The effects of mixtures of Ni–Ta and Ni–Zr powders combined with sintered solid solution TaB2 –NbC during spark-plasma sintering of compositions at a pressing loading of 60 MPa in the range 1200 – 1600°C on the phase composition, percent content of Ti, Ta, and Zr in the sialon; microstructure; grain sizes of crystalline phases; relative density; linear shrinkage; physical-mechanical properties; linear correlation of the elasticity modulus and fracture toughness of mullite–β–SiAlON–TiC and mullite–β–SiAlON–c-ZrO2 samples are shown. Synthesized powders of β-SiAlON and TiC are characterized by extensive crystallization of β-SiAlON and TiC. c-ZrO2 spark-plasma sintered at 1400°C and solid solution TaB2 –NbC, at 1800°C, show extensive crystallization of c-ZrO2 and (Nb,Ta)C, B phases. The microstructure of solid solution TaB2 –NbC is crystalline, partially nonuniform, and almost completely sintered. Sintered samples with Ta and Zr additives show extensive mullitization; active crystallization of β-SiAlON, (Nb,Ta)C, B, and Ni(Nb,Ta); and varying crystallization of NiTi, NiTa, NiZr, and NiZr2 phases in the range 1200 – 1600°C. The microstructures of ceramic phases of samples with Ta additive are more uniformly and densely sintered. Particles of NiTi and NiTa in samples with Ta additive are uniformly and densely packed, unlike particles of NiTi, NiZr and NiZr, NiZr2 in samples with Zr additive. Sintering of compositions with Ta additive proceeds uniformly and extensively with formation of polydisperse compositions of crystalline phase grains. Samples with Ta additive show active growth and larger values of physical-mechanical properties, higher crack resistance, and better linear correlation of elasticity modulus and fracture toughness in the range 1200 – 1600°C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Spark-plasma sintering of solids with added powders of metals or their mixtures is the main method for producing solid and impact-resistant ceramics and cermets [1,2,3,4, – 5]. The nonuniform evolution of this sintering affects the phase composition and microstructure of boundary layers of oxide–oxide- free and oxide–oxide-free–metal crystalline phases and, as a result, causes variations in the elasticity modulus, fracture toughness, and hardness of these materials [2,3,4, – 5]. The degree of crystallinity, brittleness or density of crystalline phases, homogeneity of the microstructure, and width and ratio of brittle and elastic properties of boundary layers are responsible for the relationship between the phase composition, microstructure, and properties of the boundary layers of crystalline phases [2,3,4, – 5]. Less dense crystalline phases, in particular h-BN, β-WC, β-WB, and β-Zr, Ta, form nonuniform, broad, and brittle intermediate layers while amorphous carbon is extensively incorporated and accumulated between grains. As a result, the plastic properties of these boundary layers are degraded; regions of dislocations, microcracks, and boundary layers are generated; and the boundary structure of the material grains becomes brittle [2, 3, 5]. Dense crystalline phases, e.g., c-BN, β-MoC, β-MoB, and β-Ta, Zr, form uniform, narrow, and strong boundary layers that promote uniform and complete distribution of the plastic properties and propagation of microcracks along a tortuous trajectory at slow speed or hinder the advance of microcracks in boundary layers, as a result of which the boundary structure of the material grains densifies and strengthens [2,3,4, – 5]. These properties of the boundary layers are important because they determine how extensively and evenly ceramic and cermet materials absorb, accumulate, and dissipate impact energy and the ratio of absorption and dissipation/accumulation of different amounts of impact energy [2,3,4, – 5].
A method in which c-ZrO2 or solid solution TiC–ZrC is added to spark-plasma sintered ceramic and cermet compositions to densify and strengthen the boundary grain structure has been used [6,7, – 8]. Such additives have low efficiencies because they have dense structures and cause nonuniform and incomplete solid-state diffusion at the boundaries of the grains being sintered [6,7, – 8]. Also, the densification and strengthening of the grain boundary structure with added solid solution TiC–ZrC depends considerably on the structure and percent content of an actual component in this solid solution [7, 8].
A method for densification and strengthening the grain boundary structure by producing solid solution ZrB2–TaSi2 during spark-plasma sintering of the ceramic composition ZrB2–SiC–TaSi2 is known [9]. The sintering evolves and the solid solution forms simultaneously, which facilitates more extensive and complete incorporation of the solid-solution crystalline phase into the grain boundary structure to form uniform and narrow boundary layers between oxide-free crystalline phases and solid solutions in addition to dense intermediate layers between solid-solution crystalline phases [9]. This mechanism of densification and strengthening of the grain boundary structure is realized through solid-state sintering and depends on the structure and percent content of an actual component in the solid solution [9].
Another method for densification and strengthening of the grain boundary structure relates to spark-plasma sintering of cermet compositions using a nickel melt with added molybdenum powder [10,11, – 12]. As a result, diffusion through the formed low-melting eutectic is accelerated and evenly evolved to produce solid solutions of cermet and metallic phases of various compositions and stoichiometries [10,11, – 12]. The percent ratio of Ni and Mo in the sintered cermet compositions is important and determines the type, mechanism, and rate of sintering and the extent of side processes, in particular recrystallization and coalescence of particles of solid solutions of cermet and metallic phases [10,11, – 12]. Embrittlement (crumbling) to form microcracks occurs or the material grain boundary structure densifies and strengthens depending on the sintering specifics [10,11, – 12].
The goal of the present work was to study the effect of mixtures of Ni,Ta and Ni,Zr powders combined with sintered solid solution TaB2–NbC during spark-plasma sintering of compositions at a pressing loading of 60 MPa in the range 1200 – 1600°C on the phase composition; percent content of Ti, Ta, and Zr in the sialon; microstructure; grain size of crystalline phases; relative density, linear shrinkage, and physical-mechanical properties; and linear correlation of the elasticity modulus and fracture toughness of mullite–β-SiAlON–TiC and mullite–β-SiAlON–c-ZrO2 samples.
EXPERIMENTAL
Mixtures of Al2O3 and SiO2 powders were prepared using Al2O3 (Aldrich, Belgium, 97.5% pure) and SiO2 (Merck, Germany, 97.5% pure). These components were carefully weighed in the weight proportion (3:2) corresponding to stoichiometric mullite (Table 1) and mixed in a planetary ball mill (Retsch, PM 400) for ~10 min.
Powders of β-SiAlON, TiC, TaB2, and NbC were synthesized at 1660°C in a plasma-chemical unit under vacuum for 1 h through the reactions:
Sintered c-ZrO2 was spark-plasma synthesized at 1400°C under vacuum at a pressing loading of 35 MPa for 2 min using starting components (Table 1) in the ratio corresponding to the phase diagram of the biphasic system ZrO2–Y2O3 (Brown and Odell, Fan Fu-K’ang and Keler) [13]. The masses of the components (97 mol% ZrO2/3 mol% Y2O3) was 94.62/5.38 g per 100 g of mixture; ratio ZrO2/Y2O3, 17.58/1.
Sintered solid solution TaB2–NbC was prepared at 1800°C by spark-plasma sintering in vacuum at a pressing loading of 60 MPa for 5 min using TaB2 and NbC. The masses of the components (mol% TaB2/mol% NbC) was 65.64/34.36 g per 100 g of mixture; ratio TaB2/NbC, 1.91/1.
Sintered c-ZrO2 and solid solution TaB2–NbC were ground in a planetary ball mill (Retsch PM 400) for 30 min to produce powders of particle sizes from 5 to 10 μm.
Powders of β-SiAlON, TiC or sintered c-ZrO2 powder, and solid solution TaB2–NbC with a mixture of Ni, Ta or Ni, Zr additive were mixed in a planetary ball mill (Retsch PM 400) for ~10 min (Table 2).
The obtained mixture of Al2O3 and SiO2 powders was mixed with the prepared groups of powder mixtures (β-SiAlON/TiC/Ni/Ta/TaB2–NbC, β-SiAlON/TiC/Ni/Zr/TaB2–NbC, β-SiAlON/c-ZrO2/Ni/Ta/TaB2–NbC, and β-SiAlON/c-ZrO2/Ni/Zr/TaB2–NbC) in a planetary ball mill (Retsch PM 400) for ~10 min for further sintering.
The obtained mixtures of components were loaded into a graphite press die of diameter 30 mm and spark-plasma sintered (SPS, Sumimoto, Model SPS 825. CE, Dr. Sinter, Japan) at 1200 – 1600°C and heating rate 100°C/min under vacuum (6 Pa) at a pressing loading of 60 MPa for 2 min.
Phase compositions of synthesized powders and sintered samples, microstructure, relative density ρrel, linear shrinkage l, elasticity modulus E, Vickers hardness HV, and fracture toughness K1c were determined by well-known methods [7]. The theoretical density (g/cm3) of the components were mullite 3.17, -Si3Al3O3N5 3.09, TiC 4.93, c-ZrO2 6.27, TaB2 11.70, NbC 7.82, Ni3Ti 7.9, Ni3Ta 12.0, Ni3Zr 8.3, NiTi 6.6, NiTa 9.56, NiZr 7.4, and NiZr2 7.2.
RESULTS AND DISCUSSION
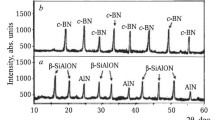
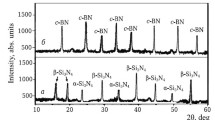
The phase compositions of the synthesized sialon and TiC powders showed strong diffraction maxima for β-SiAlON and TiC with small amounts of unreacted AlN and titanium oxycarbide, respectively (Fig. 1). The crystalline phase TiCxOy was nonstoichiometric TiC. It contained unreacted TiO2 and C. The obtained TaB2 and NbC powders consisted of developed TaB2 and NbC crystalline phases.
Sintered c-ZrO2 was characterized by distinct diffraction maxima of this phase (Fig. 2). This was explained by incorporation (intercalation) of Y3+ cations into the crystal structure of tetragonal ZrO2 that stimulated rearrangement of tetragonal into cubic ZrO2 with formation of the solid solution of (cubic) ZrO2 at 1400°C at a pressing load of 35 MPa according to the equilibrium phase diagram of the ZrO2–Y2O3 system (Brown and Odell, Fan Fu-K’ang and Keler) [13].
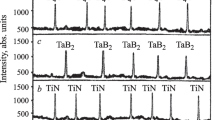
The x-ray pattern of sintered solid solution TaB2–NbC showed stronger diffraction maxima of (Nb,Ta)C,B and weaker, of (Ta,Nb)B,C (Fig. 3). This was explained by more active incorporation (intercalation) of small-sized Ta4+ into the dense (cubic) NbC structure as compared to the less vigorous incorporation of large-sized Nb4+ into the less dense (hexagonal) TaB2 structure. The difference in the intensities of the (Nb,Ta)C,B and (Ta,Nb)B,C diffraction maxima was explained by the large difference in the sizes of the Ta4+ and Nb4+ cations and, as a result, the different extents of the structure-formation processes of the solid solutions in the solids at a pressing loading of 70 MPa. The size effect of Ta4+ and Nb4+ was proportional to the development of the (Nb,Ta)C,B and (Ta,NB)B,C crystalline phases and corresponded to the ratio of TaB2 and NbC. The microstructure of sintered solid solution TaB2–NbC was crystalline, partially nonuniformly sintered, and variously agglomerated with a small amount of variously sized pores, in particular, in the crystalline (Nb,Ta)C,B region in which densely sintered (Ta,Nb)B,C particles were noticeable (Fig. 4). This microstructure was due to active incorporation of the small Ta4+ cation into the dense (cubic) NbC structure, as a result of which TaB2 stimulated sintering to a lesser extent and was mainly consumed for crystallization of (Nb,Ta)C,B (Fig. 3). This slowed solid-state sintering of (Nb,Ta)C,B and (Ta,Nb)B,C grains. Densely sintered particles of (Ta,Nb)B,C with a crystalline (Nb,Ta)C,B region formed because of the less developed crystallization of (Ta,Nb)B,C (Fig. 4), during the course of which mainly NbC stimulated sintering in combination with dense compaction of sintered TaB2 and NbC particles. As a result, TaB2 and NbC particles were more uniformly and strongly sintered. Boundary grains of these solid solutions were densified and strengthened as compared to formation of an intermediate layer of (Nb,Ta)C,B in the boundary parts of sintered TaB2 and NbC particles. The brittleness and extent of developed elastic properties at the boundaries of (Nb,Ta)C,B and (Ta,Nb)B,C particles were significantly reduced (Fig. 5).
Figure 6 shows the phase composition of samples spark-plasma sintered in the range 1200 – 1600°C.
Phase composition of M20SiAl3TiC25Ni25Ta27(50TaB2–50NbC) (a), M20SiAl3TiC25Ni25Zr27(50TaB2–50NbC) (b ), M20SiAl3ZrO225Ni25Ta27(50TaB2–50NbC) (c), and M20SiAl3ZrO225Ni25Zr27(50TaB2–50NbC) (d ) sintered in the range 1200 – 1600°C: M is mullite (3Al2O3·2SiO2); Ni(Nb,Ta), solid solution of nickel niobium tantalum; NixC, NixB, nonstoichiometric compositions of nickel carbide and boride; Ni3Ti, trinickel titanium; Ni3Ta, trinickel tantalum; Ni3Zr, trinickel zirconium; NiTi, nickel titanium; NiTa, nickel tantalum; NiZr, nickel zirconium; NiZr2, nickel dizirconium.
Samples with Ni, Ta and Ni, Zr mixtures were characterized by extensive mullitization and greater crystallization of (Nb,Ta)C,B than the less developed crystalline (Ta,Nb)B,C in the range 1400 – 1600°C. Mullite appeared because of active dissolution of Al2O3 and SiO2 in the nickel melt with the formation and structuring of stoichiometric mullite via this melt. Copious crystallization of (Nb,Ta)C,B was explained by the poorer dissolution of the less dense (Nb,Ta)C,B in the nickel melt in the range 1400 – 1600°C. Extensive crystalline Ni(Nb,Ta) was observed in the samples, the development of which was similar in amount to crystalline (Nb,Ta)C,B. Also, the lack of crystalline Ni(Ta,Nb) was notable in the range 1400 – 1600°C (Fig. 6a – d). This was related to the low solubility of (Ta,Nb)B,C in the nickel melt. Crystalline Ni(Nb,Ta) had a different composition than (Nb,Ta)C,B in the range 1400 – 1600°C (Fig. 6a – d). In particular, it contained only metallic components. Hence, crystalline Ni(Nb,Ta) was a metallic phase. The phase Ni(Nb,Ta) was initiated at 1400°C in the nickel melt and developed in the nickel melt saturated (eutectic) in the range 1400 – 1500°C and supersaturated (peritectic) in the range 1500 – 1600°C at a pressing loading of 60 MPa via the reactions:
(Nb,Ta)C,B + Nimelt
→ Ni2+(Nb,Ta)C,B(soln. in Ni melt 1400–1600°C)
→ NiTa + NiNb + Nimelt
→ Ni2+(Nb1–x, xTa2+)(main process 1400–1600°C)
+ Ni2+(Ta1–x, xNb2+)(secondary process 1400 – 1600°C) + Nimelt
→ Ni(Nb1–x, xTa)(exten. cryst., stoi. comp. dense struct.)
+ Ni(Ta1–x, xNb)(little crystl., nonstoi. comp. loose struct.)
+ Nix C, NixB(little crystl.).
Different amounts of crystalline β-SiAlON were noticeable in the samples. It was more abundant in samples with TiC, Ta and TiC, Zr mixtures than in samples with c-ZrO2, Ta and c-ZrO2, Zr mixtures in the range 1200 – 1600°C (Fig. 6). This was due to more active incorporation of smaller Ti4+ and Ta4+ cations and larger Zr4+ cations into the tetrahedral AlNxO4–x structure and partially into the tetrahedral SiNxO4–x structure of sialon with better dissolution of TiC, Ta and TiC, Zr mixtures in the nickel melt as compared to gradual dissolution of c-ZrO2, Ta and c-ZrO2, Zr in it.
The samples showed weak peaks for crystalline Ni3Ti, Ni3Ta, and Ni3Zr in the range 1200 – 1300°C (Fig. 6a – d). Crystalline Ni3Ti formed through congruent melting of Ni3Ti3 + NiTi↔Ni3Ti at 1140°C; crystalline Ni3Ta, through peritectic reaction Ni8Ta↔Nis + Ni3Ta at 1150°C and eutectic reaction Ni8Tamelt↔Nis + Ni3Ta at 1230°C; crystalline Ni3Zr, through peritectic reactions Ni7Zr2 + Ni21Zr8↔Ni3Zr and Ni7Zr2 + Ni5Zr2↔Ni3Zr at 1170°C in the equilibrium diagrams of biphasic Ni–Ti, Ni–Ta, and Ni–Zr [14,15, – 16]. As a result, development of crystalline TiC and c-ZrO2 in the range 1200 – 1300°C was somewhat slowed (Fig. 6a – d).
A slight ingrowth of crystalline NiTi was observed in the samples in the range 1500 – 1600°C (Fig. 6a and b ). It formed through the peritectic reaction melt + Ni3Ti↔NiTi at 1400°C. This agreed with the equilibrium diagram of the biphasic Ni–Ti system [14]. Poor development of crystalline NiTi was due to more active incorporation of Ti4+ into the tetrahedral AlNxO4–x structure of sialon (Fig. 6a and b ).
A large amount of crystalline NiTa was observed in the samples in the range 1500 – 1600°C (Fig. 6a and c). It formed through the eutectic reaction melt↔Ni2Ta + NiTa at 1350°C with initiation of NiTa crystallization at 1400°C. The occurrence of this reaction assisted dissolution of Ni3Ta in the melt and formation of Ni2Ta through the peritectic reaction melt + Ni3Ta↔Ni2Ta at 1400°C. These processes agreed with the equilibrium diagram of the biphasic Ni–Ta system [15, 17].
However, weaker diffraction maxima of NiZr were noticeable in the samples in the range 1400 – 1600°C (Fig. 6b – d). This phase was initiated through the eutectic reaction Ni11Zr9↔Ni10Zr7 + NiZr in the range 1200 – 1300°C and developed through the eutectic reaction Ni3Zr + melt↔NiZr in the range 1300 – 1400°C (Fig. 6). Crystallization of NiZr through the eutectic reaction was more vigorous than development of crystalline Ni3Zr up to 1300°C (Fig. 6b and d ). This was related to the different formation reactions of crystalline Ni3Zr and NiZr in the solid phase. Crystallization of NiZr slowed above 1400°C while crystallization of NiZr2 was initiated (Fig. 6b and d ) through the eutectic reaction melt↔NiZr + NiZr2 at 1400°C with formation of this melt through the eutectic reactions Ni7Zr2 + Ni11Zr9↔melt and Ni10Zr7 + Ni21Zr8↔melt. Crystalline NiZr2 was more developed than crystallization of NiZr in the range 1500 – 1600°C (Fig. 6b and d ). This was related to dissolution of NiZr in the eutectic melts and formation of a melt of Ni11Zr9 through the peritectic reaction melt + NiZr↔Ni11Zr9 at 1500°C. These reactions agreed with the equilibrium diagram of the biphasic Ni–Zr system [16, 18].
Reactions of mullite, β-SiAlON, and c-ZrO2 with TiC, solid solutions, and intermetallic compounds of various compositions were not observed because decomposition products of mullite and sialon and oxidation of sialon; TiC; (Nb,Ta)C,B; Ni(Nb,Ta); (Ta,Nb)B,C; NiTi; NiTa; NiZr; and NiZr2 were missing in the range 1200 – 1600°C. Also, reactions between TiC; c-ZrO2; (Nb,Ta)C,B; and (Ta,Nb)B,C with Ta and Zr powders were not observed. This was due to the better dissolution of Ta and Zr powders in the nickel melt. As a result, additional diffraction maxima and their shifting in the range 1200 – 1600°C were not observed.
Figure 7 shows the composition, peak intensities of elements of crystalline phases, and the effects of Ti4+, Ta4+, and Zr4+ on the growth of sialon Si and Al peaks of samples sintered at 1500°C as studied by energy dispersive x-ray spectroscopy.
The intensities of the element peaks of the crystalline phases (Fig. 7) agreed with the development of the crystalline phases of the sample at 1500°C (Fig. 6a – d). The Si and Al peaks had different intensities in the samples sintered at 1500°C because the mullite and sialon crystalline phases were actively structured by the nickel melt (Fig. 6a – d). The positions of the Si and Al peaks in mullite and sialon coincided because the Si and Al cations in both compounds were identically coordinated and located in similar polyhedral positions of the crystal structures of these phases. On the other hand, the more active incorporation of Ti4+, Ta4+ and Ti4+, Zr4+ into the tetrahedral AlNxO4–x sialon structure because of extensive dissolution of TiC, Ta and TiC, Zr mixtures in the nickel melt facilitated formation of strong Si and Al peaks, in contrast to the weaker Si and Al peaks for less active incorporation of Zr4+, Ta4+ and Zr4+, Zr4+ into the tetrahedral AlNxO4–x sialon structure because of gradual dissolution of cZrO2, Ta and cZrO2, Zr mixtures in the nickel melt. The strong Ti peak (4.5 keV) was due to active incorporation of Ti4+ into the tetrahedral AlNxO4–x sialon structure. As a result, the percent content of Si and Al, Ti/Ta, and Ti/Zr was noticeably greater in sialon of samples with TiC, Ta and TiC, Zr mixtures, in contrast with the percent content of Si and Al, Zr/Ta, and Zr/Zr in sialon of samples with c-ZrO2, Ta and c-ZrO2/Zr mixtures (Table 3). The strong Ta peak (1.70 keV) and weaker Nb peak (2.2 keV) were due to extensive crystallization of (Nb,Ta)C,B and Ni(Nb,Ta) and less crystalline (Ta,Nb)B,C at 1500°C, respectively (fig. 6a-d). The strong Ni peaks (0.85 and 7.5 keV) in combination with medium peaks for Ti (0.5 and 4.9 keV), Ta (8.18 keV), and Zr (2.2 keV) were due to formation of crystalline NiTi, NiTa, and NiZr, respectively, at 1500°C (Fig. 6a – c). A stronger and narrower Zr peak (2.2 keV) was noticeable in the sample with the c-ZrO2, Zr mixture. This was explained by dissolution of NiZr in the eutectic melts formed from Ni7Zr2 + Ni11Zr9 and Ni10Zr7 + Ni21Zr8 with formation of a peritectic melt of composition N11Zr9, through which NiZr2 crystallized at 1500°C (Fig. 6d ). Simultaneously, the Nb peak (2.2 keV) was stronger than the Zr peak (2.2 keV) in the samples. This was due to greater crystallization of (Ta,Nb)B,C as compared to lesser crystallization of NiZr and NiZr2 at 1500°C (Fig. 6b – d). Also, the observed weak Ti (5.17 and 5.52 keV) and Ta (7.18 keV) peaks were related to little and incomplete crystallization of NiTi and NiTa phases, respectively, at 1500°C (Fig. 6a – c) because of extensive incorporation of Ti4+ and Ta4+ into the tetrahedral AlNxO4–x sialon structure during dissolution of TiC and Ta in the nickel melt at 1500°C (Fig. 6a – c).
The different effects of Ti4+, Ta4+, and Zr4+ on the evolution of the sialon Si and Al peaks had different effects on the percent content of Si and Al, Ti, Ta, and Zr in the sialon samples at 1500°C (Table 3).
The microstructure of the ceramic phase of the sample with the TiC, Ta mixture was more uniformly and densely sintered, finely grained, and pore-free (Fig. 8a ) because of the equally vigorous and complete incorporation of Ti4+ and Ta4+ into the tetrahedral AlNxO4–x sialon structure (Fig. 6a ). This was consistent with rapid diffusion through the sialon oxide component and uniform sintering of sialon particles with (Ta,Nb)B,C; (Nb,Ta)C,B; and Ni(Nb,Ta) particles. The microstructure of the ceramic phase in the sample with the c-ZrO2, Ta mixture was about the same (Fig. 8c ) and contained separately positioned agglomerates of various sizes and a slightly greater number of small pores. The smaller agglomerates consisted of weakly sintered particles of sialon and (Ta,Nb)B,C; large agglomerates, of highly sintered sialon; (Nb,Ta)C,B; and Ni(Nb,Ta) particles. These agglomerates formed because of the more nonuniform sintering of these particles resulting from slower diffusion through the sialon oxide component during nonuniform incorporation of Zr4+ and Ta4+ into the tetrahedral AlNxO4–x sialon structure (Fig. 6c ). Such sintering led to incomplete pore filling.
Samples with the TiC, Zr and c-ZrO2, Zr mixtures had a noticeably more uniformly sintered ceramic-phase microstructure with a significant content of agglomerates of various sizes consisting of variously sintered particles of (Nb,Ta)C,B; Ni(Nb,Ta); and (Ta,Nb)B,C and large pores (Fig. 8b and d ). Denser monolithic agglomerates of (Nb,Ta)C,B and Ni(Nb,Ta) of sizes up to 5 – 8 μm formed, in contrast to the less dense agglomerates of (Ta,Nb)B,C of sizes 2.5 – 5 μm. This was related to the different extents of recrystallization of (Nb,Ta)C,B, Ni(Nb,Ta), and (Ta,Nb)B,C particles and dissolution of Zr powder with TiC and c-ZrO2 in the Ni melt, during which a eutectic melt of NiZr and a peritectic melt of NiZr2 of different viscosities formed in the sintered compositions with TiC, Zr and c-ZrO2, Zr. This affected the uniformity and rate of diffusion through the nickel melt and; therefore, the rate of recrystallization of the particles and the homogeneity of the shapes and sizes of the agglomerates of the solid solutions of the ceramic and metallic phases. As a result, the microstructure of the ceramic phase of the sample with c-ZrO2, Zr was less uniform and highly agglomerated (Fig. 8d ).
The microstructure of the metallic phases (Fig. 8, a1 and c1) corresponded to the microstructure of the ceramic phases of the samples with the TiC, Ta and c-ZrO2, Ta mixtures (Fig. 8a and c). However, several differences were seen in the microstructure of the metallic phases, in particular, the NiTi particles were more densely sintered as soft agglomerates of sizes 2 – 5 μm upon dissolution of TiC, c-ZrO2 in the nickel melt (Fig. 8a1), in contrast to the separately positioned and large NiZr particles of sizes 2 – 2.5 μm, between which pores were observed (Fig. 8c1).
Partially sintered NiTa particles formed during dissolution of Ta powder in the nickel melt as soft agglomerates consisting of smaller NiTa particles (Fig. 8a1) than separately positioned small rounder NiTa particles (Fig. 8c1). The structures and shapes of the NiTi, NiZr, and NiTa particles differed because of the different formation reactions of crystalline NiTi, NiZr, and NiTa phases in the corresponding NiTi peritectic melt and NiZr and NiTa eutectic melts at 1500°C.
In turn, the microstructures of the metallic phases (Fig. 8b1 and d1) were more uniform and contained many pores when compared to the microstructures of the ceramic phases of the samples with TiC, Zr and c-ZrO2, Zr mixtures (Fig. 8b and d ). Weakly sintered NiTi particles as soft agglomerates of sizes ~2.5 μm (Fig. 8b1) were noticeable in the microstructure of the metallic phases upon dissolution ofTiC and c-ZrO2 in the nickel melt, in contrast to more rounded smaller NiZr particles of sizes 0.5 – 1 μm (Fig. 8d1). The NiTi and NiZr particles were separated from each other by pores. The differences in the structures and shapes of the NiTi and NiZr particles were related to the different formation reactions of the crystalline NiTi and NiZr/NiZr2 phases in the peritectic NiTi melt and the eutectic/peritectic NiZr/NiZr2 melts at 1500°C. As a result, the compounds diffused unevenly between the sintered NiTi and NiZr particles with nonuniform and incompletely filled pores (Fig. 8b1 and d1).
The NiZr particles that formed in the presence of NiTi during dissolution of Zr powder in the nickel melt were more rounded and smaller (to 1 μm) (Fig. 8b1) than the NiZr particles that were more agglomerated and larger (1 – 1.5 μm) in the presence of NiZr (Fig. 8d1). This was explained by the different formation reactions of the crystalline NiTi and NiZr/NiZr2 phases in the NiTi peritectic melt and the NiZr/NiZr2 eutectic/peritectic melt at 1500°C. Separately positioned dense NiZr2 agglomerates of various sizes were observed in the sample with the c-ZrO2, Zr mixture (Fig. 8d1). This was related to dissolution of particles of crystalline NiZr phase in eutectic melts at 1300°C with formation of the Ni11Zr9 peritectic melt at 1500°C, in which nonuniform recrystallization (growth) of NiZr2 particles occurred, slowed most the sintering of NiZr and NiZr2 particles, and caused irregular and incomplete pore filling.
Figures 9 – 13 show the grain sizes of the crystalline phases, ρrel and ∆l in the range 1200 – 1600°C, the microstructure of the boundary regions of oxide and oxide- free crystalline phases at 1500°C, the physical-mechanical properties in the range 1200 – 1600°C, and photographs of indentations at 1500°C of samples with TiC, Ta; c-ZrO2, Ta; TiC, Zr; and c-ZrO2, Zr mixtures.
The quantities ρrel and ∆l of samples with TiC, Ta and c-ZrO2, Ta mixtures changed dramatically and uniformly in the range 1200 – 1600°C because β-SiAlON rapidly crystallized, approximately equal to crystallization of the (Nb,Ta)C,B and Ni(Nb,Ta) phases (Fig. 6a and c); particles of sialon, TiC, and c-ZrO2 sintered uniformly with particles of (Nb,Ta)C,B, Ni(Nb,Ta), and (Ta,Nb)B,C; evenly and densely sintered microstructures of ceramic phases formed in the nickel melt (Fig. 8a and c); and a polydisperse composition of crystalline phase grains developed (Fig. 9). The composition with the TiC, Ta mixture sintered somewhat more extensively with large increases of ρrel and ∆l than the composition with the c-ZrO2, Ta mixture. This correlated with several differences in the microstructures of the ceramic and metallic phases (Fig. 8a, c, a1, c1) and the grain size distribution of the crystalline phases (Fig. 9). These differences were explained by faster diffusion processes in the peritectic NiTi melt and eutectic NiTa melt, in contrast to slower diffusion in the eutectic NiZr and NiTa melts. As a result, pores in the ceramic and metallic phases of sintered compositions filled differently (Fig. 8a, c, a1, c1). The ρrel and ∆l values of the sample with the c-ZrO2, Ta mixture were close to those of the sample with the TiC, Ta mixture in the range 1500 – 1600°C. This was due to the formation of NiZr and NiTa particles of a different shape (Fig. 8c1) and a more polydisperse grain composition of the crystalline phases (Fig. 9) than of the NiTi and NiTa particles as soft agglomerates (Fig. 8a1).
The ρrel and ∆l values of the sample with the TiC, Zr mixture increased in the range 1200 – 1600°C because diffusion processes in the solid phase during congruent melting with Ni3Ti3 and peritectic reactions with Ni7Zr2, Ni21Zr8 and Ni7Zr2, Ni5Zr2 were unequal and incomplete in the range 1200 – 1300°C, a nonuniformly sintered microstructure of the ceramic phase with agglomerates and many variously sized pores formed (Fig. 8b ), diffusion processes in the metallic phase were slower and variously sintered NiTi and NiZr particles of various shapes and sizes formed (Fig. 8b1), and a relatively monodisperse grain composition of the crystalline phases developed (Fig. 9).
The quantities ρrel and ∆l of the sample with the c-ZrO2, Zr mixture evolved nonuniformly. In particular, the values of these properties increased smoothly up to 1400°C and decreased in the range 1400 – 1600°C. The properties increased smoothly up to 1400°C because of unequal and incomplete diffusion in the solid phase during peritectic reactions with Ni7Zr2, Ni21Zr8 and Ni7Zr2, Ni5Zr2 in the range 1200 – 1300°C. The ρrel and ∆l values decreased in the range 1400 – 1600°C because of the formation of a more nonuniformly sintered ceramic phase microstructure with recrystallization of (Nb,Ta)C,B, Ni(Nb,Ta), and (Ta,Nb)B,C particles (Fig. 8d ); slower diffusion in the metallic phase with partial recrystallization of NiZr particles; rapid recrystallization of NiZr2 particles (Fig. 8d1); and formation of a more monodisperse grain composition of the crystalline phases (Fig. 9).
The evolution of the physical-mechanical properties of samples with added Ta, Zr corresponded to sintering of these compositions in the range 1200 – 1600°C. The quantities E and K1c increased most for samples with TiC, Ta and c-ZrO2, Ta mixtures. The HV values were observed to increase evenly with minor differences in samples with the TiC, Zr mixture. The difference was much more substantial for the E values of these samples than for the K1c values. The sample with the c-ZrO2, Zr mixture showed nonuniform evolution of the properties in the range 1200 – 1600°C.
The more developed E and K1c values of samples with TiC, Ta and c-ZrO2, Ta mixtures were due to the variety of crystalline formations; the different shapes and sizes of NiTi, NiTa, and NiZr particles (Fig. 8a1, c1); the polydisperse grain composition of the crystalline phases (Fig. 9); and the positioning of regions of NiTi, NiTa, and NiZr of different sizes in crystalline structures of the ceramic and metallic phases, in their boundary layers (Fig. 11a2, a3, c2, c3), and at grain boundaries as thin intermediate layers, separate regions, and grains between them (Fig. 11a2-0, a3-0, c2-0, c3-0). The uniform microstructures (Fig. 8a1, c1) and polydisperse grain composition of the crystalline phases (Fig. 9) promoted densification of differently sized particles and increased the structural rigidity. Finely disperse particles with dense NiTi (cubic), NiTa, and NiZr (rhombohedral) structures hardened regions of the ceramic and metallic phases and boundary layers and stimulated more uniform and complete dissipation of stresses in boundary particles of the ceramic and metallic phases and even distribution of plastic properties in the structures of NiTi, NiTa, and NiZr crystalline phases and in boundary layers. As a result, homogeneous and narrow boundary layers of mullite–β-SiAlON–c-ZrO2 and (Ta,Nb)B,C– (Nb,Ta)C,B–Ni(Nb,Ta) were observed (Fig. 11a – a2, c – c2) and helped to densify, strengthen, and evolve the plastic properties of these boundary layers. Expanding microcracks interacted extensively with particles of these phases and NiTi and NiTa as soft agglomerates during the most even and complete dissipation of stresses in boundary particles of the ceramic and metallic phases, in contrast to the less vigorous interaction of the microcracks with separately positioned large NiTa and NiZr particles. High crack resistance without microcracks was noticed in the sample with the TiC, Ta mixture (Fig. 13a1) as compared to slight damage and several shallow chips around the indentation of the sample with the c-ZrO2, Ta mixture (Fig. 13c1). The crack resistance of these samples was much greater at 1500°C than at 1300°C, where rapid propagation of several microcracks along linear (Fig. 13a) and tortuous paths with large chips around the indentation (Fig. 13c) were observed. This was related to nonuniform and incomplete solid-state diffusion processes because of congruent melting with Ni3Ti3 at 1140°C, a peritectic reaction with Ni8Ta at 1150°C, and a eutectic reaction with Ni8Ta at 1230°C in the sintered composition with the TiC, Ta mixture (Fig. 6a ) and a peritectic reaction with Ni7Zr2, Ni21Zr8 and Ni7Zr2, Ni5Zr2 at 1170°C, a peritectic reaction with Ni8Ta at 1150°C, and a eutectic reaction with Ni8Ta at 1230°C in the sintered composition with the c-ZrO2, Ta mixture (Fig. 6c ) caused by their nonuniform sintering in the range 1200 – 1300°C (Fig. 10). In general, these results correlated with the change of K1c and HV in the range 1200 – 1600°C.
The nonuniform and slower evolution of the physical-mechanical properties of the samples with the TiC, Zr and c-ZrO2, Zr mixtures was explained by the nonuniformly sintered microstructure of the ceramic phases that consisted of variously sintered agglomerates of (Ta,Nb)B,C, (Nb,Ta)C,B, and Ni(Nb,Ta) (Fig. 8b and d ); weakly sintered NiTi and NiZr particles of various shapes and sizes (Fig. 8b1); large NiZr grains and variously sized dense NiZr2 agglomerates (Fig. 8d1); the different monodisperse composition of grains of crystalline phases (Fig. 9); the location of NiTi, NiZr, and NiZr2 regions at different distances from the boundary layers of crystalline ceramic and metallic phases (Fig. 11b2, b3, d2, d3) with intermediate layers of various widths on the boundary grains and separate regions between them (Fig. 11b2-0, b3-0, d2-0, d3-0). The nonuniformity of the microstructures (Fig. 8b, d, b1, d1) and the different monodisperse composition of crystalline phase grains (Fig. 9) caused uneven densification of the particles and reduction of the structural rigidity. Particles with dense NiTi (cubic), NiZr (rhombohedral), and NiZr2 structures (tetragonal) caused regions of ceramic and metallic phases and boundary layers to have different hardness values. The hardness was greater with NiTi and NiZr, NiZr2 particles than with NiZr and NiZr2 particles. The sample with the TiC, Zr mixture had a less monodisperse composition of crystalline phase grains (Fig. 9) that helped to dissipate more uniformly and completely stresses on boundary particles of the ceramic and metallic phases and to distribute evenly the plastic properties in regional structures of NiTi and NiZr crystalline phases in boundary layers. However, these processes were nonuniformly evolved and slower in the sample with the c-ZrO2, Zr mixture because of the various shapes, degree of sintering (Fig. 8d1), and large sizes of NiZr and NiZr2 particles (Fig. 9). As a result, nonuniform and broad boundary layers of mullite–β-SiAlON–c-ZrO2 and (Ta,Nb)B,C– (Nb,Ta)C,B–Ni(Nb,Ta) were noticeable and densified and strengthened these layers differently (Fig. 11b – b2, d – d2). Expanding microcracks interacted differently with particles and/or local regions of stresses around particles of these phases during different dissipation of stresses on boundary particles of the ceramic and metallic phases, in particular, rapidly in the presence of NiTi and NiZr, NiZr2 particles and slower in the presence of NiZr and NiZr2 particles. This determined the various crack resistances of the samples. Propagation of microcracks along a short tortuous path with a small chip around the indentation was noticeable in the sample with the TiC, Zr mixture with developed plastic properties (Fig. 13b1) that was caused by active interaction of finely disperse particles of NiTi and NiZr, NiZr2 with local stressed regions along the (Nb,Ta)C,B boundary layer. On the other hand, the microcracks were tortuous because of interaction of shallow point dislocations in the Ni(Nb,Ta) structure and with local stressed regions around ceramic and metallic particles and (Nb,Ta)C,B boundary layers (Fig. 13b1-0). Contact points of regions of ceramic and metallic phases and the (Nb,Ta)C,B boundary layer were densified and strengthened as a result of these processes. The (Nb,Ta)C,B boundary layer slowed the advance of microcracks (Fig. 13b1-1). Linear propagation of microcracks in various directions with much damage around the indentation at 1300°C (Fig. 13b ) was observed in this sample. The sample with the c-ZrO2,Zr mixture had much lower crack resistance. Extensive propagation of microcracks along a long linear trajectories and formation of slight damage and small microcracks along the indentation (Fig. 13d1) related to uneven and incomplete interaction of highly disperse NiZr and NiZr2 particles with local stressed regions along the (Nb,Ta)C,B boundary layer and its embrittlement were observed in it. This was related to the different types of dislocations. In particular, dislocations combined at the boundaries of NiZr, NiZr2 particles. Dislocations layered at the boundaries of NiZr, NiZr2,(Nb,Ta)C,B particles (Fig. 13d1-0). Combination of dislocations, in contrast to layering, was associated with great densification and strengthening of the structure, i.e., more developed plastic properties than layered dislocations. As a result, stresses were more evenly dissipated in combined dislocations than in layered dislocations where this process was nonuniform and limited. Thus, plastic properties and hardness evolved more at the boundaries of NiZr, NiZr2 particles than at boundaries of NiZr, NiZr2, (Nb,Ta)C,B particles. A narrower short microcrack advanced tortuously through a combined dislocation. A broader microcrack propagated linearly and rapidly through a layered dislocation (Fig. 13d1-1). The crack resistance of the sample with the c-ZrO2, Zr mixture at 1500°C was slighter greater than that of the sample at 1300°C and had much damage and many microcracks around the indentation (Fig. 13d, d1). The combination of the above processes had different effects on the linear correlation of E and K1c of the samples in the range 1200 – 1600°C (Fig. 14).
Microstructure of boundary regions of mullite;, β-SiAlON; c-ZrO2; (Nb,Ta)C,B; (Ta,Nb)B,C; Ni(Nb,Ta); NiTi; NiTa; NiZr; NiZr2 and grain boundaries of ceramic and metallic phases of M20SiAl3TiC25Ni25Ta27(50TaB2–50NbC) (a – a3, a2-0, a3-0), M20SiAl3TiC25Ni25Zr27(50TaB2–50NbC) (b – b3, b2-0, b3-0), M20SiAl3ZrO225Ni25Ta27(50TaB2–50NbC) (c – c3, c2-0, c3-0), and M20SiAl3ZrO225Ni25Zr27(50TaB2–50NbC) (d – d3, d2-0, d3-0) sintered at 1500°C: A is boundary layer (Nb,Ta)C,B.
Photos of indentations for measuring HV of M20SiAl3TiC25Ni25Ta27(50TaB2–50NbC) (a, a1), M20SiAl3TiC25Ni25Zr27(50TaB2–50NbC) (b, b1), M20SiAl3ZrO225Ni25Ta27(50TaB2–50NbC) (c, c1), and M20SiAl3ZrO225Ni25Zr27(50TaB2–50NbC) (d, d1) sintered at 1300 (a – d) and 1600°C (a1 – d1) indicating types of dislocations and microcrack propagation trajectories in samples with TiC, Zr (b1-0, b1-1) and c-ZrO2, Zr mixtures (d1-0, d1-1).
The R2 value differed insignificantly by 0.02 and 0.01 in samples with the Ta, Zr additive. The E and K1c values correlated almost exactly relative to the lines of the samples with the Ta additive in the range 1200 – 1600°C and differed in the range 1500 – 1600°C. As a result, the positions of the lines relative to the E and K1c values differed in the range 1500 – 1600°C as compared to the samples with the Ta additive up to 1500°C. The correlation of the property values was less exact relative to the lines in samples with the Zr additive in the range 1200 – 1600°C, where the property values correlated well for the sample with the TiC, Zr mixture in the range 1200 – 1600°C. However, the position of the lines relative to the E and K1c values of samples with the Zr additive were similar in the range 1200 – 1600°C because the difference of the R2 values was minimal, indicating a close correlation of the property values relative to each other and positions of lines on the same line of the samples with the Zr additive in the range 1200 – 1600°C.
The correlation of the E and K1c values was about the same relative to the lines of samples with the Ta additive up to 1500°C. This was explained by solid-state diffusion through congruent melting with Ni3Ti3 at 1140°C, a peritectic reaction with Ni8Ta at 1150°C, a eutectic reaction with Ni8Ta at 1230°C in the sintered composition with the TiC, Ta mixture (Fig. 6a ), a peritectic reaction with Ni7Zr2, Ni21Zr8 and Ni7Zr2, Ni5 Zr2 at 1170°C and with Ni8Ta at 1150°C, a eutectic reaction with Ni8Ta at 1230°C in the sintered composition with the c-ZrO2/Ta mixture (Fig. 6c ), the polydisperse composition of the crystalline phase grains (Fig. 9), and the equal crack resistance (Fig. 13a and c). Differences in the correlation of the property values relative to the lines of samples with the Ta additive in the range 1500 – 1600°C were related to the difference and extent of crystalline phase formation (Fig. 6a and c), the form and degree of sintering (Fig. 8a1, c1), the small reduction of polydispersion of crystalline phase grains (Fig. 9), and the difference in the crack resistance (Fig. 13a1, c1). These results were responsible for the reduced correlation of the E and K1c values relative to the lines and the small differences of rather high R2 values of samples with the Ta additive in the range 1200 – 1600°C. This was due to the position of NiTi, NiTa, and NiZr regions of different sizes in the crystal structures of the ceramic and metallic phases; in boundary layers of cermet and metallic phases (Fig. 11a2, a3, c2, c3) at grain boundaries as thin intermediate layers, separate regions, and grains between them (Fig. 11a2-0, a3-0, c2-0, c3-0) that helped to densify, strengthen, and evolve the plastic properties of boundary layers of mullite–β-SiAlON–c-ZrO2 and (Ta,Nb)B,C–(Nb,Ta)C,B–Ni(Nb,Ta) (Fig. 11a – a2, c – c2).
The correlations of the property values relative to the lines of samples with the Zr additive up to 1500°C were similar because of diffusion through congruent melting with Ni3Ti3 at 1140°C, a peritectic reaction with Ni7Zr3, Ni21Zr8 and Ni7Zr2, Ni5 Zr2 at 1170°C in the sintered composition with the TiC, Zr mixture (Fig. 6b ) and with Ni7Zr2, Ni21Zr8 and Ni7Zr2, Ni5 Zr2 at 1170°C in the sintered composition with the c-ZrO2, Zr mixture (Fig. 6d ), the almost equal size distributions of crystalline phase grains (Fig. 9), and the practically identical crack resistance (Fig. 13b and d ). Differences in the correlation of the property values relative to the lines of samples with the Zr additive in the range 1500 – 1600°C were related to the difference and extent of crystalline phase formation (Fig. 6b and d ), differences in sintering form and degree (Fig. 8b1, d1), the size distribution of crystalline phase grains (Fig. 9), the position of NiTi, NiZr, and NiZr2 regions at different distances from crystalline boundary layers of ceramic and metallic phases (Fig. 11b2, b3, d2, d3) with intermediate layers of different widths on grain boundaries and separate regions between them (Fig. 11b2-0, b3-0, d2-0, d3-0), the significant difference in crack resistance (Fig. 13b1, d1), and the different types of dislocations (Fig. 13b1-0, d1-0) and the different propagation directions of microcracks caused by them (Fig. 13b1-1, d1-1). These processes and differences in the E and K1c correlations above 1500°C decreased the R2 values of the samples with the Zr additive in the range 1200 – 1600°C, where the R2 value was lowest in the sample with the c-ZrO2, Zr mixture, in which these processes caused great brittleness and low crack resistance in the boundary layer structures of the crystalline phases and significant destruction of the structure of this sample.
Conclusion
The effects of Ni,Ta and Ni,Zr powder mixtures in combination with sintered solid solution TaB2–NbC during spark-plasma sintering of the compositions at a pressing loading of 60 MPa in the range 1200 – 1600°C on the phase composition; percent content of Ti, Ta, and Zr in sialon; the microstructure; the grain sizes of crystalline phases; ρrel; ∆l; physical-mechanical properties; and linear correlation of E and K1c of mullite–β-SiAlON–TiC and mullite–β-SiAlON–c-ZrO2 samples were shown.
The synthesized powders of β-SiAlON and TiC were characterized by extensive crystallization of β-SiAlON and TiC. c-ZrO2 spark-plasma sintered at 1400°C and solid solution TaB2–NbC, at 1800°C, showed extensive crystallization of c-ZrO2 and (Nb,Ta)C,B phases. The microstructure of solid solution TaB2–NbC was crystalline, partially nonuniform, and practically completely sintered.
Sintered samples with the Ta, Zr additive showed extensive mullitization; active crystallization of β-SiAlON, (Nb,Ta)C,B, and Ni(Nb,Ta); and varying crystallization of NiTi, NiTa, NiZr, and NiZr2 in the range 1200 – 1600°C. The microstructure of the ceramic phases with the Ta additive was more evenly and densely sintered with uniform and dense NiTi and NiTa particles, in contrast to the variously densified particles of NiTi, NiZr and NiZr, NiZr2 in samples with the Zr additive. Sintering of compositions with the Ta additive formed polydisperse compositions of crystalline phase grains and developed uniformly and rapidly. Samples with the Ta additive showed active growth and high physical-mechanical properties, good crack resistance, and a good linear correlation of E and K1c in the range 1200 – 1600°C.
Change history
26 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11148-021-00609-1
References
A. V. Hmelov, “Producing and properties of mullite–sialon–ZrB2 materials obtained using a spark-plasma technique,” Refract. Ind. Ceram., 59(6), 633 – 641 (2019); Nov. Ogneupory, No. 12, 22 – 30 (2018).
A. V. Hmelov, “Mullite–TiC–c-BN–c–ZrO2 materials produced by spark-plasma sintering and their properties,” Refract. Ind. Ceram., 60(1), 86 – 91 (2019); Nov. Ogneupory, No. 2, 23 – 29 (2019).
A. V. Hmelov, “Development of oxide-free oxide materials under spark-plasma sintering conditions of a mixture of oxide-free components and various metal powder additives,” Refract. Ind. Ceram., 61(1), 73 – 81 (2020); Nov. Ogneupory, No. 2, 17 – 25 (2020).
R. Vedant, “Development of ZrB2–B4C–Mo ceramic matrix composite for high temperature applications,” Thesis, National Inst. Technol. Rourkela, 2014, pp. 1 – 61.
A. V. Hmelov, “Development of dense materials by plasmaspark sintering of oxide–oxide-free components with different mixtures of metal powders,” Refract. Ind. Ceram., 61(3), 313 – 321 (2020); Nov. Ogneupory, No. 6, 27 – 36 (2020).
D. Chakravarty and G. Sundararajan, “Microstructure, mechanical properties and machining performance of spark plasma sintered Al2O3–ZrO2–TiCN nanocomposites,” J. Eur. Ceram. Soc., 33(13/14), 2597 – 2607 (2013).
A. V. Hmelov, “Preparation of mullite–TiC–ZrC ceramic materials by a plasma-ARC method and their properties,” Refract. Ind. Ceram., 57(6), 645 – 650 (2017); Nov. Ogneupory, No. 12, 36 – 41 (2016).
A. V. Hmelov, “Strengthening oxide–oxide-free materials by incorporation of TiC–ZrC solid solutions into their structure during spark plasma sintering of initial powder mixtures under high compression load,” Refract. Ind. Ceram., 60(5), 486 – 494 (2020); Nov. Ogneupory, No. 10, 18 – 26 (2019).
H. Chunfeng, S. Yoshio, and T. Hidehiko, “Microstructure and properties of ZrB2–SiC composites prepared by spark plasma sintering using TaSi2 as sintering additive,” J. Eur. Ceram. Soc., 30(4), 2625 – 2631 (2010).
S. Cardinal, V. Garnier, and G. Fantozzi, “Microstructure and mechanical properties of TiC–TiN based cermets for tool applications,” Int. J. Refract. Met. Hard Mater., 27(2), 521 – 527 (2009).
T. Yang, C. Huang, H. Liu, and B. Zou, “Effect of (Ni, Mo) and (W, Ti)C on the microstructure and mechanical properties of TiB2 ceramic tool materials,” Mater. Sci. Forum, 723(4), 233 – 237 (2012).
G. Zhang, W. Xiong, Q. Yang, and Z. Yao, “Effect of Mo addition on microstructure and mechanical properties of (Ti,W)C solid solution based cermets,” Int. J. Refract. Met. Hard Mater., 43, 77 – 82 (2014).
N. A. Toropov, V. P. Barzakovskii, and R. V. Lapin, Phase Diagrams of Silicate Systems, Nauka, (1979), pp. 437 – 439.
P. Franke, “Thermodynamic properties of inorganic materials (phase diagram of Ni–Ti) — group IV physical chemistry,” SpringerMaterials, 19, Part 4, 1, 2 (2018).
A. Nash and P. Nash, “The Ni–Ta (nickel–tantalum) system,” J. Alloy Phase Diagrams, 5(3), 259 – 265 (1985).
P. Nash and C. S. Jayanth, “The Ni–Zr (nickel–zirconium) system,” J. Alloy Phase Diagrams, 5(2), 143 – 146 (1985).
F. Predel, “Thermodynamic properties of Ni–Ta (nickel–tantalum) system — phase equilib., crystal. and thermodyn. data of binary all. — physical chemistry,” SpringerMaterials, 12, 122, 123 (2016).
P. Franke, “Thermodynamic properties of inorganic materials (phase diagram of Ni–Zr) — group IV physical chemistry,” SpringerMaterials, 19, Part 4, 1 – 4 (2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 2, pp. 14 – 29, February, 2021.
Rights and permissions
About this article
Cite this article
Hmelov, A.V. Stimulation of Spark-Plasma Sintering of Mixtures of Oxide–Non-Oxide Components by Adding a Solid Solution TaB2–NbC and Through a Nickel Melt in Mixtures of Metal Powders. Refract Ind Ceram 62, 74–88 (2021). https://doi.org/10.1007/s11148-021-00563-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-021-00563-y











 ) 1200°C;
) 1200°C;  ) 1300°C;
) 1300°C;  ) 1400°C;
) 1400°C;  ) 1500°C;
) 1500°C;  ) 1600°C.
) 1600°C.
 )), M20SiAl3TiC25Ni25Zr27(50TaB2–50NbC) (
)), M20SiAl3TiC25Ni25Zr27(50TaB2–50NbC) ( )), M20SiAl3ZrO225Ni25Ta27(50TaB2–50NbC) (
)), M20SiAl3ZrO225Ni25Ta27(50TaB2–50NbC) ( )), and M20SiAl3ZrO225Ni25Zr27(50TaB2–50NbC) (
)), and M20SiAl3ZrO225Ni25Zr27(50TaB2–50NbC) ( )) sintered i n the range 1200 – 1600°C.
)) sintered i n the range 1200 – 1600°C.

 )), M20SiAl3TiC25Ni25Zr27(50TaB2–50NbC) (
)), M20SiAl3TiC25Ni25Zr27(50TaB2–50NbC) ( )), M20SiAl3ZrO225Ni25Ta27(50TaB2–50NbC) (
)), M20SiAl3ZrO225Ni25Ta27(50TaB2–50NbC) ( )), and M20SiAl3ZrO225Ni25Zr27(50TaB2–50NbC) (
)), and M20SiAl3ZrO225Ni25Zr27(50TaB2–50NbC) ( )) sintered in the range 1200 – 1600°C.
)) sintered in the range 1200 – 1600°C.
