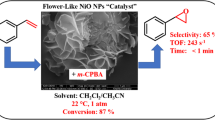
Abstract
Flower-like mesoporous NiO nanoparticles (NPs) were synthesised by surfactant-assisted chemical precipitation process and evaluated as a catalyst for the epoxidation of cyclohexene using meta-chloroperoxybenzoic acid (m-CPBA) as an oxidizing agent at room temperature and 1 atm. The mesoporous NiO (MesoNiO) showed an exceptional catalytic activity for the epoxidation of cyclohexene to produce cyclohexene oxide in the optimized conditions, exhibiting an immediate reaction at room temperature with high conversion (91%) and medium selectivity (53%), using m-CPBA/cyclohexene ratio of 1.5 in CH3CN/CH2Cl2 (1:1 v/v) as a solvent. The catalytic activity of MesoNiO was compared to bulk NiO (BulkNiO). Only 65% conversion with 58% selectivity were obtained with BulkNiO. The reusability of MesoNiO catalyst has been also investigated. During four successive cycles, the conversion of cyclohexene decreased gradually to 63% for MesoNiO and to 50% for Bulk NiO with a constant cyclohexene oxide selectivity for both materials.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, transition metal oxides (TMOs) nanoparticles (NPs) have attracted much attention in different fields including catalysis, [1] due to their interesting electronic, magnetic, and catalytic properties. Many reports [2] showed their good performance as catalysts for different reactions in terms of reactivity, and selectivity. To extend the applications of TMOs NPs in catalysis many efforts have been done to tailor their size, to improve their morphology, and to hence their surface area. Nickel oxide (NiO) is one of the most promising TMOs. NiO NPs have been applied as heterogeneous catalyst for many organic transformations [3, 4]. Since these NPs are eco-friendly, inexpensive, easy to synthesis and reusable, they may be considered as very promising heterogeneous catalysts.
One of the most important sectors in present-day chemical industry consists of catalytic oxidation processes [5]. The epoxidation reaction of olefins represents an important and significant oxidation process for the industrial production of many commodity chemicals.[6, 7]. Among the most important epoxides is the cyclohexene oxide, which has been used for many important applications such as the preparation of polymers, dyes, pharmaceuticals, pesticides, perfumery products [8].
In last decade, great efforts have been made to develop efficient and selective catalysts for the epoxidation of cyclohexene to produce pure cyclohexene oxide. Various type of catalysts have been developed and investigated such as supported and unsupported metal and metal oxides [9,10,11,12,13,14], supported and unsupported transition metal complexes [15,16,17,18], clays [19, 20], polyoxometalates [21,22,23], metal–organic frameworks (MOFs) [24], N-doped carbon nanotubes [25], and enzymes [26]. However, the epoxidation of cyclohexene using metal oxides as catalysts still suffers from some drawbacks, such as low conversion and selectivity, low reaction rate, and the necessity for heating. In addition, during the catalytic epoxidation of cyclohexene different side reactions can occur, such as oxidation of allylic position, epoxide rearrangement, ring-opening by solvolysis or hydrolysis, or even total fission of C=C double bond [9,10,11, 13, 16]. Therefore, more research work is still needed to develop simple and efficient catalysts for the preparation of cyclohexene oxide from cyclohexene with high conversion and selectivity, and in mild conditions.
In last decade, mesoporous TMOs have attracted much attention in many areas, such as catalysis, energy storage, and sensing [27,28,29]. These materials usually possess high surface area with narrow pore size distribution, which make them a versatile and promising catalysts. However, only few articles reported the utilization of mesoporous TMOs for the epoxidation of cyclohexene.
NiO is one of the most studied TMOs. Several works have reported the synthesis of mesoporous NiO with different morphologies and its applications in catalysis [30,31,32]. However, to the best our knowledge, the utilization of NiO NPs, particularly with mesoporous structure, as a catalyst for the epoxidation of cyclohexene has not yet investigated.
Recently, we have reported the synthesis and characterization of highly ordered flower-like mesoporous NiO (MesoNiO) NPs and their application in photocatalysis [30]. This material was prepared via surfactant-assisted chemical precipitation, using cetyltrimethylammonium bromide (CTAB) as structure directing agent, and in the presence of diethanolamine (EDA) as a complexing agent.
In the current work, this flower-like MesoNiO NPs were, for the first time, applied as a catalyst for the epoxidation of cyclohexene in the presence of m-CPBA as oxidizing agent in ambient conditions. This material catalyst has displayed an immediate reaction at room temperature, with high conversion and medium selectivity.
Experimental
Materials
Cyclohexene (99%), cyclohexene oxide (98%), m-CPBA (≤ 77%), nickel(II) acetate tetrahydrate (98%), DEA (98%), CTAB (98%), ammonium hydroxide solution (30–33% NH3 in H2O), 1-propanol (99.7%), acetonitrile, and dichloromethane were all purchased from Sigma-Aldrich, and used without any further purification.
Synthesis of flower-like mesoporous NiO NPs and bulk NiO (BulkNiO) powder.
Typical procedure for the synthesis of flower-like MesoNiO catalyst prepared with m-CPBA and the BulkNiO was described in our previous paper [30].
Catalytic activity measurement
Procedure was adopted from the literature [17]. In a lab test tube 10 mg of MesoNiO or no-mesoporous NiO catalysts were dispersed in 3 mL of CH3CN/CH2Cl2 (1:1 v/v), then 0.06 mL (0.6 mmol) of cyclohexene were added. After stirring this mixture at room temperature for 5 min, 156 mg of m-CPBA (1 equivalent, 0.9 mmol) was added. The mixture was stirred for 12 h at room temperature. The reaction was monitored by gas chromatography (GC). Samples were withdrawn from the reaction mixture periodically starting immediately after the addition of m-CPBA. This reaction was run twice, and the average yields, conversions are presented. Leaching measurement were performed by removing the filtrate after centrifugation of the mixture in the end of the reaction using atomic absorption spectroscopy (AAS). The solvent of the filtrate was evaporated in the oven at 100 °C, and nickel was extracted with deionized water and used for AAS. The remaining catalyst was reused four times, and Ni leached was measured after each cycle.
Results and discussion
As reported in our previous work, [30] the flower-like MesoNiO microspheres were prepared by surfactant-assisted chemical precipitation using diethanolamine (DEA) as a complexing agent. The surfactant used was cetyltrimethylammonium bromide (CTAB). The utilization of CTAB was necessary to reduce the NiO microspheres from 5 to 2 µm. The structure and morphology of the obtained NiO NPs were fully characterized in our previous work, using different techniques such as SEM, high-resolution (HR) TEM, EDX, XRD, TGA/DTA, FT-IR, BET and DR UV–Vis. The mesoporosity of this material was confirmed by the N2 adsorption–desorption measurement, where the adsorption–desorption isotherm showed a type-IV isotherm indicating the presence of mesoporous material and surface area of 21 m2/g and pore size of ⁓ 6 nm. SEM micrographs illustrated clearly the fairly flower-like shape of the NiO microspheres with uniform size distribution (2 µm), and each microsphere is formed by many thin nanoflakes (10–100 nm) arranged in random directions (Fig. 1, micrographs A and B). Further the surface structure of nanoflakes has been investigated by HR-TEM. The investigation of the nanoflakes by HR-TEM showed a highly ordered nanostructure of NiO NPs (Fig. 1, images C and D).
The obtained MesoNiO material was used as a catalyst for the epoxidation of cyclohexene oxide, using m-CPBA as an oxidizing agent in CH2Cl2/CH3CN (1:1 v/v) at room temperature. When 1 equivalent of m-CPBA was added to the reaction mixture in the presence of 10 mg of MesoNiO, the GC analysis performed after 60 min revealed the formation of tree major products, which are cyclohexene oxide, 2-cyclohexenone and 2-cyclohexanone, and cyclohexane-1,2-diol as a minor product, with cyclohexene conversion of 50% (Fig. 2).
To optimize the applied oxidant/cyclohexene ratio, three reactions were performed with varying the ratio from 1:1, 1.2:1 to 1.5:1 mol/mol. The reaction was performed for 60 min with a special attention to the first minute of the reaction. The obtained results are plotted in Fig. 3, which shows a fast reaction (almost immediate) whatever the applied oxidant/cyclohexene ratio. The ratio 1:1 showed a conversion of 47.5% in the first minute and it reached 49.2% in the end of the reaction. Moreover, the ratio 1.2:1 showed also a very fast conversion of cyclohexene (70.1%) and after 60 min the conversion reached 72.2%. Finally, when the applied ratio was 1.5:1, the conversion was 91% after the first minute, and it reached 95.5% in the end of the reaction. The selectivity of cyclohexene oxide was constant at 53% for the tree oxidant/cyclohexene ratios. The GC analysis performed for the immediate samples revealed the formation of cyclohexene oxide as the major product with 2-cyclohexenol as a minor product. The formation of 2-cyclohexenone and 2-cyclohexanone observed after 60 min can be explained by the degradation of 2-cyclohexenol [9]. Therefore, the obtained results strongly suggest that the ratio of 1.5 oxidant: 1 cyclohexene is the perfect ratio to accomplish the reaction.
In order to investigate the role of the prepared catalyst in the epoxidation of cyclohexene reaction, the reaction was allowed to be performed without catalyst using 1.5 equivalent of m-CPBA. In the non-catalyzed reaction, the calculated yield (the conversion multiplied by the epoxide selectively) was zero (Fig. 4). The same result was obtained when the reaction was performed without m-CPBA. This result shows that the reaction was not performed in the absence of oxidant or the catalyst. However, in the presence of MesoNiO and 1.5 equivalent of m-CPBA under ambient conditions, almost 91% of cyclohexene was converted in to cyclohexene oxide with a selectivity of 53%. Whereas, the conversion of cyclohexene decreased to 65% when the reaction was catalyzed by BulkNiO with a slight increase in the selectivity (58%). The high conversion obtained with MesoNiO compared to BulkNiO is related to the high surface area of MesoNiO so more available active sites. However, the low selectivity for both materials can be attributed to a competing acid-catalyzed decomposition of the obtained epoxide by the formed meta-chlorobenzoic acid (m-CBA). For MesoNiO there is an additional acid species could catalyze the decomposition of the epoxide, which are the residual Ni-OH species remaining after the calcination of Ni(OH)2 which can explain the slightly low selectivity of MesoNiO compared to BulkNiO.
The reusability of heterogeneous catalysts is an important parameter. Therefore, the reusability of MesoNiO catalyst was investigated, and for comparison, the reusability of BulkNiO was also studied, the obtained results are presented in Fig. 5. After four successive cycles the catalytic activity of MesoNiO and BulkNiO was decreased from 91 to 63%, and from 65 to 50%, respectively. This result could be attributed to the relatively fragile structure of MesoNiO, its aggregation and partial dissolution in the reaction. Similar behavior was observed for NiO NPs in the epoxidation of styrene with TBHP [27].
One of the most efficient catalysts reported in the literature used for the epoxidation of cyclohexene and exhibited a high conversion and selectivity are the Schiff base ligand complex of transition metals. Kim et al. [17] (Table 1, Entry 1) reported a fast and quantitative epoxidation of cyclohexene and other cycloalkenes with high selectivity using nickel (II) porphyrin complex as a catalyst, and m-CPBA as an oxidizing agent at room temperature. However, the utilization of this catalyst in homogeneous conditions has many drawbacks such as difficulty in separating the catalysts and the product(s) form the reaction mixture, no reusability. Therefore, the heterogenization of theses metal complexes by incorporating them in a solid matrix will increase their stability, facilitate their separation and make their reusability possible. Gupta et al. [16] reported recently the preparation of Schiff base ligand complex of copper (II) supported on monolith and its catalytic activity in the epoxidation of cyclohexene (Entry 2). This heterogeneous catalyst exhibited a high conversion and selectivity, but heating the reaction mixture to 40 °C during 24 h was necessary in addition to difficulty to prepare the support and the catalyst, followed by the incorporation of the catalyst in the support. Therefore, the development of a simple an efficient heterogeneous catalyst is still necessary. TMOs constitute the largest family of catalysts in heterogeneous catalysis, are not expensive and easy to prepare. However, only few publications reported the utilization of some TMOs as catalysts for the oxidation of alkenes. Yoshikawa et al. [33] reported the utilization of 1% RuO2-loaded mesoporous TiO2 as a catalyst for the epoxidation of cyclohexene, using H2O2 as an oxidizing agent (Entry 3). Heating to 60 °C during 3 h was not enough to exceed 35% conversion. The activation of TiO2 with V2O5, using TBHP (tert-Butyl hydroperoxide) as an oxidizing agent, and increasing temperature (80 °C) and time (6 h) increased the conversion of cyclohexene to 48% while maintaining the same selectivity (Entry 4). NiO showed a catalytic activity for the epoxidation of cyclohexene about fifteen years ago. A BulkNiO powder was used for comparison purpose with porous nickel phosphate VSB-5, but exhibited a low catalytic activity toward the epoxidation reaction of cyclohexene using H2O2 as an oxidant (Entry 5) [34]. Ebadi et al. [35] reported the utilization of NiO NPs supported on mesoporous MCM-41 with air at high temperature (280 °C) (Entry 6). But cyclohexene was converted only to 2-cyclohexene-1-ol and 2-cyclohexene-1-one (62%), and cyclohexene oxide was not observed.
In this study, MesoNiO with m-CPBA has exhibited an immediate and high catalytic activity toward the epoxidation of cyclohexene in ambient conditions, with 91% conversion (Entry 7) compared to only 65% for BulkNiO powder (Entry 8), with medium selectivity.
Proposed mechanism
Based on previous mechanistic investigations, trapping experiments and theoretical studies of the epoxidation reactions of olefins catalyzed by late transition metals, using peroxycarboxylic acids such as m-CPBA as oxygen donor, this reaction probably operates by redox mechanism involving the formation of NiII-OOAr and Ni-Oxo activated species (Scheme 1), which transfer the oxygen to the olefin either directly or by the intermediacy of a metallacycle, a radical, cation species, or via concerted transition state [17, 36,37,38].
Therefore, the following reaction pathway (Scheme 1, path 1) has been proposed for the epoxidation reaction of cyclohexene over NiO using m-CPBA as oxygen donor. This reaction starts by the immediate adsorption of m-CPBA on the surface of NiO (MesoNiO or BulkNiO), followed by deprotonation of m-CPBA and formation of NiII-OOAr intermediate. The O–O bond in NiII-OOAr bond would cleave to form the activated NiIV-Oxo and meta-chlorobenzoic acid (m-CBA). NiIV-Oxo thereafter interacts with cyclohexene with eventual transfer of the oxygen atom to the double bond through concerted transition state. Then the cyclohexene oxide is formed and the catalyst is recovered.
Beside this pathway, there is a competing acid-catalyzed decomposition of the epoxide and the formation of cyclohexane-1,2-diol (path 2). The acid could be the formed m-CBA (ArOH) (path 2, A) or the remaining Ni-OH species (path 2, B) after the formation of MesoNiO from the Ni(OH)2 precursors. Thus, the slightly high selectivity of BulkNiO could be explained by the absence of reaction B (path 2).
Conclusions
Flower-like Mesoporous NiO microspheres have been successfully prepared via surfactant-assisted chemical precipitation method. Flowers size was around 2 µm, with nanoflakes size ranges from 10 to 100 nm. This material has been utilized as a catalyst for the epoxidation of cyclohexene in CH2Cl2/CH3CN—m-CPBA system with 1.5 equivalent of m-CPBA, at room temperature and at 1 atm. The highly ordered MesoNiO NPs have shown an immediate reaction with high conversion (91%) and medium selectivity (53%) compared to bulk NiO which exhibited only 65% conversion with 58% selectivity. The reusability of both catalysts, MesoNiO and BulkNiO, has been also studied. During four successive cycles, the conversion of cyclohexene decreased gradually from 91 to 63% for MesoNiO, and from 65 to 50% for BulkNiO. This decrease in the activity can be attributed to the relative fragility, aggregation and partial dissolution of NiO particles in the reaction mixture. To overcome some drawbacks of NiO particles as catalyst, NiO will be incorporated and dispersed into an adequate host matrix, such as mesoporous silica, and the obtained results will be reported in a future work.
References
Védrine JC (2017) Catalysts 7:341–366
Pergament A (2014) Oxide Electronics and Functional Properties of Transition Metal Oxides. Nova, New York
Nasseri MA, Kamali F, Zakerinasab B (2015) RSC Adv 5:26517–26520
Sachdeva H, Dwivedi D, Bhattacharjee RR, Khaturia S, Saroj R (2013) J Chem. https://doi.org/10.1155/2013/606259
Centi G, Cavani F, Trifirò F (2001) Selective Oxidation by Heterogeneous Catalysis. Springer, Boston
Cavani F, Teles JH (2009) Chemsuschem 2:508–534
Whittall J, Roberts SM (2007) Regio- and Stereo-Controlled Oxidations and Reductions. Wiley, Chichester
Kim I, Kim SM, Ha C-S (2004) Rapid Commun 25:888–893
Lahcene D, Choukchou-Braham A (2018) J Chin Chem Soc 65:1529–1535
Denekamp IM, Antens M, Slot TK, Rothenberg G (2018) ChemCatChem 10:1035–1041
Rogers O, Pattisson S, Macginley J, Engel RV, Whiston K, Taylor SH, Hutchings GJ (2018) Catalysts 8:311–321
Denekamp IM, Antens M, Slot TK, Rothenberg G (2017) ChemCatChem 10:1035–1041
Dou J, Tang Y, Nguyen L, Tong X, Thapa PS, Tao FF (2016) Catal Lett 147:442–452
Ghiami S, Nasseri MA, Allahresani A, Kazemnejadi M (2019) React Kinet Mech Catal 126:383–398
Farahmand S, Ghiaci M (2019) Microporous Mesoporous Mater 288:109550–109560
Moghe K, Sutar AK, Kang IK, Gupta KC (2019) RSC Adv 9:30823–30834
Ahn HM, Bae JM, Kim MJ, Bok KH, Jeong HY, Lee SJ, Kim C (2017) Chem Eur J 23:11969–11976
Li X, Ma D, Cao B, Lu Y (2017) New J Chem 41:11619–11625
Dali A, Rekkab-Hammoumraoui I, El Korso S, Boudjema S, Choukchou-Braham A (2019) Bull Chem React Eng Catal 14:614–624
Boudjemaa S, Rabaha H, Choukchou-Braham A (2017) Catal Acta Phys Polonica A 132:469–472
Radman R, Aouissi A, Al Kahtani A, Mekhamer W (2017) Pet Chem 57:79–84
Liu Y, Murata K, Inaba M, Nakajima H, Koyay M, Tomokuni K (2004) Chem Lett 33:200–201
Masteri-Farahani M, Alavijeh MK, Hosseini M-S (2020) React Kinet Mech Catal 130:303–315
Maksimchuk N, Lee JS, Ayupov A, Chang J-S, Kholdeeva O (2019) Catalysts 9:324–337
Cao Y, Yu H, Peng F, Wang H (2014) ACS Catal 4:1617–1625
Wunschik DS, Ingenbosch KN, Süss P, Liebelt U, Quint S, Dyllick-Brenzinger M, Zuhse R, Menyes U, Hoffmann-Jacobsen K, Opwis K, Gutmann JS (2020) Enzyme Microb Technol 136:109512
Huang K, Wang Z, Wu D (2018) J Chem Sci 130:62
Hassan HMA, Betiha MA, Elshaarawy RFM, El-Shall MS (2017) Appl Surf Sci 402:99
Li Z, Wu S, Yang C, Ma Y, Fu X, Peng L, Guan J, Kan Q (2017) Mol Catal 432:267
Abboud M, Abu-Haija M, Bel-Hadj-Tahar R, Mubarak AT, Ismail I, Hamdy MS (2020) New J Chem 44:3402–3411
Xu X, Li L, Yu F, Peng H, Fang X, Wang X (2017) Mol Catal 441:81–91
Tong S, Zheng M, Lu Y, Lin Z, Li J, Zhang X, Shi Y, He P, Zhou H (2015) J Mater Chem A 3:16177–16182
Sreethawong T, Yamada Y, Kobayashi T, Yoshikawa S (2005) J Mol Catal A 24:23–32
Jhung SH, Lee J-H, Cheetham AK, Férey G, Chang J-S (2006) J Catal 239:97–104
Ebadi A, Mozaffari M, Shojaei S (2014) J Chem Sci 126:989–996
Ted Oyama S (2008) Mechanisms in Homogeneous and Heterogeneous Epoxidation Catalysis, 1st edn. Elsevier, Oxford
Agarwala H, Ehret F, Chowdhury AD, Maji S, Mobin SM, Kaim W, Lahiri GK (2013) Dalton Trans 42:3721
Hu R, Yang P, Pan Y, Li Y, He Y, Feng J, Li D (2017) Dalton Trans 46:13463–13471
Acknowledgements
The author extends his appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a research group project number R.G.P1/89/40. The author would like to express his gratitude to King Khalid University, Abha, Saudi Arabia for providing administrative and technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abboud, M. Immediate epoxidation of cyclohexene at room temperature using mesoporous flower-like NiO nanoparticles. Reac Kinet Mech Cat 131, 781–792 (2020). https://doi.org/10.1007/s11144-020-01864-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01864-y