The ZrB2–CrB composites with a ceramic bond content of 80 wt.% and a relative density of 0.85 – 0.90 were obtained by the method of electrothermal explosion under pressure. It is shown that the mechanical activation of the source powder mixture decreases its heterogeneity and increases its reactivity.We also obtained a finely divided ceramic composite with homogeneous microstructure containing needle-like ZrB2 grains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The process of self-propagating high-temperature synthesis (SHS) proves to be an efficient method for getting refractory compounds. In the course of this process, the initial reagents are transformed into refractory compounds and a great amount of heat is released. The composition and structure of the final products depend on the characteristics of the source heterogeneous mixture and the temperature of synthesis [1, 2].
To increase the reactivity of the source heterogeneous condensed mixture, it is customary to perform the so-called mechanical activation (MA). It is accompanied by the destruction of oxide films, accumulation of defects in the crystal lattice, and grinding of particles, which leads to an increase in the specific area of the surface of mixture and to a more homogeneous mixing of the components [3, 4].
The present work is devoted to the synthesis of ZrB2–CrB ceramic composites by the method of electrothermal explosion (ETE) under the conditions of quasiisostatic compaction. The advantages of the ETE method are connected with the combination of exothermal synthesis with pressing (single-stage process), high productivity (the duration of composite synthesis is equal to several seconds), and the possibility to control over the thermal mode of exothermal reaction. The investigated specimens pressed from mixtures of zirconium, chromium, and boron powders were heated by the direct transmission of electric current up to the inflammation temperature at which thermal equilibrium is violated as a result of heat release in the course of the exothermal reaction of synthesis of the ZrB2 and CrB refractory compounds and the hot product is pressed [5]. In the course of ETE, we measured the electric current and voltage and, by using these quantities, determined the electric resistance of the specimen depending on its phase composition and microstructure. The indicated resistance affects the rate of heating of the heterogeneous mixture and the regularities of ETE: the lower the electric resistance, the higher the heating rate. Therefore, in order to decrease the duration of synthesis and obtain homogeneous composites, the original components were subjected to mechanical activation. The aim of the present work is to study the influence of the mechanical activation of zirconium and chromium powders on the formation of microstructure of ZrB2–CrB composites.
Experimental Procedure
We carried out the procedure of synthesis of ceramic composites according to the following reaction:
(1 − x)(Zr + 2B) + (xCr + B) → (1 − x)ZrB2 + xCrB,
where x is the mass fraction of Cr + B in the mixture.
The scheme of reaction includes the formation of a two-phase product in the form of well wetted ZrB2 and CrB. In this case, CrB plays the role of ceramic binder [6]. For the synthesis of a composite with 80 wt.% of ceramic binder, we chose the following composition of reaction mixture (wt.%): 16.17 Zr, 66.23 Cr, and 17.60 B. To prepare this mixture, we used powders of PTsÉ-ZR zirconium, PKh1-M chromium, and amorphous boron. The particle sizes of Zr and Cr were smaller than 25 μm, while the particle sizes of boron did not exceed 0.2 μm.
We prepared the reaction mixture in two stages. In the first stage, we performed the MA of zirconium and chromium powders taken in the weight ratio 1/4 for 1 h in an AGO-2 mill. The mass of mixture was equal to 20 g and the ratio of masses of the milling balls and the mixture was equal to 20/1. Boron powder was not introduced in the mixture in order to exclude its interaction with Zr [7, 8].
In the diffraction pattern of mixture after MA, there are no peaks corresponding to the Zr phase. At the same time, we observe wider peaks of the Cr phase (Fig. 1). This fact shows that Zr is in the amorphous state but Cr has partially preserved its crystal structure due to its higher mechanical strength.
In the second stage, amorphous boron was added to the activated Zr and Cr powders. They were mixed in an agate mortar up to obtaining a homogeneous mass. From this reaction mixture, we pressed cylindrical specimens (10 g in mass and 20 mm in diameter) under a pressure of 110 MPa up to a relative density of 0.5. The specimens were placed into a reaction press-tool, compacted under a pressure of 96 MPa, and heated by the direct transmission of electric current.
The phase composition of the obtained materials was studied with the help of a DRON-3 diffractometer (in the monochromatic Cu K𝛼-radiation) by using the Crystallographica Search Match computer program, and the database on diffraction Power Diffraction File (2011). The microstructural investigations of the composites were performed by the method of scanning electron microscopy in a Carl Zeiss Ultra Plus field-emission scanning electron microscope of ultrahigh resolution.
Results and Discussion
In Fig. 2, we present the dependence of changes in the electric parameters in the course of the ETE of reaction mixture obtained under a pressure of 96 MPa and an electric voltage of 11.5 V. The electrothermal explosion procedure includes the stages of pre-explosion heating, thermal explosion, and post-processes. In the stage of pre-explosion heating, the electric parameters remain practically constant and the electric current does not exceed 0.1 kA. After the inflammation, we observe sharp changes in the electric parameters: the current strength increases from 0.1 to 5 kA, while the electric resistance decreases from 70 to 2 mΩ. This is explained by the increase in the area of contact surface between the particles of the product. After thermal explosion, the electric current continues to grow, which is connected with the increase in the density of composite in the course of its hot pressing [9,10,11,12,13].
The duration of pre-explosion heating of the activated mixture is one-third as large as the time of pre-explosion heating of the source mixture for the same voltages. This fact shows that the procedure of MA noticeably increases the reactivity of mixture. The density of the obtained ceramic composites was equal to 5 – 5.5 g/cm3 or 0.85 – 0.90 of the theoretical density (Fig. 3).
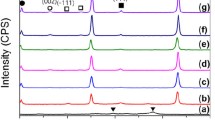
Ceramic composites contain the phases of ZrB2 (zirconium diboride) and CrB (chromium monoboride). In the course of reaction, the initial reagents are completely transformed into the ZrB2 and CrB refractory compounds (Fig. 4).
The analyzed composite contains ZrB2 particles and the CrB ceramic binder (Fig. 5). In composite obtained without MA, the grain size is equal to 2 μm. After the procedure of MA, we observe the formation of ZrB2 grains of needle-like shape up to 50 μm in length. Good mutual wetting of borides guarantees the realization of close contact between the CrB ceramic binder and the ZrB2 dispersed phase.
Conclusions
We study the influence of mechanical activation of heterogeneous mixtures of Zr and Cr powders on the formation of microstructure of ZrB2–CrB ceramic composites obtained by the ETE method under pressure. It is shown that the MA of the mixture leads to the formation of a finely divided composite with a more homogeneous microstructure and needle-like grains. We experimentally studied the regularities of ETE for a mixture of Zr, Cr, and B powders. It was established that the MA of the mixture makes the time of pre-explosion heating three times smaller than the time of pre-explosion heating of nonactivated mixture.
We also studied the phase composition of the products of ETE of Zr, Cr, and B powders. It was shown that the exothermal reaction leads to the complete transformation of the source reagents into the ZrB2 and CrB refractory compounds.
References
A. G. Merzhanov, Combustion Processes and Synthesis of Materials [in Russian], Chernogolovka, Izd. ISMAN (1998).
A. G. Merzhanov and A. S. Mukas’yan, Solid-Flame Combustion [in Russian], Torus Press, Moscow (2007).
K. Brunelli, M. Dabala, R. Frattini, et al., “Electrochemical behavior of Cu–Zr and Cu–Ti glassy alloys,” Alloys Comp., 317, 595 – 602 (2001).
N. F. Shkodich, A. S. Rogachev, S. G. Vadchenko, et al., “Formation of amorphous structures and their crystallization in the Cu–Ti system by high-energy ball milling,” Russ. J. Non-Ferrous Metals, 59(5), 543 – 549 (2018).
A. V. Shcherbakov, V. Yu. Barinov, A. S. Shchukin, et al., “Synthesis of the TiB2 – 30ChrB composite by the method of electrothermal explosion under pressure,” Fundament. Issled., No. 11 – 2, 344 – 349 (2017).
L. Han, S.Wang, J. Zhu, et al., “Hardness, elastic, and electronic properties of chromium monoboride,” Appl. Phys. Lett., 106(22), 221902 (2015).
T. Tsuchida and S. Yamamoto, “Mechanical activation assisted self-propagation high-temperature synthesis of ZrC and ZrB2 in air from Zr/B/C powder mixtures,” J. Europ. Ceram. Soc., 24(1), 45 – 51 (2004).
A. L. Chamberlain,W. G. Fahrenholtz, and G. E. Hilmas, “Reactive hot pressing of zirconium diboride,” J. Europ. Ceram. Soc., 29(16), 3401 – 3408 (2009).
A. L. Chamberlain, W. G. Fahrenholtz, and G. E. Hilmas, “Pressureless sintering of zirconium diboride,” J. Amer. Ceram. Soc., 89(2), 450 – 456 (2006).
W. G. Fahrenholtz, G. E. Hilmas, I. G. Talmy, et al., “Refractory diborides of zirconium and hafnium,” J. Amer. Ceram. Soc., 90(5), 1347 – 1364 (2007).
S. Q. Guo, T. Nishimuna, Y. Kagawa, et al., “Spark plasma sintering of zirconium diborides,” J. Amer. Ceram. Soc., 91(9), 2848 – 2855 (2008).
A. L. Chamberlain,W. G. Fahrenholtz, and G. E. Hilmas, “Reactive hot pressing of zirconium diboride,” J. Europ. Ceram. Soc., 29(16), 3401 – 3408 (2009)
L. Silvestroni and D. Sciti, “Densification of ZrB2 – TaSi2 and HfB2 – TaSi2 ultra-high-temperature ceramic composites,” J. Amer. Ceram. Soc., 94(6), 1920 – 1930 (2011).
The present work was financially supported by the Russian Foundation for Fundamental Research (Grant 17-58-04081 Bel mol a) and performed with the use of equipment of the Distributed Center of Collective Use (RTsKP) ISMAN.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 4, April, pp. 61 – 64, 2019
Rights and permissions
About this article
Cite this article
Shcherbakov, A.V., Shcherbakov, V.A., Barinov, V.Y. et al. Influence of the Mechanical Activation of Reaction Mixture on the Formation of Microstructure of ZrB2–CrB Composites Obtained by Electrothermal Explosions under Pressure. Refract Ind Ceram 60, 223–226 (2019). https://doi.org/10.1007/s11148-019-00340-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-019-00340-y