28–40.
Duntas LH. Chemical contamination and the thyroid. Endocrine. 2015;48:53–64. doi:10.1007/s12020-014-0442-4.
Beyer A, Biziuk M. Environmental fate and global distribution of polychlorinated biphenyls. Rev Environ Contam Toxicol. 2009;201:137–58. doi:10.1007/978-1-4419-0032-6_5.
Brucker-Davis F, Hiéronimus S, Fénichel P. Thyroid and the environment. Presse Med. 2015. doi:10.1016/j.lpm.2015.06.015.
Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, et al. Metabolism and metabolites of polychlorinated biphenyls (PCBs). Crit Rev Toxicol. 2015;45:245–72. doi:10.3109/10408444.2014.999365.
Montaño M, Cocco E, Guignard C, Marsh G, Hoffmann L, Bergman A, et al. New approaches to assess the transthyretin binding capacity of bioactivated thyroid hormone disruptors. Toxicol Sci. 2012;130:94–105. doi:10.1093/toxsci/kfs228.
Grimm FA, Lehmler HJ, He X, Robertson LW, Duffel MW. Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ Health Perspect. 2013;121:657–62. doi:10.1289/ehp.1206198.
Yang H, Chen H, Guo H, Li W, Tang J, Xu B, et al. Molecular mechanisms of 2, 3′, 4, 4′, 5-pentachlorobiphenyl-induced thyroid dysfunction in FRTL-5 cells. PLoS One. 2015;10:e0120133. doi:10.1371/journal.pone.0120133.eCollection2015.
Guo H, Yang H, Chen H, Li W, Tang J, Cheng P, et al. Molecular mechanisms of human thyrocyte dysfunction induced by low concentrations of polychlorinated biphenyl 118 through the Akt/FoxO3a/NIS pathway. J Appl Toxicol. 2015;35:992–8. doi:10.1002/jat.3032.
Xu B, Yang H, Sun M, Chen H, Jiang L, Zheng X, et al. 2,3′,4,4′,5-Pentachlorobiphenyl induces inflammatory responses in the thyroid through JNK and Aryl hydrocarbon receptor-mediated pathway. Toxicol Sci. 2015.
Liu C, Li L, Ha M, Qi S, Duan P, Yang K. The PI3K/Akt and ERK pathways elevate thyroid hormone receptor β1 and TRH receptor to decrease thyroid hormones after exposure to PCB153 and p, p'-DDE. Chemosphere. 2015;118:229–38. doi:10.1016/j.chemosphere.2014.09.023.
Langer P. The impacts of organochlorines and other persistent pollutants on thyroid and metabolic health. Front Neuroendocrinol. 2010;31:497–518. doi:10.1016/j.yfrne.2010.08.001.
Rádiková Z, Tajtáková M, Kocan A, Trnovec T, Seböková E, Klimes I, et al. Possible effects of environmental nitrates and toxic organochlorines on human thyroid in highly polluted areas in Slovakia. Thyroid. 2008;18:353–62. doi:10.1089/thy.2007.0182.
Schug TT, Blawas AM, Gray K, Heindel JJ, Lawler CP. Elucidating the links between endocrine disruptors and neurodevelopment. Endocrinology. 2015;156:1941–51. doi:10.1210/en.2014-1734.
Brucker-Davis F, Ganier-Chauliac F, Gal J, Panaïa-Ferrari P, Pacini P, Fénichel P, et al. Neurotoxicant exposure during pregnancy is a confounder for assessment of iodine supplementation on neurodevelopment outcome. Neurotoxicol Teratol. 2015;51:45–51. doi:10.1016/j.ntt.2015.07.009.
Romano ME, Webster GM, Vuong AM, Thomas Zoeller R, Chen A, Hoofnagle AN, et al. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: the HOME study. Environ Res. 2015;138:453–60. doi:10.1016/j.envres.2015.03.003.
Geens T, Dirtu AC, Dirinck E, Malarvannan G, Van Gaal L, Jorens PG, et al. Daily intake of bisphenol A and triclosan and their association with anthropometric data, thyroid hormones and weight loss in overweight and obese individuals. Environ Int. 2015;76:98–105. doi:10.1016/j.envint.2014.12.003.
Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol. 2011;45:7896–905. doi:10.1021/es200422b.
Roberts SC, Bianco AC, Stapleton HM. Disruption of type 2 iodothyronine deiodinase activity in cultured human glial cells by polybrominated diphenyl ethers. Chem Res Toxicol. 2015;28:1265–74. doi:10.1021/acs.chemrestox.5b00072.
Dong W, Macaulay LJ, Kwok KW, Hinton DE, Stapleton HM. Using whole mount in situ hybridization to examine thyroid hormone deiodinase expression in embryonic and larval zebrafish: a tool for examining OH-BDE toxicity to early life stages. Aquat Toxicol. 2013;132–133:190–9. doi:10.1016/j.aquatox.2013.02.008.
Bach CC, Bech BH, Brix N, Nohr EA, Bonde JP, Henriksen TB. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit Rev Toxicol. 2015;5:53–67. doi:10.3109/10408444.2014.952400.
Webster GM, Rauch SA, Ste Marie N, Mattman A, Lanphear BP, Venners SA. Cross-sectional associations of serum perfluoroalkyl acids and thyroid hormones in U.S. adults: variation according to TPOAb and iodine status (NHANES 2007–2008). Environ Health Perspect. 2015.
Berg V, Nøst TH, Hansen S, Elverland A, Veyhe AS, Jorde R, et al. Assessing the relationship between perfluoroalkyl substances, thyroid hormones and binding proteins in pregnant women; a longitudinal mixed effects approach. Environ Int. 2015;77:63–9. doi:10.1016/j.envint.2015.01.007.
Appleby AP, Müller F, Carpy S. Weed control. Ullmann’s encyclopedia of industrial chemistry. Weinheim: Wiley-VCH; 2002. doi:10.1002/14356007.a28_165.
Goldner WS, Sandler DP, Yu F, Shostrom V, Hoppin JA, Kamel F, et al. Hypothyroidism and pesticide use among male private pesticide applicators in the agricultural health study. J Occup Environ Med. 2013;55(10):1171–8.
Goldner WS, Sandler DP, Yu F, Hoppin JA, Kamel F, Levan TD. Pesticide use and thyroid disease among women in the agricultural health study. Am J Epidemiol. 2010;171(4):455–64.
Wilson AG, Thake DC, Heydens WE, Brewster DW, Hotz KJ. Mode of action of thyroid tumor formation in the male long-Evans rat administered high doses of alachlor. Fundam Appl Toxicol. 1996;33(1):16–23.
Acquavella JF, Delzell E, Cheng H, Lynch CF, Johnson G. Mortality and cancer incidence among alachlor manufacturing workers 1968–99. Occup Environ Med. 2004;61(8):680–5.
Yaglova NV, Yaglov VV. Mechanisms of disruptive action of Dichlorodiphenyltrichloroethane (DDT) on the function of thyroid follicular epitheliocytes. Bull Exp Biol Med. 2015;160(2):231–3. doi:10.1007/s10517-015-3136-x.
Li C, Cheng Y, Tang Q, Lin S, Li Y, Hu X, et al. The association between prenatal exposure to organochlorine pesticides and thyroid hormone levels in newborns in Yancheng, China. Environ Res. 2014;129:47–51.
Freire C, Koifman RJ, Sarcinelli PN, Simões Rosa AC, Clapauch R, Koifman S. Long-term exposure to organochlorine pesticides and thyroid status in adults in a heavily contaminated area in Brazil. Environ Res. 2013;127:7–15.
Bloom MS, Jansing RL, Kannan K, Rej R, Fitzgerald EF. Thyroid hormones are associated with exposure to persistent organic pollutants in aging residents of upper Hudson River communities. Int J Hyg Environ Health. 2014;217:473–82.
Liu C, Shi Y, Li H, Wang Y, Yang K. p, p ′ -DDE disturbs the homeostasis of thyroid hormones via thyroid hormone receptors, transthyretin, and hepatic enzymes. Horm Metab Res. 2011;43(6):391–6.
Herin F, Boutet-Robinet E, Levant A, Dulaurent S, Manika M, Galatry-Bouju F, et al. Thyroid function tests in persons with occupational exposure to Fipronil. Thyroid. 2011;21(7):701–6.
Hurley PM. Mode of carcinogenic action of pesticides inducing thyroid follicular cell tumors in rodents. Environ Health Perspect. 1998;106(8):437–45.
Grimalt JO, Sunyer J, Moreno V, Amaral OC, Sala M, Rosell A, et al. Risk excess of soft-tissue sarcoma and thyroid cancer in a community exposed to airborne organochlorinated compound mixtures with a high hexachlorobenzene content. Int J Cancer. 1994;56(2):200–3.
Lope V, Pérez-Gómez B, Aragonés N, López-Abente G, Gustavsson P, Plato N, et al. Occupational exposure to chemicals and risk of thyroid cancer in Sweden. Int Arch Occup Environ Health. 2009;82:267–74.
Zaidi SS, Bhatnagar VK, Gandhi SJ, Shah MP, Kulkarni PK, Saiyed HN. Assessment of thyroid function in pesticide formulators. Hum Exp Toxicol. 2000;19(9):497–501.
Roques BB, Leghait J, Lacroix MZ, Lasserre F, Pineau T, Viguié C, et al. The nuclear receptors pregnane X receptor and constitutive androstane receptor contribute to the impact of fipronil on hepatic gene expression linked to thyroid hormone metabolism. Biochem Pharmacol. 2013;86(7):997–1039. doi:10.1016/j.bcp.2013.08.012.
Lu M, Du J, Zhou P, Chen H, Lu C, Zhang Q. Endocrine disrupting potential of fipronil and its metabolite in reporter gene assays. Chemosphere. 2015;120:246–51. doi:10.1016/j.chemosphere.2014.07.015.
Weber J, Halsall CJ, Muir D, Teixeira C, Small J, Solomon K, et al. Bidleman T Endosulfan, a global pesticide: a review of its fate in the environment and occurrence in the Arctic. Sci Total Environ. 2010;408(15):2966–84. doi:10.1016/j.scitotenv.2009.10.077.
Freire C, Koifman RJ, Sarcinelli P, Rosa AC, Clapauch R, Koifman S. Long term exposure to organochlorine pesticides and thyroid function in children from Cidade dos Meninos, Rio de Janeiro, Brazil. Environ Res. 2012;117:68–74.
Reuber MD. Carcinogenicity of heptachlor and heptachlor epoxide. J Environ Pathol Toxicol Oncol. 1987;7(3):85–114.
Loomis D, Guyton K, Grosse Y, El Ghissasi F, Bouvard V, Benbrahim-Tallaa L, et al. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol. 2015;16(8):891–2.
Song M, Kim YJ, Park YK, Ryu JC. Changes in thyroid peroxidase activity in response to various chemicals. J Environ Monit. 2012;14(8):2121–6.
Juberg DR, Gehen SC, Coady KK, LeBaron MJ, Kramer VJ, Lu H, et al. Chlorpyrifos: wieght of evidence evaluation of patential interaction with estrogen, androgen or thyroid pathways. Regul Toxicol Pharmacol. 2013;66(3):249–63.
Slotkin TA, Cooper EM, Stapleton HM, Seidler FJ. Does thyroid disruption contribute to the developmental neurotoxicity of chlorpyrifos? Environ Toxicol Pharmacol. 2013;36(2):284–7.
De Cock M, Maas YG, van de Bor M. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Rev Acta Paediatr. 2012;101(8):811–8.
Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, et al. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104:5942–6.
Walker DM, Gore AG. Transgenerational neuroendocrine disruption of reproduction. Nat Rev Endocrinol. 2011;7:197–207. doi:10.1038/nrendo.2010.215.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have nothing to declare.
Conflict of interest
The authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Duntas, L.H., Stathatos, N. Toxic chemicals and thyroid function: hard facts and lateral thinking. Rev Endocr Metab Disord 16, 311–318 (2015). https://doi.org/10.1007/s11154-016-9331-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-016-9331-x