Abstract
This paper evaluates the photocatalytic degradation of malathion using titanium dioxide (TiO2) nanoparticles supported on sodium alginate (SA), polyvinyl alcohol (PVA), polyvinyl pyrrolidone (PVP) polymer beads and ultraviolet light as irradiation source with a wavelength of 254 nm. Six different types of beads were prepared: SA, PVA, PVP, SA/TiO2, PVA/TiO2, and PVP/TiO2 to assess the effect of the adsorbent material on the photodegradation process by optimizing an experimental design using the Taguchi method. Four factors were considered: TiO2 concentration, bead mass, polymer type, and initial malathion concentration. The response variables were the percentage of removal of the contaminant and the removal rate calculated from the first-order kinetic models. A malathion degradation of 99% was achieved after 180 min of operation when using 100 g and 200 g of SA/TiO2, PVA/TiO2, and PVP/TiO2 beads. The best operating conditions were 1 g L−1 of TiO2, 100 g of bead mass, SA polymer, and 5 mg L−1 of malathion initial concentration. This study found that the adsorption process affected negatively the photodegradation process, reducing the removal efficiency and rate. Finally, the feasibility of the use of a biopolymer-enhanced photocatalytic treatment to degrade agro-industrial contaminants was demonstrated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the agricultural industry has shown accelerated growth worldwide due to the different intensive techniques applied and different uses of agrochemicals and pesticides [1]. Organophosphorus compounds are the most used due to their high effectiveness in pest control [2]. However, due to their chemical stability, resistance to biodegradation, and high solubility in water, they are easily transported to surface and underground water bodies and terrestrial ecosystems through precipitation, irrigation, infiltration, erosion, and runoff [3].
The contamination of water resources by agricultural residues has become more important in recent years, due to its high toxicity and adverse effects on human health [4, 5], becoming a concern worldwide [6]. The current study focuses on the elimination of malathion, which is a highly used organophosphate pesticide, which is widely present in agricultural effluents and waterbodies, particularly in regions with high agricultural activity [7]. Malathion concentrations between 3.3 and 5 mg L−1 can cause cytotoxicity to cells of the human central nervous system [3]. Hepatotoxic effects have also been reported in marine species [8] and rats [9]. Despite this situation, agricultural effluents are treated through conventional techniques, such as physical and biological processes. These processes are inefficient in removing non-biodegradable and chemically stable contaminants from contaminated effluents [10, 11].
To reduce these impacts, photocatalysis emerged as a technologically viable, sustainable, and novel alternative in wastewater treatment [12,13,14], due to its main advantages such as its ability to degrade recalcitrant pollutants, and the oxidation of organic compounds to CO2 and water. In addition, the catalysts commonly used in this process, such as TiO2 and ZnO, are innocuous and can be reused [15,16,17,18]. ZnO has been widely used as a catalyst in the photodegradation of various pollutants. Farzadkia et al. [19], evaluated the photodegradation efficiency of metronidazole in an aqueous solution. They reported a maximum photodegradation of 96.55% of metronidazole and 95.42% of COD by optimizing the pH, UV irradiation time, irradiation power, and nano-ZnO load in a hydraulic retention time of 3 h. Likewise, Rodriguez-Mata et al. [20] evaluated the photo degradation of the 2, 4-dichlorophenoxyacetic acid pesticide, when implementing ZnO and SO2−4/ZnO as catalysts. They obtained a removal efficiency of 38.4% when using ZnO and 82.3% with the doped catalyst (SO2−4/ZnO) in a hydraulic retention time of 6 h, showing that photocatalysis is an alternative for mitigation of pollution problems from agricultural activities.
Recently, some studies have opted for the use of TiO2 in the photocatalysis process due to its high photocatalytic activity, large surface area, low cost, non-toxicity, and excellent stability under lighting [21,22,23,24]. Juang and Chen et al. [6], compared the photodegradation of two agricultural pollutants, Methomyl and Parathion, when using TiO2 particles as catalyst and UV light as irradiation source. Both compounds were completely degraded in 90 and 120 min of operation. Kadam et al. [25] evaluated the photocatalytic activity of nitrogen-doped titanium dioxide (N/TiO2) nanostructures in malathion degradation. They studied the effect of pH, catalyst load, and irradiation sources. Under the best process conditions (pH 6; 1 g dm−3 of N/TiO2 and 150 min of UV light irradiation), they observed a maximum degradation of 97% of malathion. Besides, they demonstrated that the by-products resulting from the process were less toxic than malathion. Surendra et al. [10] studied the effect of malathion photocatalytic degradation as a function of contact time by using Ni/TiO2 nanoparticles. They observed a maximum removal of 94% of malathion in 80 min. Akter et al. [26] investigated the photodegradation of sulfamethoxazole (SMX), metronidazole (MNZ), and ciprofloxacin (CIP) by commercial TiO2, in a reactor with a 254 nm ultraviolet lamp as the irradiation source. They obtained a maximum removal efficiency of 97% of SMX in 360 min of operation with 5 mg L−1 of initial concentration of the pollutant, 100% of MNZ in 600 min with an initial concentration of 80 mg L−1, and 89% of CIP in 600 min. with an initial concentration of 80 mg L−1. The dose of the catalyst used in the process was 0.7 g L−1. Finally, they concluded that the use of TiO2 with UV radiation is a promising process for the efficient treatment of pharmaceutical wastewater. However, when using the catalyst in the form of nanoparticles, the catalyst recovery from the treated water is difficult and requires the use of various subsequent techniques, increasing the costs of the process.
To solve this inconvenience, some studies recommend the use of a support for the catalyst. Commonly, this support can be calcium alginate, sodium alginate, palm fiber, and polyvinyl alcohol [5, 24, 27,28,29]. The use of these polymers eases the separation of the catalyst after the photocatalysis process and could be reused [15, 30,31,32,33,34,35,36]. However, there are few studies where the photocatalysis process is conducted using supports since it is a complex process where the adsorption and photodegradation phenomena occur simultaneously. These works have so far omitted the effect of the adsorption process on the photocatalysis process. The present study aims to describe the behavior of the adsorption–photocatalysis processes to figure out the effect of the adsorbent material on the photodegradation of a highly persistent pollutant such as malathion. Different supports were evaluated to perform a statistical comparison of the photodegradation efficiencies and their degradation and adsorption kinetics. The best treatment efficiency was obtained using the Taguchi statistical method. In addition, the present study contributes to the mathematical modeling of the photocatalysis process and the adsorption process.
Materials and methods
Materials
All the reagents used in the work were of reagent-grade quality. Titanium isopropoxide (C12H28O4Ti) brand Aldrich Chemistry, 97% purity. Hydrochloric Acid (HCl) Meyer brand, 38% purity. Ethanol (C2H5OH) Meyer brand, purity 99.5%. Distilled water (H2O). Malathion (C10H19O6PS2) Velsimex 1000-E brand, 87.8% purity. Sodium alginate (C6H7O6Na)n, Alquimia Brand. Polyvinyl alcohol (C2H4O)n, Alquimia brand. Polyvinyl pyrrolidone (C6H9NO)n Alquimia brand. Calcium chloride (CaCl2), Faga Lab. brand, 96% purity.
TiO2 synthesis
Titanium dioxide (TiO2) nanoparticles were obtained by the Sol–Gel synthesis method [37]. Initially, 15 mL of ethanol (C2H5OH) was poured into a beaker and then 7.4 mL of titanium isopropoxide (C12H28O4Ti) was added. The solution was kept under constant stirring and then 10 mL of distilled water (H2O) was added drop by drop until the solution was homogeneous. Stirring was continued for 10 min. Subsequently, 0.5 mL of hydrochloric acid (HCl) [0.1 M] was added. The system was heated to 70 °C until the excess water evaporated. Finally, a heat treatment was conducted in a muffle (Felisa) for 2 h at 500 °C. The catalyst obtained was TiO2 in the anatase phase (Fig. S1). Fig. S1 shows the X-ray patterns recorded in the 2θ range between 10° and 100°, exhibiting an anatase phase at the diffraction peak 2θ = 25° [38, 39]. The presence of TiO2 in the anatase phase is consistent with the diffraction pattern obtained in the X-ray diffractometer equipped with a copper objective of λ = 1.54184 Å.
TiO2 support in sodium alginate, polyvinyl alcohol, and polyvinyl pyrrolidone beads
TiO2 nanoparticles were supported on sodium alginate (SA), polyvinyl alcohol (PVA) and polyvinyl pyrrolidone (PVP) polymer beads. The method used was a modification to the proposal of Bustos-Terrones et al. [40], and Basu et al. [41] (Fig. 1). Three different solutions were prepared. The first solution was 2.5 (w v−1) sodium alginate, adding 12.5 g of SA to 500 mL of distilled water with constant stirring at room temperature. The second solution was 2.5 (w v−1) polyvinyl alcohol, adding 12.5 g of PVA to 500 mL of distilled water with constant stirring at 80 °C. The third solution was 4% (w v−1) of polyvinyl pyrrolidone, adding 20 g of PVP in 500 mL of distilled water with constant stirring at 80 °C. Subsequently, the catalyst nanoparticles (TiO2) with a concentration of 1 g L−1 were added to each solution. To homogenize the catalyst, magnetic stirring (HP-3100) and radiofrequency (Brason 5800) were alternated for 30 min. Then, the homogeneous mixtures were transferred drop by drop to a 2.5 w v−1 calcium chloride (CaCl2) solution with a peristaltic pump (INTLLAB) to form small beads. Finally, the beads were kept in the CaCl2 solution for 24 h to reinforce their solidification.
Characterization of SA/TiO2, PVA/TiO2 and PVP/TiO2 beads
SA/TiO2, PVA/TiO2, and PVP/TiO2 beads were characterized by Scanning Electron Microscopy (SEM). The characterization study of the beads was conducted before and after the photocatalysis process to make a morphological and surface comparison of them. In addition, the size of the polymer beads was measured using a Vernier caliper.
Evaluation of adsorption and photocatalytic activity
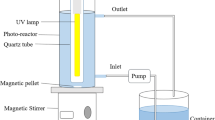
The photocatalytic activity in the degradation of malathion was evaluated using TiO2 in suspension and TiO2 supported on polymer beads (SA, PVA, and PVP) in a batch-type glass reactor (13 cm in diameter and 15 cm in height) with a volume capacity of 1 L (Fig. S2). The reactor was on a stirring plate (HP-3100), allowing the fluidization of the sample. Inside, it featured a quartz lamp irradiating UV light with a wavelength of 254 nm. The photodegradation process was studied using a UV–Vis spectrophotometer (Hach DR6000). The absorbance wavelength for this contaminant was 330 nm. Preliminary tests were carried out to determine the detection limits of the spectrophotometer. These tests involved preparing a series of standard solutions with known concentrations of malathion and measuring their absorbance. The calibration curve was used to determine the detection limit by identifying the concentration at which the signal-to-noise ratio (S/N) reached a 10:1 value [42]. Based on these tests, synthetic samples of agricultural wastewater were prepared with malathion in a concentration of 6 mg L−1. Subsequently, the photocatalysis process was evaluated with different masses of beads (50, 100, and 200 g) of the polymers with catalyst (SA/TiO2, PVA/TiO2, and PVP/TiO2), beads without catalyst (SA, PVA, and PVP) and TiO2 in suspension. Samples of 7 mL were taken every 15 min for around 3 h during malathion degradation. Samples in All experiments were performed at room temperature (25 °C). The removal efficiency was calculated using Eq. (1) [43].
Here % R = Removal efficiency, (%); C0 = initial concentration, (mg L−1); Ce = steady state concentration, (mg L−1).
Mathematical modeling
Three different processes for the elimination of malathion were evaluated: adsorption, direct photocatalysis, and the effect of adsorption–photocatalysis. First, the malathion adsorption process with the different support polymers was evaluated independently. This process was conducted with a hydraulic retention time of 1 h. In parallel, the malathion photodegradation process was evaluated without the influence of the adsorption process. UV light was irradiated right at the start of the process (time = 0) to ensure that the photocatalysis takes place at once without the adsorption process influencing the removal of malathion. The hydraulic retention time of the photocatalysis process was 3 h. In addition, the adsorption–photocatalysis process was evaluated. In this process, the adsorption process took place for 60 min. After this time, the system was irradiated with UV light for 3 h to start the photocatalysis process and identify if the contaminant adsorbed on the surface interferes with the malathion photodegradation process. Therefore, the hydraulic retention time of the adsorption–photocatalysis process was 4 h. These processes were described mathematically using the equations given below.
Adsorption kinetics
The adsorption kinetics by Lagergren’s pseudo-first-order model was evaluated in this study using Eq. (2) [21, 44].
Here qe = amount of malathion at equilibrium per unit mass of adsorbent, (mg g−1); qt = amount of malathion adsorbed per unit mass of adsorbent at time t, (mg g−1); k1 = pseudo-first-order rate constant, (min−1). This mathematical model predicts malathion pesticide adsorption through time.
First-order photodegradation kinetics
The data obtained through the malathion photodegradation process were fitted to the first-order exponential model. The kinetics of photodegradation was described following the following equation [45]:
Here: C = malathion concentration, (mg L−1); k = is the first order constant, (min−1). This mathematical model predicts the photodegradation of the pesticide malathion through time.
Adsorption isotherms
The Langmuir and Freundlich isotherms were studied because they are adsorption models adopted to study the distribution of malathion in an aqueous solution through the adsorbent phase [5, 21, 27, 43, 46]. Equation (4) describes the Langmuir isotherm model used and the Freundlich isotherm model used corresponds to Eq. (5).
Here: qe = Amount of adsorption per unit mass of adsorbent in equilibrium, (mg g−1); Ce = equilibrium concentration of the pollutant in the solution, (mg L−1); qmax = adsorption capacity per unit mass of adsorbent, (mg g−1); KL = Langmuir’s constant based on the affinity of the adsorbate binding site per adsorbent (L g−1). By plotting the experimental data of adsorption capacity (qe) against the equilibrium concentration (Ce) and fitting the data to the Langmuir isotherm equation, the values of qmax and KL were obtained; KF and n = Freundlich constants that are related to adsorption capacity and adsorption intensity.
Experimental design
The study of the malathion photodegradation process was conducted using a Taguchi L9 orthogonal array. The Taguchi method reduces the number of executions of experiments using orthogonal matrices and has been applied in different areas to establish parameters of interest [36, 47]. According to Suresh et al. [48], biopolymer-based photocatalysis is influenced by some crucial operating variables, such as biopolymer composition and mass, the wavelength and energy of light source, irradiation time, catalyst type and concentration, temperature, pH and the initial concentration of the pollutant. In this investigation, the study variables were the TiO2 concentration, the number of beads, the type of polymer, and the initial concentration of malathion. These variables have been often used to optimize the photodegradation process using biopolymers, leading to more efficient and effective degradation of targeted compounds [48]. The design matrix shows the experiments carried out in the L9 orthogonal arrangement and the operating levels for each of the control variables (Table 1).
Statistical analysis
The treatments were conducted through 9 experimental runs according to the Taguchi orthogonal arrangement. In addition, 3 replicates were performed to evaluate the adsorption process, the photodegradation process, and the adsorption–photo degradation process. The statistical analysis was performed using the removal efficiency and the removal rate as response variables. An analysis of variance (ANOVA) was carried out to compare the different processes in the removal of malathion. The software used for statistical analysis was Statgraphics.
Results and discussion
Characterization of polymer beads
The morphology of the polymer bead surface with TiO2 nanoparticles supported was studied through scanning electron microscope (SEM) before and after treatment to identify their structure (Fig. S3). The surface of SA/TiO2, PVA/TiO2, and PVP/TiO2 beads showed irregular and rough shapes, with the presence of micropores that allow the adequate and diffusive transport of contaminants present in wastewater [49]. The mean size of SA/TiO2, PVA/TiO2, and PVP/TiO2 beads was 3.27 mm. SEM images detected the presence of malathion in the micropores of the polymers, represented by the white spots of the images, which is characteristic of malathion when it is in contact with water. Likewise, TiO2 was successfully supported on the SA, PVA, and PVP polymer beads. According to SEM results, the catalyst adheres to the beads to remain suspended, preventing them from settling into the bottom of the reactor.
Photocatalytic activity of TiO2 in suspension
The photodegradation efficiency of the catalyst (TiO2) in suspension was evaluated to figure out its ability to photodegrade malathion. The initial concentration of the pollutant was 6 mg L−1 and the hydraulic retention time was 180 min. A maximum photodegradation of 75% was obtained in 180 min of treatment (Fig. S4). In addition, the experimental data was adjusted to a first-order linear kinetic model, which shows that the mean photodegradation rate of TiO2 in suspension is 0.068 min−1, with a coefficient of determination (R2) of 0.9553. These results are like those reported by Kumar et al. [50], who evaluated the photodegradation of organophosphate pesticides (dichlorvos and malathion) by graphene oxide-TiO2 nanocomposites under ultraviolet radiation. They obtained 80% degradation of fichlorvos and 90% degradation of malathion in 80 min of treatment, for an initial pesticide concentration of 0.5 mg L−1.
Polymer adsorption efficiency
The adsorption capacity of malathion was studied using different polymers (SA, PVA, and PVP) for 180 min. Different polymer masses were evaluated: 50, 100, and 200 g. The behavior of the adsorption process for each of the treatments with different masses of beads of all the polymers is presented in Fig. 2. This Fig. shows the percentage of malathion removed by adsorption through time.
The adsorption of the contaminant when using 50 g of polymer beads with a hydraulic retention time of 3 h is shown in Fig. 2a. Under this condition, the removal efficiencies were similar for the different polymers used; however, sodium alginate resulted in the highest efficiency with a removal percentage of 30% after 160 min of treatment. Polyvinyl alcohol (PVA) achieved a maximum removal of 20% in 180 min of treatment, while polyvinyl pyrrolidone (PVP) adsorbed 15% of malathion after 180 min of treatment.
When using 100 g of beads in the treatments, the adsorption efficiencies doubled, obtaining almost a removal efficiency of 50% for the SA and PVA polymers (Fig. 2b). In the case of the PVP polymer, the highest efficiency obtained was close to 30%. In this case, an unsteady variation occurred during the process, which could be attributed to the adsorption–desorption of the pollutant. This variation could be associated with the saturation of the polymer beads and their later desorption and coincides with reports by Chakhtouna et al. [44], who suggest that adsorption occurs spontaneously at the beginning due to the high availability of sites or pores in the surface of the material. The adsorbent material reaches the largest adsorption, but the desorption process takes place immediately because the pollutant molecules find it difficult to occupy the vacant pores due to the barrier between them, generating repulsive forces between the adsorbate and the adsorbent.
Fig. 2c shows the behavior of the removal efficiency of malathion through time using 200 g of polymer beads. An increase in the adsorption efficiency was noticed, where the SA was the one that presented the highest removal average efficiency, 67.86%, (p < 0.05). In the case of PVA and PVP, a similar trend was observed in both treatments, with a maximum efficiency of 40%. The adsorption–desorption processes are depicted in Fig. 2c. These results are like those by Han et al. [21], who reported a maximum removal of 98.4% of methylene blue using polyacrylonitrile (PAN) beads, coated with polyvinyl alcohol (PVA) with encapsulation of TiO2 nanoparticles.
Photocatalytic activity of SA/TiO2, PVA/TiO2 and PVP/TiO2
Table 2 and Fig. S5 show a comparison of the malathion photodegradation process when the catalyst (TiO2) was supported on polymer beads (SA, PVA, and PVP). The results showed the removal efficiencies through time by varying the mass of beads used in the treatments. The maximum efficiency using 50 g of beads was achieved with SA polymer, with a removal close to 90% during a hydraulic retention time of 180 min. The catalyst supported on PVA and PVP beads allowed removals below 53% during 180 min of treatment.
When a mass of 100 g of bead was used, a similar trend was observed for the different polymer beads since the same removal efficiencies (100%) in 180 min of treatment were achieved. No significant difference (p > 0.05) was found when using 100 g and 200 g of bead. The behavior of malathion degradation through time was similar to the one observed using 100 g of bead and a maximum malathion degradation (100%) was reached after 180 min of treatment.
Comparison of the photocatalytic activity of TiO2 in suspension and supported TiO2
Fig. 3 shows the comparison of the photocatalysis treatments when the catalyst (TiO2) was used in suspension and supported on polymer beads (TiO2/SA, TiO2/PVA, and TiO2/PVP). Fig. 3a shows the photocatalytic degradation of malathion when using 50 g of beads for each polymer. Greater efficiency was obtained using TiO2 supported on SA beads than the photocatalysis treatment with the catalyst in suspension during 180 min of treatment, even though the concentration of TiO2 in suspension used was 1 g L−1. The malathion degradation efficiency obtained was lower when the PVA and PVP beads were used. The degradation efficiencies were lower than the efficiency obtained with the SA beads and the catalyst in suspension. Fig. 3b demonstrates that the photocatalytic degradation was improved when a larger amount of bead mass (100 g) was used. Malathion degradation efficiencies above 99% were achieved after 120 min of the process. The degradation efficiencies were higher than the photocatalysis degradation using the catalyst in suspension since this treatment achieved only a 75% removal efficiency after 180 min.
Fig. 3c shows that despite increasing the mass of the polymer beads. similar efficiencies were obtained than those shown in Fig. 3b. However, the removal efficiencies obtained by using 200 g of beads showed efficiencies above 99% after 90 min of treatment. These results suggested higher degradation rates when using a larger amount of polymer mass. These results are like those reported by Mehmood et al. [18], who studied the photodegradation of triclosan and diclofenac using TiO2 supported on poly-sulfone beads. In the first five min of treatment, they identified a rapid photodegradation of the contaminants, obtaining a degradation efficiency of 60% for diclofenac and 87% for triclosan. The removal rates doubled for both contaminants, needing only 120 min and 40 min of treatment. Table 3 summarizes the most relevant photocatalysis studies with TiO2 supported in different matrices, including the present study.
Statistical analysis
A Taguchi L9 design was used to determine the optimum operating conditions of the biopolymer-based photocatalysis. The study factors in this research were TiO2 concentration, pearl mass, type of polymer, and initial concentration of malathion. Each of the factors had three levels of operation. Two response variables were used: the removal rate and efficiency of malathion. The adsorption and photocatalysis processes were evaluated separately, and then the effect of biopolymer adsorption on the photocatalysis process was also assessed. The experimental times varied according to the process: the adsorption process was evaluated for 60 min, while the photocatalysis process lasted 180 min. Finally, the biopolymer-based photocatalysis was evaluated for 240 min (60 min for adsorption and 180 min for photocatalysis). Table 4 shows the results of the removal coefficient rates and efficiencies obtained through the experiments of each process.
According to the data shown in Table 4, low adsorption rates were observed for the treatments that used initial malathion concentration of 10 mg L−1 and 15 mg L−1. This may be because the adsorbent material was quickly saturated when exposed to high concentrations of the pesticide. This situation affected the removal adsorption rates. Adsorption efficiencies of up to 20% were achieved when an initial malathion concentration of 5 mg L−1 was used (treatments 5 and 9).
The photocatalysis process was then evaluated independently, avoiding the influence of the adsorption process on the removal of malathion. In this process, it was observed that the concentrations decreased significantly over time, obtaining a maximum removal efficiency of 93.78% in 180 min of operation (treatment 9). In the adsorption/photocatalysis process, no synergistic effect was found as it was expected. If a comparison is carried out between the photocatalysis and adsorption/photocatalysis processes, it can be observed that the malathion degradation efficiency decreased within the treatments. For instance, a mean removal efficiency of 83.85% was obtained in treatment 1 when the photocatalysis was used solely, and a mean removal efficiency of 65.91% was observed in the same treatment when using the adsorption/photocatalysis process. This situation can be observed in several treatments of the Taguchi L9 design.
Fig. 4 shows the experimental data fit to the first-order exponential model. Fig. 4a shows the adsorption kinetics. The adsorption rate constant (k) varied between 0.0076 and 0.0557 min−1. Treatment 8 showed the highest malathion adsorption rate, with a value of k = 0.0557 min−1. This value is higher than the one reported by Hermosillo-Nevarez et al. [7], who found a maximum adsorption rate of 0.0347 min−1 for malathion adsorption using recycled PVC polymers. Fig. 4b shows that treatments 1, 2, 6, and 9 adjusted well to the first-order exponential model (r2 > 0.95). The degradation rate constant (k) varied from 0.0009 to 0.013 min−1. The treatments that presented the highest photodegradation rates were 6 and 9, with values of k = 0.011 min−1 and k = 0.013 min−1. In Fig. 4c the adsorption/photocatalysis treatments 1, 2, 5, 6, and 9 showed a good degree of fit to the first-order exponential model (r2 > 0.90). The degradation rate (k) was lower than the photocatalysis process solely, with a range of 0.00009 min−1 to 0.011 min−1. The treatments with the highest malathion photodegradation rates were 5 and 9, with values of k = 0.009 min−1 and k = 0.011 min−1. These values are higher than those reported by Rodriguez-Mata et al. [20], who found a value of k = 0.0051 min−1 for the photodegradation of the pesticide 2, 4-D and Fazal et al. [43], who reported a value of k = 0.0050 min−1 for the photodegradation of methylene blue.
Kinetic modeling of malathion removal under different processes: a) adsorption, b) photocatalysis and c) adsorption–photocatalysis. The experimental conditions of these processes are shown in Table 1 according to the Taguchi methodology
Li et al. [2] achieved a removal efficiency of 90% of malathion with an initial concentration of 15 μM through a photocatalysis treatment with a retention time of 30 min. The removal efficiencies reported by Li et al. [2] are like those obtained in this study, however, the malathion concentrations were lower. Kralj et al. [51] reported a malathion removal efficiency of about 90% and a degradation rate of 0.083 min−1 with a photocatalytic process with TiO2. Surendra et al. [8] achieved a removal efficiency of 95% for malathion degradation by optimizing a Box–Behnken response surface experimental design. The optimum operational conditions were pH 10, temperature = 70 °C, a degradation time of 60 min, and a weight ratio of 1.5% Ni/TiO2/volume. Yu et al. [52] reported removal efficiencies of 96% and a degradation rate of 0.0058 min−1 for the removal of malathion using TiO2 as a catalyst during 240 min of photocatalysis.
Likewise, treatment 9 showed the highest removal efficiencies in the different processes: 93.78% for photocatalysis and 95.03% for adsorption/photocatalysis. The highest rates also occurred in treatment 9, where k = 0.0127 min−1 and k = 0.0111 min−1 were obtained for the photocatalysis and adsorption–photocatalysis processes, respectively. On the contrary, treatment 8 presented the lowest removal efficiencies in the different processes: 13.41% for photocatalysis and 5.80% for adsorption–photocatalysis. These results coincided with the lowest degradation rates obtained in this study, with k values of 0.0009 min−1 and 0.0002 min−1 for the photocatalysis and adsorption–photocatalysis processes, respectively.
Adsorption isotherms
The Langmuir and Freundlich isotherms describe the adsorption process. The Langmuir adsorption isotherm is a theoretical representation of the mass of malathion adsorbed on a given mass of adsorbent. This isotherm corresponds to a distribution of the pollutant molecules on the surface of the adsorbent material as a monolayer. This means that when the pollutant molecules bind to the active site of the adsorbent, no further adsorption occurs. The Freundlich adsorption isotherm is an empirical representation that considers the multilayer adsorption of the pollutant [5]. The Langmuir and Freundlich isothermal constants were calculated using non-linear least squares fitting to the untransformed original equations according to the methodology proposed by Lente [53]. These constants are shown in Table 5. The value of R2 close to 1 indicates that the adsorption process was favorable. The results show that the SA polymer better fits the Langmuir isotherm since a higher coefficient of determination value was found (R2 = 0.9992). This result indicates a monolayer adsorption process when using SA polymer in the biopolymer-based photocatalysis process. Under these conditions, malathion is adsorbed uniformly on the superficial layer of the beads of the polymer, limiting the adsorption efficiency. These results are similar to those reported by Han et al. [21] who reported the adsorption of methylene blue by polyacrylonitrile (PAN) beads, coated with polyvinylalcohol (PVA).
The adsorption kinetic study also revealed that the PVP polymer better fitted the Freundlich isotherm with an R2 value of 0.9519, which indicates a multilayer adsorption process occurs when this polymer is used. These results are similar to those reported by Hosseini et al. [5] who also reported that the adsorption of malathion using a sodium alginate/biosilicate/magnetite (SABM) nanocomposite as adsorbent showed a good fit by Freundlich isotherm (R2 = 0.9959).
In Table 5, it is noteworthy that low R2 values were obtained for the PVA polymer when fitting experimental data to the Langmuir and Freundlich isotherm equations (R2 = 0.0698 and R2 = 0.0105, respectively). Since the fitting parameters obtained from the isotherm equations were not satisfactory, it can be inferred that a low adsorption strength or affinity was found for the PVA polymer. This situation could be desirable in the biopolymer-based photocatalysis process. Previously, this study evidenced an interference in the photocatalytic activity because of the presence of an adsorbed multilayer on the surface beads, which resulted in a lower degradation efficiency. Since the PVA biopolymer showed a low affinity for malathion, it will be less likely to form unwanted interferences, improving the degradation efficiency with a longer useful life of the polymer. This situation coincides with the results obtained in this study, where treatment 9 (using PVA biopolymer) showed the maximum degradation rate and efficiency in the L9 Taguchi experimental design.
Conclusions
This study proved the feasibility of using polymer beads (SA, PVA, and PVP) to immobilize a catalyst (TiO2) in a UV oxidation process for malathion removal. The characterization of the beads by Scanning Electron Microscopy demonstrated the presence of malathion on the surface of the polymers. In a biopolymer-based photocatalysis process, the effect of adsorption and photocatalysis were evaluated independently, but also the synergistic effect of adsorption–photocatalysis, by using the removal rate and efficiency. These processes were also compared with the photocatalysis process using TiO2 nanoparticles in suspension. The TiO2 supported on the polymer beads (SA/TiO2, PVA/TiO2, PVP/TiO2) achieved removal efficiencies of 99% after 180 min of treatment. The best operating conditions were obtained using a Taguchi L9 experimental design. The adsorption process when using SA adjusted to the Langmuir mathematical model, suggesting that a monolayer adsorption process occurs. The adsorption process when using PVP fitted the Freundlich mathematical model, suggesting that a multilayer adsorption process occurs. The statistical analysis showed that the multilayer adsorption interfered with the photocatalysis process since a higher degradation efficiency was observed when the direct photocatalysis was conducted in comparison with the adsorption–photocatalysis process. Therefore, this study suggests carrying out the photocatalysis process without waiting for the beads to adsorb malathion and/or using an adsorbent with low adsorption affinity. The results also demonstrated that the use of catalyst support (TiO2) increased the efficiency of the photocatalysis process in comparison with the photodegradation of malathion using the catalyst in suspension.
Data availability
Not applicable
References
Raju IM, Rao SKV, Divya G (2019) Poly 3-thenoic acid sensitized, copper doped anatase/brookite TiO2 nanohybrids for enhanced photocatalytic degradation of an organophosphorus pesticide. J Environ Chem Eng 7(4):103211
Li W, Zhao Y, Yan X, Duan J, Saint CP, Beecham S (2019) Transformation pathway and toxicity assessment of malathion in aqueous solution during UV photolysis and photocatalysis. Chemosphere 234:204–214
Rangel-Peraza JG, Prado MAR, Amabilis-Sosa LE, Bustos-Terrones YA, Ramírez-Pereda B (2020) Malathion removal through peroxi-electrocoagulation and photocatalytic treatments optimization by statistical analysis. Int J Electrochem Sci 15:8253–8264
Vassalini I, Gjipalaj J, Crespi S, Gianoncelli A, Mella M, Ferroni M, Alessandri I (2020) Alginate-derived active blend enhances adsorption and photocatalytic removal of organic pollutants in water. Adv Sustain Syst 4(7):1900112
Hosseini M, Kamani H, Esrafili A, Badi MY, Gholami M (2019) Removal of malathion by sodium alginate/biosilicate/magnetite nanocomposite as a novel adsorbent: kinetics, isotherms, and thermodynamic. Study Health Scope 8(4):11
Juang RS, Chen CH (2014) Comparative study on photocatalytic degradation of methomyl and parathion over UV-irradiated TiO2 particles in aqueous solutions. J Taiwan Inst Chem Eng 45(3):989–995
Hermosillo-Nevárez JJ, Bustos-Terrones V, Bustos-Terrones YA, Uriarte-Aceves PM, Rangel-Peraza JG (2020) Feasibility study on the use of recycled polymers for Malathion adsorption: isotherms and kinetic modeling. Materials 13(8):1824
Ullah S, Li Z, Hasan Z, Khan SU, Fahad S (2018) Malathion induced oxidative stress leads to histopathological and biochemical toxicity in the liver of rohu (Labeo rohita, Hamilton) at acute concentration. Ecotoxicol Environ Saf 161:270–280
Flehi-Slim I, Chargui I, Boughattas S, El Mabrouk A, Belaïd-Nouira Y, Neffati F, Najjar MF, Haouas Z, Ben CH (2015) Malathion-induced hepatotoxicity in male Wistar rats: biochemical and histopathological studies. Environ Sci Pollut Res Int 22(22):17828–17838
Surendra B, Raju BM, Srikanth KN, Choudhary GL, Francis P, Vengalapati M (2020) Synthesis and characterization of Ni doped TiO2 nanoparticles and its application for the degradation of Malathion. Mater Today 26:1091–1095
Pérez-Lucas G, El AatikAliste AM, Hernández V, Fenoll J, Navarro S (2022) Reclamation of aqueous waste solutions polluted with pharmaceutical and pesticide residues by biological-photocatalytic (solar) coupling in situ for agricultural reuse. Chem Eng J 448:137616
Tu H, Li D, Yi Y, Liu R, Wu Y, Dong X, Deng H (2019) Incorporation of rectorite into porous polycaprolactone/TiO2 nanofibrous mats for enhancing photocatalysis properties towards organic dye pollution. Compos Commun 15:58–63
Maldonado-Larios L, Mayen-Mondragón R, Martínez-Orozco RD, Páramo-García U, Gallardo-Rivas NV, García-Alamilla R (2020) Electrochemically assisted fabrication of titanium-dioxide/polyaniline nanocomposite films for the electroremediation of Congo red in aqueous effluents. Synth Met 268:116464
González-González RB, Parra-Saldívar R, Alsanie WF, Iqbal HM (2022) Nanohybrid catalysts with porous structures for environmental remediation through photocatalytic degradation of emerging pollutants. Environ Res 214:113955
Zhao L, Deng J, Sun P, Liu J, Ji Y, Nakada N, Yang Y (2018) Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: systematic review and bibliometric analysis. Sci Total Environ 627:1253–1263
Reveendran ST (2018) Application of experimental design for dyes removal in aqueous environment by using sodium alginate-TiO2 thin film. Chem Data Collect 15:32–40
Ortíz MDJR, Valencia RH, Parra GA, Morales PA (2021) Síntesis verde de materiales nanoestructurados de ZnO en la degradación de contaminantes orgánicos por medio de la fotocatálisis heterogénea. Rev Cienc Tecnol 4(4):299–313
Mehmood CT, Zhong Z, Zhou H, Xiao Y (2020) Constructing porous beads with modified polysulfone-alginate and TiO2 as a robust and recyclable photocatalyst for wastewater treatment. J Water Process Eng 38:101601
Farzadkia M, Esrafili A, Baghapour MA, Shahamat YD, Okhovat N (2013) Degradation of metronidazole in aqueous solution by nano-ZnO/UV photocatalytic process. Desalin Water Treat 52(25–27):4947–4952
Rodriguez-Mata AE, Tzompantzi FJ, Amabilis-Sosa LE, Diaz-Peña I, Bustos-Terrones Y, Rangel-Peraza JG (2018) Characterization of SO2-4/ZnO and photo degradation kinetics of 2, 4-Dichlorophenoxyacetic Acid (2, 4-D). Kinet Catal 59(6):720–726
Han Z, Jin J, Wang Y, Zhang Z, Gu J, Ou M, Xu X (2019) Encapsulating TiO2 into polyvinyl alcohol coated polyacrylonitrile composite beads for the effective removal of methylene blue. J Braz Chem Soc 30:211–223
Elbarbary AM, Gad YH (2021) Radiation synthesis and characterization of poly (vinyl alcohol)/acrylamide/TiO2/SiO2 nanocomposite for removal of metal ion and dye from wastewater. J Inorg Organomet Polym Mater 31(10):4103–4125
Hui KC, Suhaimi H, Sambudi NS (2021) Electrospun-based TiO2 nanofibers for organic pollutant photo degradation: a comprehensive review. Rev Chem Eng 38(6):641–668
Moustafa H, Karmalawi AM, Youssef AM (2021) Development of hybrid TiO2 nanocomposites coated with dapsone and their effects on UV radiation, mechanical, thermal properties and antibacterial activity of PVA bionanocomposites. Environ Nanotechnol Monit Manag 16:100482
Kadam AN, Dhabbe RS, Kokate MR, Gaikwad YB, Garadkar KM (2014) Preparation of N doped TiO2 via microwave-assisted method and its photocatalytic activity for degradation of Malathion. Spectrochim Acta A 133:669–676
Akter S, Islam MS, Kabir MH, Shaikh MA, Gafur MA (2022) UV/TiO2 photo degradation of metronidazole, ciprofloxacin and sulfamethoxazole in aqueous solution: an optimization and kinetic study. Arab J Chem 15(7):103900
Karkeh-Abadi F, Saber-Samandari S, Saber-Samandari S (2016) The impact of functionalized CNT in the network of sodium alginate-based nanocomposite beads on the removal of Co (II) ions from aqueous solutions. J Hazard Mater 312:224–233
Nouri L, Hemidouche S, Boudjemaa A, Kaouah F, Sadaoui Z, Bachari K (2020) Elaboration and characterization of photobiocomposite beads, based on titanium (IV) oxide and sodium alginate biopolymer, for basic blue 41 adsorption/photocatalytic degradation. Int J Biol Macromol 151:66–84
Lee SJ, Lim HW, Park SH (2021) Adsorptive seawater desalination using MOF-incorporated Cu-alginate/PVA beads: ion removal efficiency and durability. Chemosphere 268:128797
Dalponte I, de Sousa BC, Mathias AL, Jorge RM (2019) Formulation and optimization of a novel TiO2/calcium alginate floating photocatalyst. Int J Biol Macromol 137:992–1001
Gopinath KP, Madhav NV, Krishnan A, Malolan R, Rangarajan G (2020) Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: a review. J Environ Manag 270:110906
Sakarkar S, Muthukumaran S, Jegatheesan V (2020) Evaluation of polyvinyl alcohol (PVA) loading in the PVA/titanium dioxide (TiO2) thin film coating on polyvinylidene fluoride (PVDF) membrane for the removal of textile dyes. Chemosphere 257:127144
Ainali NM, Kalaronis D, Evgenidou E, Bikiaris DN, Lambropoulou DA (2021) Insights into biodegradable polymer-supported titanium dioxide photocatalysts for environmental remediation. Macromolecules 1(3):201–233
Sherugar P, Naik NS, Padaki M, Nayak V, Gangadharan A, Nadig AR, Déon S (2021) Fabrication of zinc doped aluminum oxide/polysulfone mixed matrix membranes for enhanced antifouling property and heavy metal removal. Chemosphere 275:130024
Rafaie HA, Shohaimi NA, Ramli NI, Ishak ZI, Rosmi MS, Mohamed MA, Hir ZA (2022) Application of hybrid polymeric materials as photocatalyst in textile wastewater. In: Polymer technology in dye-containing wastewater, pp 101–143
Balakrishnan A, Appunni S, Chinthala M (2022) Biopolymer-supported TiO2 as a sustainable photocatalyst for wastewater treatment: a review. Environ Chem Lett 20:3071–3098
Zanella R (2012) Metodologías para la síntesis de nanopartículas: controlando forma y tamaño Mundo Nano. Revista Interdisciplinaria en Nanociencias y Nanotecnología 5(1):69–81
Martínez Rojas V, Matejova L, López Milla A, Cruz G, Solís Veliz J, Gómez León M (2015) Obtención de partículas de TiO2 por sol-gel, asistido con ultrasonido para aplicaciones fotocatalíticas. Rev Soc Quím Perú 81:201–211
Al Qarni F, Alomair N, Mohamed H (2019) Environment-friendly nanoporous titanium dioxide with enhanced photocatalytic activity. Catalysts 9:799
Bustos-Terrones YA, Estrada-Vázquez R, Ramírez-Pereda B, Bustos-Terrones V, Rangel-Peraza JG (2020) Kinetics of a fixed bed reactor with immobilized microorganisms for the removal of organic matter and phosphorous. Water Environ Res 92(11):1956–1965
Basu H, Pimple MV, Saha S, Patel A, Dansena C, Singhal RK (2020) TiO2 microsphere impregnated alginate: a novel hybrid sorbent for uranium removal from aquatic bodies. New J Chem 44(10):3950–3960
Rancic SM, Nikolic-Mandic SD, Mandic LM (2005) Kinetic spectrophotometric method for gold(III) determination. Anal Chim Acta 547(1):144–149
Fazal T, Razzaq A, Javed F, Hafeez A, Rashid N, Amjad US, Rehman F (2020) Integrating adsorption and photocatalysis: a cost effective strategy for textile wastewater treatment using hybrid biochar-TiO2 composite. J Hazard Mater 390:121623
Chakhtouna H, Zari N, Bouhfid R, Qaiss A, Benzeid H (2021) Novel photocatalyst based on date palm fibers for efficient dyes removal. J Water Process Eng 43:102167
Sarkar S, Chakraborty S, Bhattacharjee C (2015) Photocatalytic degradation of pharmaceutical wastes by alginate supported TiO2 nanoparticles in packed bed photo reactor (PBPR). Ecotoxil Environ Saf 121:263–270
Chen JH, Liu QL, Hu SR, Ni JC, He YS (2011) Adsorption mechanism of Cu (II) ions from aqueous solution by glutaraldehyde crosslinked humic acid-immobilized sodium alginate porous membrane adsorbent. Chem Eng J 173(2):511–519
Bolboacă SD, Jäntschi L (2007) Design of experiments: useful orthogonal arrays for number of experiments from 4 to 16. Entropy 9(4):198–232
Suresh R, Rajendran S, Hoang TKA, Vo D-VN, Siddiqui MN, Cornejo-Ponce L (2021) Recent progress in green and biopolymer based photocatalysts for the abatement of aquatic pollutants. Environ Res 199:111324
Arikal D, Kallingal A (2021) Photocatalytic degradation of azo and anthraquinone dye using TiO2/MgO nanocomposite immobilized chitosan hydrogels. Environ Technol 42(15):2278–2291
Kumar R, George L, Jun Z, Mukherji S (2022) Photocatalytic activity of graphene oxide-TiO2 nanocomposite on dichlorvos and Malathion and assessment of toxicity changes due to photo degradation. Chemosphere 308:136402
Kralj MB, Cernigoj U, Franko M, Trebse P (2007) Comparison of photocatalysis and photolysis of Malathion, isoMalathion, malaoxon, and commercial Malathion—products and toxicity studies. Water Res 41:4504–4514
Yu H, Wang X, Sun H, Huo M (2010) Photocatalytic degradation of malathion in aqueous solution using an Au–Pd–TiO2 nanotube film Au-Pd-TiO2. J Hazard Mater 184(1–3):753–758
Lente G (2015) Deterministic kinetics in chemistry and systems biology: the dynamics of complex reaction networks. Springer, New York
Fernández JA, Cardozo MG, Carrascal AK, Salcedo JC, Pedroza AM, Daza CE (2015) Tratamiento de agua residual de microbiología usando películas delgadas de TiO2. Ingeniería Y Competitividad 17(1):35–48
Alrousan DM, Dunlop PS, McMurray TA, Byrne JA (2009) Photocatalytic inactivation of E. coli in surface water using immobilised nanoparticle TiO2 films. Water Res 43(1):47–54
Athanasekou C, Romanos GE, Papageorgiou SK, Manolis GK, Katsaros F, Falaras P (2017) Degradación fotocatalítica de contaminantes emergentes de cromo hexavalente a través de nanoestructuras de dióxido de titanio avanzadas. Revista de ingeniería química 318:171–180
Acknowledgements
The authors thank TecNM/Instituto Tecnológico de Culiacán for providing the infrastructure to carry out this work and CONAHCYT for the scholarship provided to the first author.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Estrada-Vázquez, R., Vaca-Mier, M., Bustos-Terrones, V. et al. Degradation of agricultural pollutants by biopolymer-enhanced photocatalysis: application of Taguchi method for optimization. Reac Kinet Mech Cat 137, 523–545 (2024). https://doi.org/10.1007/s11144-023-02515-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02515-8