Abstract
In this work, Bi1−xSmxFeO3 (x = 0.1, 0.08, 0.06, 0.04, 0.02) and Bi0.094 Sm0.06 Fe1−y NiyO3 nanoparticles (y = 0.05, 0.03, 0.01) were synthesized by sol–gel method. The structural, microstructural, magnetic, and optical properties of Bi1−xSmxFe1−yNiyO3 nanoparticles were characterized by using X-ray diffraction, field-emission scanning electron microscopy, the energy-dispersive X-ray analysis, vibrating sample magnetometry and UV–Vis reflectance spectra at room temperature. The structural investigations indicate that the unit cell volume decreases with Sm ion doping, while it increases with substituting Ni ions in the Bi1−xSmxFe1−yNiyO3 nanoparticles. Moreover, the magnetic properties were enhanced by doping Sm and Ni ions. The photocatalytic activities of Bi1−xSmxFe1−yNiyO3 nanoparticles were investigated to degrade methyl red (M.R.) in an aqueous solution. For this purpose, the M.R. degradation with the synthesized photocatalysis was evaluated at room temperature under dark and visible light exposure using a mercury lamp (150 W). The results indicated that the highest photodegradation of M.R. is obtained using Bi0.94Sm0.06Ni0.03Fe0.97 nanoparticles as a photocatalyst in the presence of hydrogen peroxide in an acidic media (pH 3). Besides, the photocatalytic activity of Bi1-xSmxFe1-yNiyO3 (x = 0.06, and y = 0.01, 0.03, 0.05) is higher than that of Bi1−xSmxFeO3 (x = 0.0, 0.02, 0.04, 0.06, 0.08) nanoparticles for the photodegradation of MR. Besides, the Sm and Ni co-doped BFO nanoparticles have a greater magnetic property than the Sm-doped BFO nanoparticles, leading to a simple and fast separation of catalyst using a strong magnet after the M.R. degradation. Under the optimum conditions, the M.R. degradation efficiencies under visible-light illumination for 60 and 100 min were 97.34 and 85.76% for Bi0.94Sm0.06Ni0.03Fe0.97 and Bi094Sm0.06FeO3 nanoparticles at pH 3.0.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today, a wide variety of industrial products are produced with the expansion of various industries to create human comfort. Many of these products are led to environmental pollution by an uncontrolled discharge of non-usable textiles, reactants, and reagents into the environment [1, 2]. This problem becomes acute when these pollutants are non-biodegradable and accumulate in the environment [3, 4]. These pollutants eventually enter the human and animal bodies through food chains and cause many human and animal health [5,6,7].
Photocatalytic processes are widely applied to degrade non-biodegradable pollutants[8, 9]. The advantages of the procedure include simplicity and low-cost operation, no production of secondary pollutants, and performing the process at ambient pressure and temperature[10]. It can also be utilized for large-scale practical applications [11]. Various methods were applied to enhance the photocatalytic efficiency of a photocatalyst toward pollutant degradation, including selecting a suitable photocatalyst, doping with various materials, preparing composites by mixing suitable semiconductors with proper morphology and crystallography phase, and modifing the photocatalyst surface with appropriate functional groups [12]. Among them, doping with a suitable nanoparticle, metal ion, nanocomposite, etc., significantly impacts the efficiency of the photocatalytic process, which is a critical method to improve the photocatalytic properties of semiconductors [13,14,15,16,17].
Bismuth ferrite (BFO) is a multiferroic compound with simultaneous ferromagnetic and ferroelectric properties used to manufacture spintronics, sensors, piezoelectric devices, memory, and photovoltaics [11, 18]. BFO has shown suitable photocatalytic properties under visible light for the degradation of organic material because of the narrow and low band-gap energy of 2.2 eV [18,19,20,21,22]. Besides, BFO as a photocatalyst can be quickly and easily separated from the sample solution in the presence of a strong magnet[23]. Other advantages of BFO as a photocatalyst are simple synthesis and modification, cheapness, and chemical and thermal stability. However, the photocatalytic activity of BFO is limited due to the high rate of electrons and holes combination, the narrow range of the photoresponse, and the low conduction band level[24,25,26]. A straightforward and effective way to overcome this limitation is to dope the appropriate metals into the BFO network, enhancing the absorptivity of visible light and the separation properties of charge carriers with an increase in surface area photocatalyst through heterojunction formation [11]. Recently, there has been a strong tendency to simultaneously dope two metals into the BFO structure, which leads to a further modification in the photocatalyst activity than BFO doping with a metal [10]. For example, using two metals (Ni and Nd) doped in BFO increased the transfer rate of the generated electron in the BFO network. It significantly prevented the recombination of the electron and the hole produced in the BFO structure [27, 28]. Similarly, an increase in photocatalytic activity with co-doped (La3+ and Se4+) in BFO is due to increased absorption efficiency of light, proper separation of photogenerated charge carriers, and a decrease in the recombination rate of the hole–electron pairs [29]. However, the type and percentage of dopants selected in the preparation of photocatalyst based on co-doped BFO are very effective in its activity.
In the study, two BFO derivatives including In this work, Bi1−xSmxFeO3 (x = 0.1, 0.08, 0.06, 0.04, 0.02) and Bi0.094 Sm0.06 Fe1−y NiyO3 nanoparticles (y = 0.05,0.03,0.01) were synthesized by sol–gel method. The ability of these nanoparticles as photocatalysts in the methyl red degradation process was investigated and compared. Various methods characterize the structural, morphology, and magnetic properties of these nanoparticles. The photocatalytic activities of Sm-doped BFO and Sm and Ni co-doped BFO for the degradation of methyl red in an aqueous solution were evaluated under dark and visible light conditions at room temperature. The advantages of the synthesis photocatalysis (Sm and Ni co-doped BFO nanoparticles and Sm-doped BFO nanoparticles) for the M.R. degradation include straightforward preparation, low cost, high degradation efficiency, and suitable degradation time under visible light.
Experimental
Reagents and materials
All materials used to synthesize the photocatalyst, including bismuth (III) nitrate pentahydrate, iron (III) nitrate nonahydrate, samarium (III) nitrate hexahydrate, and nickel (II) nitrate hexahydrate, nitric acid, tartaric acid, ammonium hydroxide (28%), were purchased from Merck (Germany). Methyl red (M.R.) with an analytical grade was obtained from Sigma Aldrich (USA).
Instruments
A UV–visible spectrophotometer (Varian Cary Bio 50 model) was used to determine the M.R. concentration in the aqueous solution. The morphology and structure of BiFeO3 (BFO), Bi1−xSmx FeO3, and Bi1−xSmx Fe1−yNiyO3 as a photocatalyst were investigated using X-ray diffraction spectroscopy (XRD; Bruker D8 ADVANCE, Germany), the field-emission scanning electron microscopy (FE-SEM; Mira 3 Tescan; Czech Republic), and vibration sample magnetometry (VSM; analysis under applied magnetic fields up to 20 kOe at 300 K). A mercury lamp (150 W) was utilized to study the photocatalytic activity of BiFeO3 (BFO), Bi1−xSmx FeO3, and B(1−xSmx Fe1−yNiyO3 for the M.R. degradation under visible light at a distance of about 5 cm from the M.R. aqueous solution. A Metrohm pH meter (model 780, Switzerland) was applied to adjust the pH of the M.R. aqueous solution.
Photocatalyst preparation
The nanoparticles of BiFeO3 (BFO), Bi(1−x)SmxFeO3 (x = 0.1, 0.08, 0.06, 0.04, 0.02) and Bi0.094 Sm0.06 Fe1−y NiyO3 nanoparticles (y = 0.05,0.03,0.01) as a photocatalyst were prepared according to the sol–gel technique. The photocatalyst was synthesized using a reported paper with a little modification [45]. For this purpose, an appropriate amount of bismuth nitrate, iron(III) nitrate, samarium(III) nitrate, and nickel(II) nitrate was poured into a solution containing 1.0 mL of nitric acid and 20.0 mL of distilled water. The mixture was stirred at 500 rpm for 1 h at 60 °C to dissolve all compounds, followed by tartaric acid completely. The mixture was stirred at room temperature for 2 h, and a milky color suspension was formed. While the suspension was continuously stirred, an aqueous ammonia solution (20% or 2 mol L−1) was added dropwise to the suspension until its pH reached about 3. The suspension was stirred for 9 h with an initial temperature of 80 °C, which increased to 200 °C at a rate of 1 °C min−1 and remained constant at the temperature. The mixture was finally calcined in a digital furnace at temperatures of 400 °C for 6 h and 600 °C for 5 h.
Photodegradation procedure
A homemade photocatalytic reactor was fabricated with dimensions of 75 × 50 × 40 Cm3, including two fans to control its temperature, a Hg vapor lamp, and a magnetic stirrer to perform the M.R. degradation under visible light irradiation. For this purpose, 185.0 mL of M.R. aqueous solution (2.9 mg L−1) was poured into a proper beaker, and its pH was adjusted to 3.1 using hydrochloric acid solution (0.1 mol L−1). Then, catalyst (98 mg), and H2O2 solution (0.58 mL), were added to the resulting solution. The resulting suspension was diluted to 200.0 ml with distilled water. The suspension was transferred into the photocatalytic reactor. The suspension was stirred at 600 rpm for 5.0 h at room temperature in the dark to achieve an adsorption–desorption equilibrium. The suspension was exposed for a suitable time, and sampling was carried out using a pipette every 10 min, followed by centrifuging for 10 min at 5000 pm. The mixture was filtered, and the solution was determined by the spectrophotometric method at 521.9 nm. The degradation efficiency of M.R. (E%) was calculated by the following equation[12]:
Ct and Co are the M.R. concentration at irradiation time (t > 0) and initial M.R. concentration (t = 0).
Results and discussion
Nanoparticles characterization
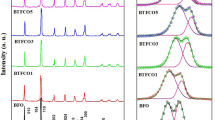
The XRD patterns of Bi1−xSmxFe1−yNiyO3 nanoparticles are presented in Fig. 1(a, b). By using X’pert software, the XRD patterns of studied samples were compared with the standard diffraction data (JCPDS file No. 01-07-2494) of the BiFeO3 sample. It is observed that all samples have the rhombohedral structure with a space group of R3c. A small amount of Bi2Fe4O9 and Bi2O3 impurity phases corresponding to the JCPDS file numbers (01-072-1832) and (01-074-1374) are observed in the XRD pattern of our samples that were displayed with symbols (*) and (#). It is clear that the amount of impurity phases decreases with increasing Sm content, but these gradually increase with increasing Ni content, again. This effect was also observed in the literature [30]. On the other hand, the doublet peaks in the XRD patterns merge gradually to one peak with increasing Sm concentration. This effect indicates that distortion occurs in the rhombohedral structure because of the difference in radii ionic between Bi3+ and Sm3+ ions. However, because of the small difference between the radii ionic of Fe3+ and Ni3+ ions, Ni substitution does not modify the XRD pattern of Bi0.94Sm0.06Fe1-yNiyO3 nanoparticles significantly. Moreover, Fig. 1c indicates the magnified major peaks of the XRD patterns. It is clear that the peaks shifted slightly toward higher angles with increasing Sm3+ concentration. Thus, based on Bragg’s law, the lattice parameters decrease. Hence, the lattice constants (a, b, and c) and unit cell volume (V) of Bi1−xSmxFe1−yNiyO3 nanoparticles were calculated using the Fullprof software. Fig. S1 illustrates the Rietveld refinement of the XRD pattern for the studied samples. Their results are listed in Table 1. The results indicate that unit cell volume decrease with Sm substitution. This occurrence can be attributed to the replacement of Bi3+ ion with radii ionic of 1.03 Å by Sm3+ ion with radii ionic of 0.958 Å [31, 32]. This result agrees with the previous report [33]. In contrast, the unit cell volume increase with Ni substituting in Bi0.94Sm0.06Fe1−yNiyO3 nanoparticles. This irregular effect may be ascribed to substituting Fe3+ ion with radii ionic of 0.645 Å in the photocatalyst composite by low valance Ni ion (Ni2+) with radii ionic of 0.69 Å [34].
The FE-SEM images of Bi1−xSmxFe1−yNiyO3 nanoparticles are presented in Fig. S2. By comparing the insets of Fig. S2, the size particle distribution histograms, can be concluded that particle size decrease gradually with Sm doping. Moreover, the shape of Bi1−xSmxFe1O3 particles does not modify with Sm substitution. On the other hand, the effect of Ni substitution in the Bi0.94Sm0.06FeO3 sample is more effective on particle size, as shown in insets of Fig. S2. Furthermore, the porosity of samples increases with Fe3+ ion substitution with Ni2+ ion.
The energy-dispersive X-ray spectroscopy spectra of Bi1−xSmxFe1−yNiyO3 nanoparticles are presented in Fig. S3. The patterns of Bi1−xSmxFe1−yNiyO3 nanoparticles (y = 0.0) were composited of four elements, including Bi, Sm, Fe, and O, without the presence of any other elements in the obtained pattern. The peak intensity of Bi in BFO was reduced with doping Sm in the BFO composite, confirming that Bi ions in BFO were successfully substituted with Sm ions. Also, five elements, Bi, Fe, Sm, Ni, and O, are observed in the pattern of Bi0.94Sm0.06 Fe1−yNiyO3 nanoparticles, which indicates any other peaks of elements are not present in the obtained pattern of these nanoparticles. Similarly, a decrease in the peak intensity of Fe ions in BFO was observed by substituting them with Ni ions. The results indicated the successful synthesis of Bi1−xSmxFe1−yNiyO3 nanoparticles with a high purity without other elements in the structure of these nanoparticles.
The magnetic hysteresis loop (M–H) of Bi1−xSmxFe1−yNiyO3 nanoparticles at room temperature (R.T.) was observed in Fig. 2. It is clear that BFO indicates an antiferromagnetic behavior with not any hysteresis loop. However, with doping Sm in BFO, the Bi1−xSmxFeO3 nanoparticles exhibit a weak ferromagnetic feature. It is known that the magnetic behaviour of BiFeO3 is due to two components. The first one consists of a short-range order due to the sublattice of Fe3+ ions. The second one includes a long-range order that has the spiral spin structure with a long wavelength of λ = 62 nm[35]. Therefore, a suppression occurs in the spiral cycloid spin structure via modifying short-range order due to structural distortion by Sm substitution. As a result, the remnant magnetization (Mr) and coercive field (H.C.) increase with Sm concentration, as shown in Table 2. On the other hand, the effect of Ni substitution on the magnetic behaviour of the Bi0.94Sm0.06FeO3 sample is different compared to that of Sm substitution in BFO. While Mr enhances with increasing Ni doping, but H.C. decline with them. As mentioned in the structural part, because the Ni ionic radii is almost similar to that of Fe, the change in magnetic behavior because of lattice distortion is weakened. Therefore, two scenarios are possible for modifying the magnetic behaviour of the Bi0.94Sm0.06Fe1−yNiyO3 sample. (i) Difference of magnetic moment between Fe ion and Ni ion; With substituting Ni ion for Fe ion, there is a non-zero contribution in magnetization because of the imbalance of the magnetic moment of Fe (5 μB) and Ni (2 μB) in B site. However, this factor is not very dominant due to the low-level Ni ion doping. (ii) Size effect; When the average particle size is less than 62 nm, this factor plays an important role in the magnetic behavior of studied samples. The microstructural result of Bi1-xSmxFe1−yNiyO3 nanoparticles indicates that the particle size decrease more with Ni doping so that it is clear, particularly in the sample with y = 0.05. Therefore, we expect that Mr increase and H.C. decrease with decreasing particle size. These results were supported by previous reports [36, 37].
In order to investigate the optical properties of Bi1-xSmxFe1-yNiyO3 nanoparticles at RT, the UV–Vis diffuse reflectance spectra were carried out in 100–1200 nm wavelength. Fig. S4 shows the changes of [F(R)hν]2 versus hν (photon energy) for Bi1−xSmxFe1−yNiyO3 nanoparticles. The adsorption [F(R)] spectra of all samples were calculated using Kabelka–Munk (K–M) equation [38]. The direct band gap of Bi1−xSmxFe1−yNiyO3 nanoparticles was determined using plotted curves in Fig. S4. The band gap of Bi1−xSmxFeO3 nanoparticles (x = 0.0, 0.02, 0.04, 0.06, 0.08) were deduced about 2.14, 2.12, 2.12, 2.06, and 2.14 eV. The decrease of band gap with Sm doping is agreed with a previous report [39]. Besides, the band gaps of Bi0.94Sm0.06Fe1−yNiyO3 nanoparticles decrease with Ni substituting up to y = 0.03 and increase afterwords (Table S1).
Optimization of photodegradation procedure
A central composite design with a random order, including 30 runs, was provided to optimize four factors that affected the M.R. photodegradation. The factors run and degradation efficiencies (E%) are presented in Table 3. In the procedure, BFO was selected as a catalyst for 120 min underexposure with a U.V. lamp. Each run was repeated three times under the same condition, and the average of the results was used to calculate E% based on Eq. 1. The obtained E% was evaluated using variance analysis (ANOVA) at a confidence interval of 95% (Table 4) [40, 41]. The p-value is a suitable parameter in the ANOVA table to investigate the significance of factors or interactions[42, 43]. Therefore, all factors significantly affect the M.R. degradation due to a p-value lower than 0.05 at 95% of the confidence interval. Besides, the p-value of interaction between the M.R. concentration and catalyst amount is equal to 0.9454, indicating this interaction has a non-significant effect on the Mr degradation. Other binary interactions are meaningful parameters with a p-value lower than 0.05. The model presented using the design is significant because the p-value of the model is lower than 0.05, while the p-value of lack of fit is equal to 0.7951. Hence, the parameter is non-significant. A quadratic equation was usually developed to describe the proposed model. This equation shows the relationship between E% with factors and interactions. The resulting equation is as follows:
The equation’s adjusted coefficient of determination (adjusted R2) and determination coefficient (R2) are 0.9250 and 0.9625, indicating that the equation fitted with the results well. Besides, an adequately predicted coefficient of determination (predicted R2) of 0.8499 showed that E% could be well predicted using the equation for future investigation. The pH of samples (B) is a critical factor with a negative effect on E% due to a high and negative coefficient, indicating that the M.R. degradation is performed more effectively under acid conditions. This may be due to the better and faster decomposition of H2O2 in acidic media to form free radicals. The next crucial factor is the H2O2 volume (D), which has a significant coefficient in the equation and positively affects the M.R. degradation. Also, the interaction between catalyst amount and H2O2 volume (CD) has the highest significant and positive effect on E%. The surface plot of this interaction (CD) is presented in Fig. S5, showing that E% was increased with a simultaneous increase in catalyst amount and H2O2 volume. The adsorption of light was increased with increasing the catalyst amount, leading to an increase in the formation of electron–hole pairs and free radicals. Also, the M.R. photodegradation was significantly increased by an increase in H2O2 volume due to an increase in the formation of *O.H. radicals in the surface of BFO or doped BFO based on the following equation [44, 45]:
This process reduces the recombination time of the electron–hole pairs and increases the M.R. degradation efficiency. The optimization of all factors was investigated based on the proposed model and equation. For this purpose, a maximum amount of E% was selected to determine the optimum amount of factors. The optimum value of M.R. concentration, pH, catalyst amount, and H2O2 volume was 2.9 mg L−1, 3.1, 98 mg, and 0.58 mol L−1 (Fig. S6).
Photocatalytic activity study
Methyl red (M.R.) was selected as a typical dye to investigate the photocatalytic activity of the Bi0.94Sm0.06 Fe0.97Ni0.03O3 nanoparticles than the Bi0.94Sm0.06FeO3 nanoparticles in an aqueous solution of M.R. under visible-light irradiation at room temperature. In the study, the M.R. concentration and the photocatalyst amount were 2.9 mg L−1and 98 mg at a pH of 3.1y (Sect. 2.4). The suspension of M.R. and photocatalyst (200.0 mL) was kept in the dark and stirred at 600 rpm for 5.0 h at room temperature to achieve an adsorption–desorption equilibrium. The suspension was exposed to visible light with a wavelength range between 350 and 700 nm at intervals of 0–100 min for the Bi0.94Sm0.06FeO3 nanoparticles and 0–60 min for the Bi0.94Sm0.06 Fe0.97Ni0.03O3 nanoparticles under magnetic stirring at room temperature. A distance of about 5 cm between the M.R. solution and the lamp was used for the light exposure. Then, the photocatalyst was separated from the suspension using a neodymium magnet. The degradation of M.R. was investigated by measuring the absorbance of the M.R. aqueous solution after separating the photocatalyst using a UV–visible spectrophotometer at a wavelength of 526.0 nm (Fig. S7). For this purpose, the absorption peak intensity of M.R. was compared with its initial intensity (Ct/C.O.).
The photocatalytic activity of the Bi1−x Smx FeO3 and the Bi0.094 Sm0.06 Fe1−y NiyO3 nanoparticles is presented in Fig. S8. According to Fig. S8, the degradation efficiency of M.R. is negligible without increasing the photocatalyst, indicating that H2O2 has a non-significant effect on the degradation of M.R. In contrast, it increases dramatically in the presence of BFO as a photocatalyst. Besides, the photocatalytic activity of the Bi1−x Smx FeO3 nanoparticles increased by increasing the amount of Sm in the BFO nanoparticles. The MR degradations of 47.89, 63.21, 69.84, 85.76, and 78.51 were obtained for x = 0.00, 0.02, 0.04, 0.06 and 0.08 in the Bi1−x Smx FeO3 nanoparticles for 100 min. Therefore, the highest degradation of M.R. was determined using the Bi0.094Sm0.06FeO3 nanoparticles as a photocatalyst. Then, the effects of changing the Ni amount on the structure of the Bi0.094 Sm0.06 Fe1−y NiyO3 nanoparticles were evaluated as photocatalysts for the M.R. degradation (Fig. S8b). The results indicated that the M.R. degradations of 61.64, 80.24, 97.34, and 84.44% were obtained for y = 0.00, 0.01, 0.03, 0.05 in the Bi0.094 Sm0.06 Fe1-y NiyO3 nanoparticles for 60 min. According to Fig. S8 b, the M.R. degradation was increased with increasing Ni2+ ion up to 0.03% and decreasing afterward. Because Sm3+ and Ni2+ ions can act as good electron acceptor species in the BFO structure, forming trapping sites, possibly leading to easy separation of the formed electron–hole pairs and prolonging the charge carrier lifetime[46, 47]. Excessive amounts of Sm3+ and Ni2+ dopants can form the recombination centers in the BFO lattice; as a result, the photoactivity of the prepared nanoparticles is reduced [47]. Therefore, the highest M.R. degradation of 97.34% was observed for the Bi0.94 Sm0.06 Fe0.097 Ni0.03O3 nanoparticles as a photocatalyst at a time of about 60 min. The energy gaps of BFO, Bi1−x Smx FeO3, and Bi0.96Sm0.06 Fe1−yNiyO3 were studied to investigate the photocatalytic activity of these nanoparticles (Table S1 and Fig. S4). The energy gap for BFO was 2.14 eV, which decreased with increasing Sm (III) ions up to 0.06 as a dopant to 2.06 eV and increased afterward. Therefore, the highest photocatalytic activity was obtained for Bi0.94 Sm0.06 FeO3 with the lowest energy band gap. Besides, investigation of energy gaps of Bi0.96Sm0.06 Fe(1−y)NiyO3 indicated that a reduction in the energy gaps was displayed with increasing Ni(II) ions as co-dopant in up to 0.03 Bi0.94 Sm0.06 FeO3. Then, the energy gaps were increased for higher amounts of Ni (II) ions (Fig. S4). These results confirm that BFO photocatalytic activity to degrade M.R. increases dramatically with increasing Sm3+ and Ni2+ ions in its structure and decreases the M.R. degradation time.
The kinetics of the M.R. photodegradation in the presence of the Bi1−x Smx FeO3 and Bi0.094 Sm0.06 Fe1−y NiyO3 nanoparticles were investigated using the Langmuir–Hinshelwood model through the determination of the pseudo-first-order rate constant for the M.R. photodegradation. For this purpose, the pseudo-first-order rate constant for the M.R. photodegradation was determined based on the following nonlinear equation[48, 49]:
where A, and k are the absorbance of M.R. in irradiation time of t, and the pseudo-first-order rate constant,. X and E are the amplitude and the endpoint of the photodegradation process. The photodegradation rate constant (k) for a period of 100 min in presence of the Bi1-x Smx FeO3 were 0.00643, 0.0103, 0.0115, 0.0199, and 0.0118 Cm−1 for x = 0.00, 0.02, 0.04, 0.06, and 0.08 at pH 3.0. Besides, the K values of the Bi0.094 Sm0.06 Fe1-y NiyO3 nanoparticles as a photocatalysts for a period of 60 min were 0.0173, 0.0287, 0.0604, 0.0324 Cm−1 for y = 0.0, 0.01, 0.03, and 0.05 (Table 5). The results showed that the M.R. degradation reaction rate increases significantly with the Sm and Ni dopants in the BFO lattice structure.
Conclusion
In this research, two BFO derivatives including, Bi(1−x)SmxFeO3 (x = 0.1, 0.08, 0.06, 0.04, 0.02) and Bi0.094 Sm0.06 Fe1−y NiyO3 nanoparticles (y = 0.05, 0.03, 0.01) were synthesized by sol–gel method and their application as photocatalyst for methyl red degradation were investigated. The study of the photocatalytic activities of Sm-doped BFO and Sm and Ni co-doped BFO for the M.R. degradation was performed under dark and visible light in hydrogen peroxide in an acidic media (pH 3.0). The photocatalytic activity of Sm and Ni co-doped BFO nanoparticles is higher than the Sm-doped BFO nanoparticles, showing the energy band-gap of the Sm and Ni co-doped BFO nanoparticles is lower than the Sm-doped BFO nanoparticles. Besides, the M.R. degradation time in the presence of Bi0.94Sm0.06Ni0.03Fe0.97 nanoparticles (about 60 min) is dramatically less than Bi094Sm0.06FeO3 nanoparticles (about 100 min). The greater magnetic property of the Sm and Ni co-doped BFO nanoparticles compared to the Sm-doped BFO nanoparticles leads to a simple photocatalytic separation using a strong magnet after the M.R. degradation and removal of the centrifuge step. Under the optimum conditions, The MR degradation efficiencies under visible-light illumination for 60 and 105 min at pH 3.0 were 97.34 and 85.76% for Bi0.94Sm0.06Ni0.03Fe0.97 and Bi094Sm0.06FeO3 nanoparticles.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Ponraj C, Vinitha G, Daniel J (2017) A review on the visible light active BiFeO3 nanostructures as suitable photocatalyst in the degradation of different textile dyes. Environ Nanotechnol Monit Manag. 7:110–120
Singh S, Sharma R, Khanuja M (2018) A review and recent developments on strategies to improve the photocatalytic elimination of organic dye pollutants by BiOX (X= Cl, Br, I, F) nanostructures. Korean J Chem Eng 35(10):1955–1968
Islam MA (2019) Emergent environmental pollutants removal using metal oxide nanoparticles. Cell 1818:482533
Mathew S, Radhakrishnan E (2019) Polymer nanocomposites alternative to reduce environmental impact of non-biodegradable food packaging materials. In: Ahmed S, Chaudhry SA (eds) Composites for Environmental Engineering. Wiley, New Jersey
Gordi Z, Ghorbani M, Ahmadian Khakhiyani M (2020) Adsorptive removal of enrofloxacin with magnetic functionalized graphene oxide@ Metal-organic frameworks employing D-optimal mixture design. Water Environ Res 92:1935–1947
Nowakowska M, Szczubiałka K (2017) Photoactive polymeric and hybrid systems for photocatalytic degradation of water pollutants. Polym Degrad Stab 145:120–141
He D, Luo Y, Lu S, Liu M, Song Y, Lei L (2018) Microplastics in soils: analytical methods, pollution characteristics and ecological risks. TrAC, Trends Anal Chem 109:163–172
Paliwal A, Banu R, Ameta R, Ameta SC (2017) Photocatalytic degradation of methylene blue using undoped and Co-doped bismuth ferrite. J Applicable Chem 6:967–975
Liu H, Wang C, Wang G (2020) Photocatalytic advanced oxidation processes for water treatment: recent advances and perspective. Chem Asian J 15:3239–3253
Lam S-M, Sin J-C, Mohamed AR (2017) A newly emerging visible light-responsive BiFeO3 perovskite for photocatalytic applications: a mini review. Mater Res Bull 90:15–30
Bhoi YP, Mishra B (2018) Photocatalytic degradation of alachlor using type-II CuS/BiFeO3 heterojunctions as novel photocatalyst under visible light irradiation. Chem Eng J 344:391–401
Irfan S, Shen Y, Rizwan S, Wang HC, Khan SB, Nan CW (2017) Band-gap engineering and enhanced photocatalytic activity of Sm and Mn doped BiFeO3 nanoparticles. J Am Ceram Soc 100:31–40
Xie W, Li R, Xu Q (2018) Enhanced photocatalytic activity of Se-doped TiO2 under visible light irradiation. Sci Rep 8:1–10
Gong Y, Zhao X, Zhang H, Yang B, Xiao K, Guo T, Zhang J, Shao H, Wang Y, Yu G (2018) MOF-derived nitrogen doped carbon modified g-C3N4 heterostructure composite with enhanced photocatalytic activity for bisphenol A degradation with peroxymonosulfate under visible light irradiation. Appl Catal B 233:35–45
Liang S, Zhang D, Yao X, Han R, Zhang Q, Jin C, Pu X, Geng Y (2020) Deposition-precipitation synthesis of Yb3+/Er3+ co-doped BiOBr/AgBr heterojunction photocatalysts with enhanced photocatalytic activity under Vis/NIR light irradiation. Sep Purif Technol 238:116450
Yousefi R, Beheshtian J, Seyed-Talebi SM, Azimi H, Jamali-Sheini F (2018) Experimental and theoretical study of enhanced photocatalytic activity of Mg-doped ZnO NPs and ZnO/rGO nanocomposites. Chem Asian J 13:194–203
Bui TS, Bansal P, Lee B-K, Mahvelati-Shamsabadi T, Soltani T (2020) Facile fabrication of novel Ba-doped g-C3N4 photocatalyst with remarkably enhanced photocatalytic activity towards tetracycline elimination under visible-light irradiation. Appl Surf Sci 506:144184
Si Y-H, Xia Y, Shang S-K, Xiong X-B, Zeng X-R, Zhou J, Li Y-Y (2018) Enhanced visible light driven photocatalytic behavior of BiFeO3/reduced graphene oxide composites. Nanomaterials 8:526
de Lima AF (2020) Optical properties, energy band gap and the charge carriers’ effective masses of the R3c BiFeO3 magnetoelectric compound. J Phys Chem Sol 144:109484
Verma MK, Kumar A, Das T, Kumar V, Singh S, Rai VS, Prajapati D, Sonwani RK, Sahoo K, Mandal K (2020) BiFeO3 perovskite as an efficient photocatalyst synthesised by soft chemical route. Mater Technol 36:1–9
Naeimi A, Dehghan E, Khoshnoud DS, Gholizadeh A (2015) Enhancment of ferromagnetism in Ba and Er co-doped BiFeO3 nanoparticles. J Magn Magn Mater 393:502–507
Rezaei M, Sanavi Khoshnoud D, Dehghan E (2016) Study of structural and magnetic properties of BiFeO3 nanoparticles co-doped with Ba and La. Iranian J Crystallogr Miner 24:563–572
Yang Y, Kang L, Li H (2019) Enhancement of photocatalytic hydrogen production of BiFeO3 by Gd3+ doping. Ceram Int 45:8017–8022
Zhang L, Feng W, Wang B, Wang K, Gao F, Zhao Y, Liu P (2017) Construction of dual-channel for optimizing Z-scheme photocatalytic system. Appl Catal B 212:80–88
Tang J, Wang R, Liu M, Zhang Z, Song Y, Xue S, Zhao Z, Dionysiou DD (2018) Construction of novel Z-scheme Ag/FeTiO3/Ag/BiFeO3 photocatalyst with enhanced visible-light-driven photocatalytic performance for degradation of norfloxacin. Chem Eng J 351:1056–1066
Rahimkhani M, Khoshnoud DS, Ehsani M (2018) Origin of enhanced multiferroic properties in Bi0. 85− xLa0. 15HoxFeO3 nanopowders. J Magn Magn Mater 449:538–544
Vanga PR, Mangalaraja R, Ashok M (2015) Effect of (Nd, Ni) co-doped on the multiferroic and photocatalytic properties of BiFeO3. Mater Res Bull 72:299–305
Dehno RT, Khoshnoud DS (2022) Multiferroic properties in Sm1-xErxFeO3 ceramics. J Magn Magn Mater 541:168515
Irfan S, Li L, Saleemi AS, Nan C-W (2017) Enhanced photocatalytic activity of La3+ and Se4+ co-doped bismuth ferrite nanostructures. J Mater Chem 5:11143–11151
Khoshnood RS, Khoshnoud DS (2019) Structural, magnetic, and photocatalytic properties in Bi0. 83− xLa0. 17YxFeO3 nanoparticles. Appl Phys A 125:1–10
Ghosh MP, Mukherjee S (2019) Disordered surface spins induced large exchange anisotropy in single-phase Sm3+ ions substituted nickel ferrite nanoparticles. J Magn Magn Mater 489:165320
Yang YL, Yang XC, Yuan JY, Li T, Fan YT, Wang L, Deng Z, Li QL, Wan DY, Zhao JT (2021) Time-Resolved Bright Red to Cyan Color Tunable Mechanoluminescence from CaZnOS: Bi3+, Mn2+ for Anti-Counterfeiting Device and Stress Sensor. Adv Opt Mater 9:2100668
Li Q, Bao S, Liu Y, Li Y, Jing Y, Li J (2016) Influence of lightly Sm-substitution on crystal structure, magnetic and dielectric properties of BiFeO3 ceramics. J Alloy Compd 682:672–678
S. Ahammod Ali, (2018) Fabrication and characterization of Ni 1-x CoxFe2O4 electromagnetic ceramic. http://lib.buet.ac.bd:8080/xmlui/handle/123456789/5732
Raevski I, Kubrin S, Pushkarev A, Olekhnovich N, Radyush Y, Li G, Chou C-C, Raevskaya S, Titov V, Malitskaya M (2019) Magnetic phase transitions in solid solutions of Fe-containing perovskite multiferroics. Ferroelectrics 542:36–44
Puhan A, Bhushan B, Satpathy S, Meena S, Nayak A, Rout D (2019) Facile single phase synthesis of Sr, Co co-doped BiFeO3 nanoparticles for boosting photocatalytic and magnetic properties. Appl Surf Sci 493:593–604
Nadeem M, Khan W, Khan S, Shoeb M, Husain S, Mobin M (2018) Significant enhancement in photocatalytic performance of Ni doped BiFeO3 nanoparticles. Mater Res Expr 5:065506
Zedek R, Djedjiga H, Megherbi M, Belkaid MS, Ntsoenzok E (2021) Effects of slight Fe (III)-doping on structural and optical properties of TiO2 nanoparticles. J Sol-Gel Sci Technol 100:44–54
Haruna A, Abdulkadir I, Idris S (2020) Photocatalytic activity and doping effects of BiFeO3nanoparticles in model organic dyes. Heliyon 6:e03237
Thammanu S, Marod D, Han H, Bhusal N, Asanok L, Ketdee P, Gaewsingha N, Lee S, Chung J (2021) The influence of environmental factors on species composition and distribution in a community forest in Northern Thailand. J For Res 32:649–662
Liao C, Tian Q, Liu F (2021) Nitrogen availability regulates deep soil priming effect by changing microbial metabolic efficiency in a subtropical forest. J For Res 32:713–723
Ghorbani M, Ariavand S, Aghamohammadhasan M, Seyedin O (2021) Synthesis and optimization of a green and efficient sorbent for removal of three heavy metal ions from wastewater samples: kinetic, thermodynamic, and isotherm studies. J Iran Chem Soc 18:1947–1963
Mohammadi P, Ghorbani M, Keshavarzi M, Rastegar A, Pakseresht M, Mohammadi M (2022) Dispersive micro solid-phase extraction as a green procedure for extracting prostate anti-cancer drugs in real water and biological samples and optimization of sorbent composite with an optimal mixture design. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2022.2045588
Rahmati R, Nayebi B, Ayati B (2021) Investigating the effect of hydrogen peroxide as an electron acceptor in increasing the capability of slurry photocatalytic process in dye removal. Water Sci Technol 83:2414–2423
Neshin MV, Khoshnood RS, Khoshnoud DS (2021) Enhanced photocatalytic activity of Ni-doped BiFeO3 nanoparticles for degradation of bromophenol blue in aqueous solutions. React Kinet Mech Catal 134:951–970
Choi W, Termin A, Hoffmann MR (2002) The role of metal ion dopants in quantum-sized TiO2: correlation between photoreactivity and charge carrier recombination dynamics. J Phys Chem 98:13669–13679
Hu Z, Chen D, Wang S, Zhang N, Qin L, Huang Y (2017) Facile synthesis of Sm-doped BiFeO3 nanoparticles for enhanced visible light photocatalytic performance. Mater Sci Eng, B 220:1–12
Lente G (2015) Deterministic kinetics in chemistry and systems biology: the dynamics of complex reaction networks. Springer, Cham
Lente G (2018) Facts and alternative facts in chemical kinetics: remarks about the kinetic use of activities, termolecular processes, and linearization techniques. Curr Opin Chem Eng 21:76–83
Acknowledgements
The Azad University of Mashhad, Iran and Semnan University, Iran, are appreciated.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. AS, RSK, DSK and ZE: Material preparation, data collection and analysis were performed. AS: The first draft of the manuscript was written. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Disclosure of potential conflicts of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saghafi, A., Khoshnood, R.S., Khoshnoud, D.S. et al. Magnetic properties and photocatalytic activity of Bi1-xSmxFe1-yNiyO3 nanoparticles for methyl red degradation. Reac Kinet Mech Cat 135, 3375–3391 (2022). https://doi.org/10.1007/s11144-022-02281-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02281-z