Abstract
This research aims to produce a novel biodiesel fuel with standard quality from the non-edible oil with Lithium doped Calcium Oxide (Li–CaO) based heterogeneous nanocatalyst derived from Musa balbisiana Root ash. The characterization of the prepared nanocatalysts was achieved by X-ray diffractometer (XRD), Brunauer–Emmett–Teller (BET), Fourier transform infrared (FTIR), energy dispersive X-ray (EDX), scanning electron microscopy (SEM), X-Ray photoelectron spectroscopy (XPS) and transmission electron microscopy (TEM) techniques. Moreover, the production time and yield were optimized by a novel Hybrid Response Surface Methodology along with African Buffalo Optimization (HRSM-ABO) algorithm. The proposed method simulation was done on the Matlab platform. According to the simulation and experimental outcomes, the optimum biodiesel yield of nearly 97.8% was achieved at the conditions that the 4 wt% of catalyst amount, 15:1 methanol to the oil of molar ratio, reaction time of 150 min and the reaction temperature of 65 °C with Amplitude of 75%. Consequently, reusability investigation proved that there the catalytic action of the improved catalyst was moderately decreased after 7 cycles. The mechanism of the proposed transesterification process was understood by the estimation of kinetic study. Furthermore, the physiochemical properties of the proposed biodiesel were measured. This result signified that the newly prepared Li–CaO was the most suitable catalyst to make biodiesel, which can be utilized in diesel engines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent times, an exhaustion of fossil fuel reserves, increasing demand of energy and different ecological factors have led for exploring the renewable based environment-friendly alternative resources such as solar, wind, and biomass [1]. Among, these different renewable energy sources, biodiesel are selected as the substitute fuel to diesel that could effectively fulfil the problem of fossil fuel shortage [2]. Because biodiesel produced from 100% of renewable assets compared with the other fuels. Biodiesel offers many advantages due to its renewable, perishable, non-toxic, clean combustion properties, superior lubricity, and diminished CO2 emissions [3]. Moreover, this green fuel is the most advantageous resource because of its characteristics such as flash and cloud point, viscosity, etc., likewise traditional diesel. Biodiesel also termed as fatty acid methyl esters (FAME), which is formed via transesterification of nonedible oils, fats of animal, or vegetable oil composed with alcohol or methanol in the existence of a suitable catalyst [4]. In biodiesel production, for efficiency enhancing and transesterification speedup homogeneous or heterogeneous of inorganic catalyst or enzymes of organic catalyst can be used [5]. The homogeneous of the inorganic catalyst has non-reusability properties and a vast quantity of waste liquid will generate while catalyst residues elimination in biodiesel generation [6]. However, the heterogeneous of inorganic catalysts have certain merits such as the catalyst could easily improve as well as reutilized without any loss in the reaction [7]. In the presence of an acid/base catalyst, transesterification can typically produce biodiesel. The acid based catalyst requires high temperature and takes more time; this will lead to an increase the production price. Hence, the alkaline metal oxide based inorganic heterogeneous catalyst is the well-recognized method for biodiesel fabrication [8]. Nevertheless, because of the huge price of biodiesel manufacture is the major reason for non-commercialized worldwide. Therefore, several researchers are interested in reducing the price of biodiesel generation using edible oils and nonedible oils namely coconut oil, Peanut oil, rubber oil, Palm oil, soybean oil, curcas oil, and rapeseed oil [9]. Conversely, the edible oil based biodiesel formation can conquer and resist plantation, this leads to the lack of food and deforestation. For this reason, the nonedible oils heterogeneous catalysts based biodiesel generation plays a remarkable part due to its numerous advantages [10]. Furthermore, in a huge amount of modeling and experiments, optimization of transesterification condition is utilized to forecast the finest parameters under each reaction. Conventionally, the various materials and catalysts in biodiesel production optimization had been effectively done using the RSM method, machine learning techniques, and heuristic optimizations [11].
Recently, different heterogeneous based catalysts have been analyzed for making of biodiesel in the works of literature [12]. The utilization of fossil fuel causes environmental pollution and global warming because it releases greenhouse gases and this leads to the invention of replacing fuel like biodiesel [13]. For this reason, Seffati et al. [14] introduced the biodiesel from chicken fat by CaO/CuFe2O4 nanocatalyst in transesterification. This method of biodiesel production supported under the molar ratio 15:1, catalyst 3 wt% at 70 °C for 4 h of reaction time, and attained 94.52% of yield. Moreover, the pure diesel physical properties for example, density, kinematic viscosity, flash and cloud points were investigated. Moreover, a heterogeneous based alkaline earth metal oxide (CaO) catalyst derived from waste date pits was prepared by Muhtaseb et al. [15]. The optimized biodiesel yield of 97% was attained under the molar ratio of 12 and 4.5 wt% of catalyst quantity at 70 °C for around 120 min. The acid value and viscosity of the produced biodiesel were measured as 3.98 mm2/s and 0.26 mgKOH/g, which is in the assortment of ASTM and EN standard limit. Mazaheri et al. [16] investigated the rice bran oil-based transesterification into diesel is prepared using the CaO catalyst. Consequently, Coreus brunneus of shell resultant the CaO catalyst [17] under the molar ratio 30:1 of rice brain oil, 0.4 wt% of catalyst at 1100 °C for 120 min and the yield obtained as 93%. The characteristics of catalysts from seashells were analyzed using FTIR, XRD, BET, and TEM. Moreover, the Artificial Neural Network (ANN) along with Ant Colony Optimization (ACO) techniques was combined for enhancing the transesterification model. This method of biodiesel production was obtained with the reduced kinematic viscosity as 4.42 mm2/s, which was confirmed within ASTM standard condition. Hoseini et al. [18] investigated a new form of biodiesel generation using Ailanthus Altissima seed Oil (AAO) referred to as a feedstock and potassium carbonate (KOH) as a catalyst. Moreover, the RSM model optimization was utilized for improving the obtained biodiesel yield. Under optimized condition, the molar ratio of the seed oil is 8.50:1, 1.01 wt% of catalyst amount at 160 °C for 4.71 min, and achieved 92.26% of yield. Furthermore, the Euonymus Maackii Seed Oil (EMSO) was utilized as a fresh probable non-edible seed oil for biodiesel introduced by Ju-Zhao Liu et al. [19]. In this EMSO based finest biodiesel production, the methanol to oil of molar ratio was 10:1, 2 wt% of catalyst quantity at 60 °C for 40 min and 94.74% of utmost yield was obtained. The constant rate of reactions was estimated by the pseudo-first-order kinetic method. Furthermore, the fuel and thermodynamic belongings of the biodiesel formation were considered and validated with the standards. Besides, Jeon et al. [20] introduced an acid with a base combination of hybrid heterogeneous catalysts for enhanced biodiesel preparation using core shell with rapid seed oil. This method has great enlargement in the formation of biodiesel due to the surface area properties and 98.02% of high FAME yield. Teo et al. [21] prepared effective biodiesel from Jatropha curcus oil using core shell of CaSO4/Fe2O3-SiO2 magnetic nanoparticle. In this method, the preparation of the material, morphology, and its recyclable properties were studied [22]. Moreover, the performance of the material was investigated during the transesterification process using the characteristics techniques such as BET, TEM, and SEM. Consequently, 94% of FAME yield was obtained under the methanol to oil of molar ratio was 9:1 and 5.1 wt% of catalyst amount at 120 °C for 240 min. Fard RGZ, et al. [23] introduced the Barioum Oxide (BaO) catalyst from beef tallow for making biodiesel as fuel. Here, Taguchi method was studied for the effect of parameter optimization. Therefore, the methanol to oil of molar ratio 16:1 was attained, catalyst quantity of 4 wt% at 50 °C temperature for five hours. Recycle capability of the catalyst was examined that the BaO based catalyst was recovered after 5 cycles. Nevertheless, this produced biodiesel cannot be utilized in cold areas. Kesserwan, et al. [24] developed a hybrid form of heterogeneous catalyst named as CaO/Al2O3 aerogel and waste cooking oil of transesterification for the formation of biodiesel. The catalyst characteristics were performed using SEM, FTIR, and XRD methods. Furthermore, the 11:1 molar ratio for 1wt% quantity of catalyst at 600 °C for 4 h was obtained as the finest state and 89.9% of utmost yield was attained. Besides, the renewable heterogeneous acid based catalyst using stimulated carbon obtained as of Oil Palm Empty Fruit Bunch (OPEFB), which was introduced by Wan-Ying, et al. [25]. Under the finest condition, the 50:1 methyl acetate to oleic acid of molar ratio and the catalyst weight as 10 wt% at 100 °C for 8 h, thus 50.5% of FAME yield was obtained. Hossain, et al. [26] investigated reliable biodiesel from the seed oil of papaya due to its extraordinary oil content. Here, the Generalized Linear Model (GLM) and RSM methods were utilized to the mathematical ideal progression. Besides, Crow Search Algorithm (CSA) was utilized for enhancing the reaction condition and attained 29.05% of yield at 6.5 h of execution time with 0.85 nm of particle size.
Various heterogeneous catalysts have been investigated in this recent literature to be energetic for biodiesel generation. From this, CaO based heterogeneous catalyst plays a significant part in biodiesel production due to its harmless environmental factors and low price. Previously, CaO [27] had been produced from various natural products like waste eggshells [42], mussel shells [43], mud crab shells and oyster shells [28], which minimized the entire price of biodiesel production. Hence, it was stated that the CaO catalysts synthesized from waste shells have certain benefits in biodiesel formation because of its easy production, recycle property, and less loss properties [29]. Recently, biodiesel production was prepared using Li incorporated CaO from sunflower oil [30]. The entire emphasis had been making the CaO catalyst as renewable resources. The key contribution of this research is summarized below:
-
The aim of this investigation is to study the novel finest reaction state for transesterification of nonedible oil with Musa balbisiana root using Li–CaO nanocatalyst for the biodiesel development. This is the novel research of biodiesel development using Musa ash derived CaO as the catalyst.
-
In biodiesel formation process, the nonedible oil of Lesquerella fendlei and Borage oil were utilized as feedstock.
-
Furthermore, the characteristics of the catalyst were performed by certain characterized techniques. Consequently, the production yield and time were modeled and optimized using a novel HRSM-ABO approach.
-
Subsequently, the impact of numerous parameters in the catalytic reaction process like methanol to oil of molar ratio, catalyst amount, amplitude reaction time and temperature were investigated.
-
Besides, the recycle property of this catalyst was assessed for minimizing the price of biodiesel. Consequently, the proposed work is compared with existing researches. Thus, the results show that the proposed method has reusability, easy forming, low cost, and high yield properties.
The organization of this paper is structured as follows: "Materials and methods" section encloses materials used for catalyst preparation and methods adopted such as catalyst preparation, catalyst synthesis from Musa and characterization, synthesis of biodiesel, modeling optimization technique, and kinetic model. "Results and discussion" section elaborates the result and discussion along with the comparison and "Conclusion" describes the conclusion of the paper.
Materials and methods
Materials
In this present work, Lesquerella fendleri along with Borage seed oil of nonedible oil and Li–CaO based heterogeneous catalyst resultant from the ash root of Musa balbisiana colla was used for the biodiesel production. Here, Lesquerella fendleri and Borage seed oil were utilized as a feedstock. The Borage oil has 20% of gamma linolenic acid content and high γ-linolenic acid value. Moreover, Lesquerella fendleri has 20 to 28% of oil content with around 62% lesquerolic acid and 21% triglyceride oil. The oil from the seeds was prepared by cold pressed seed process [31] and subsequently, the seeds were under alkali refined, deodorized, and bleached. The initial free fatty acid (FFA) of Borage and Lesquerella fendleri was 0.11% and 0.16%. The physical properties of borage and Lesquerella fendleri seed oil were illustrated in Table 1.
Catalyst preparation and characterization
In this research, Li–CaO was produced using the wetness impregnation technique. The Li–CaO catalyst developed from the Musa balbisiana colla root ash, which has been devised to be used as the heterogeneous catalyst and more effective catalyst for transesterification [32] due to its higher basicity index. The roots of Musa were sliced into thin pieces and it was dried into sunny sunlight for more than 15 days. Then the catalyst was developed by the calcination process at 11000C with a warming degree concerning 10 °C/min for 4 h. During calcination, the remaining components of ash except the CaO was decomposed, thus subsequently produces pure CaO [33]. Furthermore, the proximate study and known weight were estimated for the material balance. The fresh Musa balbisiana catalyst includes moisture content 91.3% at 210 °C, volatile material 7.45% at 700 °C and carbon value 0.14% at 1100 °C.
Besides, the physical characteristics of the proposed catalyst was analyzed by dissimilar methods such as XPS, TEM, FTIR, SEM, XRD, and BET. SEM was used to predict the surface structure of the catalyst and employed at 10 kV of accelerating voltage. FTIR spectrophotometer was utilized to record the FTIR spectra of KBr pallets in a Nicolet and the wavenumber limit of 4000–400 cm−1, which is to specify the functional groups of catalysts. Moreover, XRD (Rigaku Miniflex, Japan) was used to evaluate the structural survey of the catalyst by means of Cu Kα (λ = 1.541 Å) radiation at 30 kV in the 2ϴ assortment of 20 to 70°. The particle size and elemental analysis of the catalyst were estimated by TEM and SEM–EDX. BET was utilized to estimate the pore size, surface area along with pore volume catalyst. Consequently, the XPS was utilized to estimate the chemical structure of the proposed catalyst.
Biodiesel Synthesis
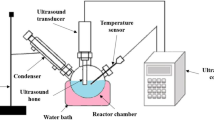
The transesterification technique was utilized to formulate the biodiesel from Borage and Lesquerella fendleri oil. In our research, the FFA content of borage was 0.11% and the FFA content of Lesquerella fendleri was 0.16%, respectively. Therefore, a single-step transesterification method has been adopted. The ultrasonic transesterification method [34] was used to prepare biodiesel from Borage and Lesquerella fendleri oil. The transesterification responses were performed in the probe type ultrasonic reactor which consists of a converter, horn, and reactor. In this research, Borage oil and Lesquerella fendleri oil were utilized and its characteristics of viscosity, FFA and density were studied as per the standard of ASTM and procedure adopted in the past literature [35]. The reaction started, once the quantitative amount of Li–CaO [36] derived from root ash catalyst liquefied in methanol liquor. Then the dissolved solution flowed into the heated reactor containing a blend of two seed oils of Borage and Lesquerella fendleri oil. In horn form of reactor, the horn was connected to the transducer which creates ultrasonic radioactivity in the combination, the frequency was kept fixed at 20 kHz, amplitude range varies from 20 to 90%, the ultrasonication time duration ranging from 3 to 30 min (Total reaction time including ultrasonication time varied from 40 to 200 min).
Besides, the supplied ultrasonic power was 1500 W and the methanol to oil of molar ratio mixture was changed from 6:1 to 15:1. The reaction was performed by ultrasonic irradiation from the audio bar horn integrated with the transducer. Moreover, Cavities were formed by the ultrasonic power irradiation with required energy in immiscible solution, which causes minute fine bubbles and these bubbles were miscible at different locations of the reactor which caused in the emulsification of mixture. Then, the reaction temperature was maintained between 40 and 65 °C. After the achievement of the separation process, biodiesel (methyl ester) was visible and settled in the upper coating, glycerol was settled in the lower coating and the middle coating contains the reusable catalyst. This remaining catalyst was filtered out and reused again in a recycle process of transesterification and finally got the yield. Hence, the percentage of biodiesel yield was estimated by Eq. 1:
Here, \(B_{A}\) is the amount of biodiesel and \(O_{A}\) is the amount of oil, respectively. Consequently, optimization is examined for biodiesel production using the finest catalyst acquired once repeats the progression.
Hybrid RSM-ABO for statistical optimization
The processes in every value of Li–CaO are utilized to improve the mathematical model that represents a correlation among the yield of Li–CaO and the variables for process via first and second orders. Sequentially, their interface terms consistent with the polynomial Eq. (2),
Here, \(Y^{\prime}\) is the yield of Li–CaO [37], \(a_{0}\) is the offset period, \(a_{j}\) is the linear outcome, \(a_{ij}\) is the first order interface effect, and \(a_{jj}\) is the squared consequence, \(x_{i}\) and \(x_{j}\) are the coded factors. The transesterification of catalyst parameters was investigated using HRSM and ABO technique. For this, the self-governing variables namely Methanol to oil of molar ratio (A), catalyst quantity (B), reaction temperature (C), reaction time (D), and amplitude (E) coded values were illustrated in Table 2. Furthermore, the superiority of the proposed method was validated using the estimation of correlation \((R^{2} )\) and statistical analysis.
Moreover, the function of the optimization technique is to choose the best solution for the predicted results [38]. Therefore, the ABO algorithm was utilized to optimize the production yield and production time. This algorithm was actualized reliant on the exploration and exploitation characteristics of African buffalos. Primarily in optimization proposes, initializing the reaction time, molar ratio, catalyst amount, amplitude, reaction temperature, and yield in ABO method for optimizing the production yield and production time. After the initialization of the input parameters, the best exploration was estimated by the Eq. 3. Here, the best solution denotes the optimized highest yield.
Here \(\hat{m}_{\mu }\) is the exploitation moves and \(\hat{w}_{\mu }\) is the exploration changes of the \(\mu^{th}\) parameters \({ (}\mu { = 1,2,}.............{\text{N) }}\) respectively, \(l_{1}\) and \(l_{2}\) are the learning features used to control the parameters, \(x_{1}\) and \(x_{2}\) are random numbers between [0, 1], \(r_{g\max .\mu }\) is the group’s finest fitness and \(r_{p\max .\mu }\) denotes the best specific outcome. After the each iteration, production time was optimized using Eq. 4.
After the completion of this process, the process is to be stopped and optimal yield was obtained. From this, \(\hat{m}_{\mu + 1}\) is the exploitation used for optimizing the yield of production and \(\hat{w}_{\mu + 1}\) is the exploration used for optimizing the production time. In this algorithm, the variables like reaction time, methanol to seed oil of molar ratio, reaction temperature, catalyst amount and amplitude were initialized and the yield was analyzed at different reaction parameters. Subsequently receiving the biodiesel yield at various values of variable factors, the forecasted standards like time and yield are kept in the reminiscence. Moreover, updating of this process and maintaining was continued until all the oil and catalyst were depleted. In iteration, the outcome will stored in the memory and compare the predicted time and yield with the stored outcome. Finally, the optimized output was obtained.
Kinetic modeling
In the transesterification process, the kinetic study of the reaction parameters was investigated. Moreover, the Tri-glycerides (TG) were extensively converted into methyl ester \((R_{ME} )\) and glycerol (G) in the occurrence of catalyst and methanol [39]. The reverse process can be disregarded due to the additional methanol was utilized to move the stability of reaction to the production. The pseudo-first order was considered for the kinetic study and expressed by the Eq. 5:
Here \(R\) is the reaction rate for transesterification, \(c\) is the constant rate and \(t^{\prime}\) is represented as reaction time. Moreover, from mass equilibrium is articulated in Eq. (6),
This expression was rewritten by the integration in Eq. 7,
Then, \(c\) at dissimilar temperature can be attained from the experimental data. Furthermore, the Arrhenius derivation was utilized for the frequency factor \((c_{0} )\) in \((kJ/mol)\) and activation energy \((E^{\prime}_{a} )\) in \((\min^{ - 1} )\) estimation in Eq. 8:
Here, \(R\) is denoted as the gas constant. Then the natural logarithm is applied for the Eq. (8) and it is expressed in Eq. 9,
Finally, from the intercept and slopes, the \(E^{\prime}_{a}\) and \(c_{0}\) values are estimated.
Results and discussion
The transesterification process was implemented in MATLAB R2018b Simulink for that the input parameters were applied to the input of the transesterification process and after this process, the utmost yield of biodiesel was attained at various effects of input parameters. Then, the conversion yield at different conditions like temperature and production time was optimized by HRSM-ABO. Moreover, the Simulink model consists of an ultrasonic reactor block and allows the input parameters like catalyst quantity, seed oil to methanol of molar ratio, reaction temperature and time. The lower and higher temperature adjustment block was available. Using these adjustments, the input parameters were adjusted for the corresponding catalyst and oil types. The Simulink model was combined with the HRSM-ABO to predict the maximum optimal yield. The output of Simulink was in terms of optimal yield and production time concerning the amplitude, catalyst amount, seed oil to methanol of molar ratio proportion, process temperature and time. The modeled process was validated using actual experimentation based on the strategy of the experiment using the Design-Expert software.
Structural and Characteristics Analysis of the Catalyst
The composition analysis of the Musa balbisiana ash developed at 1100 °C was evaluated using suitable techniques. From this, the Sulfur trioxide (SO3) has 2.10% of mass fraction, potassium oxide (K2O) has 25.05%, silicon dioxide (SiO2) has 35.92%, Magnesium Oxide (MgO) has 10.04%, lime (CaO) has 10%, phosphorous pentoxide (P2O5) has 4.47%, alumina (Al2O3) has 4%, nickel oxide (NiO) has 0.045 × 10−3%, iron oxide (Fe2O3) has 1.88% and copper oxide (CuO) has 0.072 × 10−3%. The functional group of organic and inorganic elements was estimated by FTIR. Moreover, the FTIR was identified as the covalent links on metallic ligands. The extents below the peaks established from the exploration were relative to the model concentration and utilized for the quantitative capacities. The sample of FTIR spectra was attained by KBr approach at the extent temperature and spectra were verified at 400–4000 cm−1 illustrated in Fig. 1.
The spectrum in this method has no impurity. The absorption ensembles at around 3415 cm−1 in the sample relate to the O–H group, which checked humidity on the samples. The FTIR spectra for two sharp peaks for the Li–CaO at 3415 and 1474 cm−1. This was due to the physisorbed water molecule on the surface of the CaO. Thus the present FTIR spectra result concluded that has pure material compared with the other works of literature [23, 16, 14, and 12].
Moreover, the crystalline property of the catalyst was estimated by XRD pattern, which was denoted by the sharp peak. The secondary phase excluding Li–CaO is to be captured in the XRD pattern. Therefore, the XRD studies of CaO and 1.5 wt% of Li–CaO have been executed and achieved the intense peaks. The CaO attained several intense peaks at 2θ ~ 8.31°, 10.90°, 14.20°, 22.10°, 32.56°, 44.16°, 46.28°, 53.62°, 55. 6° and 57.18° keep up a correspondence to the d-values of 2.39, 5.91, 2.75, 1.69, 2.45, 2.16, 4.92, and 3.43 respectively, shown in Fig. 2. Nevertheless, the Li–CaO attained some of the intense peaks at 2θ ~ 9.75°, 15.56°, 23.6°, 30.98°, 42.11°, 47.62°, 53.75°, 55.73°, and 57.2° correspond to d-values of 2.31, 4.10, 5.01, 3.20, 3.62, 2.71, 1.58 and 2.69, as illustrated in Fig. 2.
Consequently, using the BET analysis in the proposed catalyst, the surface area density is 39.42 m2 g−1, pore size is equal to 4.04 nm and pole volume is 0.43 cm3 g−1. Thus the value shows that the proposed catalyst has considerably improved than different described literature [16, 21]. Moreover, the pore volume value proven that the material has both macroporosity and mesoporosity properties. Therefore, this proposed Li–CaO catalyst was tremendous for the reaction of transesterification.
The surface structure of the CaO particles have high porosity and shapeless in structure and produce a nodule mass because of the interconnection between CaO-CaO atoms [40]. After doping, the SEM graph shows that Li–CaO nanocatalyst gives a slight thickening of the pore structure distribution. The widely held of the CaO nanocatalyst prepared from the wetness impregnation method and the root ash is prepared from the deep drying of Musa root using bright sunlight. SEM was utilized to detect the exterior structure of the catalyst because each catalyst has a different crystal structure is illustrated in Fig. 3a. Therefore, the characteristics of elemental composition were estimated using SEM–EDX shown in Table 3. The related observation was described in [41] for Musa balbisiana.
The exterior configuration of the catalyst was measured using TEM examination. Besides, it approves the fitness as a catalyst for energy storage and adsorption. The TEM illustration for the structural information of the catalyst was investigated shown in Fig. 3b.
The surface of catalyst
Characterization was analyzed by XPS method illustrated in Fig. 4. From the graph illustrated that the 580 eV for CaO and 582.7 eV for Li–CaO of binding energies were observed. Furthermore, due to the Li doped to CaO, the binding energy transference of CaO 2.7 eV was observed from CaO for 580 eV to Li–CaO for 582.7 eV. The higher binding energy moves were attained when the concentration of CaO was increased. The binding energy of proposed research is superior from the literatures [15].
Optimization by RSM-ABO method
Based on this experimental study, the transesterification process was optimized using MATLAB Simulink. Furthermore, it optimizes the process parameters for maximum yield and minimum production time. The recommended qualified yield was estimated by the Eq. (10),
Here A is represented as methanol to oil of molar ratio, B is the quantity of catalyst, C is represented as the reaction temperature, D is represented as the reaction time in min, and E is amplitude. The experimental design for production and predicted yield outcome was illustrated in Table 4. Moreover, Eq. 10 in terms of coded features can be utilized to create forecasts about the reaction for certain levels of each aspect. By default, the coded features for a high level as a positive one and the coded features for low level as a negative one. The factors impact between the factor coefficients was identifying by this implied equation. Furthermore, this coded equation used for yield as an objective function. Consequently, based on the finest function of HRSM-ABO the production yield and time were further optimized. From Table 4, the highest yield 97.8% of biodiesel was obtained under the 15:1 methanol to oil of molar ratio, 4 wt% of catalyst amount at 65 °C for 150 min and 75% of amplitude. Nevertheless, the lowest 68.45% of biodiesel yield was attained under the methanol to oil of molar ratio was 9:1, 2 wt% of catalyst quantity at 45 °C for 60 min, and 20% of amplitude, respectively. Statistical parameter for the outcome of variables on produced biodiesel was presented in Table 5. Here, the correlation R squared (R2) of 0.8241 was attained for the experimental data and the predicted R squared (R2) of 0.9475 was obtained. The value of R2 should be near to 1.00 in the correlation between the estimated and predicted standards. Nevertheless, the obtained R2 value recommended the high level of correlation between the experimental value and the predicted value.
It has been observed that the actual yield obtained by the values of the parameters suggested using RSM was 91.002% and suggested by HRSM-ABO was around 98%. Thus the conversion yield by HRSM-ABO was around 10% enhancement successfully. The total gain in biodiesel yield obtained through the Hybrid RSM-ABO approach in comparison to the simple RSM approach was 6.79% and the relative error in predicted and actual experimental values has reduced to 0.7%. Thus the result shows that HRSM-ABO approach was much more effective and precise in compared to a simple RSM approach for biodiesel conversion yield optimization. Besides, the methanol to seed oil of molar ratio series as of 9:1 to 15:1 increased the production of biodiesel. Furthermore, the p-value was 0.0009 for methanol to oil of molar ratio shown in Table 5, which was presented in the p-value range of less than 0.001, thus the effect of this attained parameter is highly significant.
After the complete analysis of the report, the noted values were given to the input of the MATLAB Simulink and started to begin the process and generated the appropriate plot.
Effect of reaction parameters
The finest optimization for the synthesis of biodiesel was completed by Li–CaO catalyst along with borage oil and Lesquerella fendleri seed oil using HRSM-ABO catalyst under various parameters namely, catalyst amount, effect of reaction temperature, effect of amplitude, methanol to oil of molar ratio, effect of reaction time and number of cycles.
Effect of catalyst amount
In transesterification procedure, the catalyst quantity was utilized as the significant parameter, which can affect the reaction rate. The impacts of catalysts in various amounts such as 0.5 wt% to 4 wt% on base transesterification for the synthesis of with methanol to oil of molar ratio 15:1 at the temperature 65 °C for 150 min were examined. Moreover, catalyst content rises from the 0.5 to 4 wt% was improved and the yield of biodiesel from 68 to 98%. Nevertheless, it was noticed that the yield was reduced when the catalyst amount exceeds 4 wt%. The catalyst amount versus conversion yield was shown in Fig. 5a. In the current study, it was measured that when the catalyst quantity raises from 0.5 wt% to 4 wt% and the yield of biodiesel enhanced progressively attained a maximum of 97.8% yield at 4 wt%. Therefore, the optimum catalyst amount 4 wt% was qualified to oil content. Besides, the statistical parameters were illustrated in Table 5 found that the weight percentage of Musa balbisiana based catalyst for biodiesel production plays a highly significant parameter.
The effect of constraints on biodiesel synthesis. a Catalyst amount (reaction time of 150 min, reaction temperature of 65 °C, oil to methanol ratio of 1:15 and amplitude of 75%); b Methanol to oil of molar ratio (reaction temperature of 65 °C, Catalyst amount of 4wt%, reaction time of 150 min, and amplitude of 75%); c Reaction temperature (1:15 oil to methanol of molar ratio, catalyst amount of 4 wt%, reaction time of 150 min, and amplitude of 75%); d Reaction time (catalyst amount of 4 wt%, reaction temperature of 65°, 1:15 oil to methanol ratio of molar ratio, and amplitude of 75%)
Effect of oil to methanol ratio
This conversion process was a reversible response and also it necessitates an excessive quantity of methanol to move in the way of the products. An overabundance measure of methanol has been utilized to improve the miscibility of the reactants, move the balance towards a high biodiesel change, and permit its stage detachment from the glycerol shaped. The reaction was accepted out by changing the methanol to oil of molar ratio varies in the range of 9:1 to 15:1 increases gradually and beyond 15:1, the conversion yield was reduced under persistent catalyst concentration at 60 °C for 150 min. The methanol to oil ratio versus conversion yield was illustrated in Fig. 5b. Hence, the finest methanol to oil of molar ratio played a significant part in enhancing the profit of biodiesel production amid other reaction constraints. Consequently, the 15:1 methanol to oil of molar ratio was utilized for the finest transesterification process. Thus, the statistical parameter from Table 5, the p-value shows that the molar ratio of Musa balbisiana based catalyst for making biodiesel was a highly important parameter.
Effect of reaction temperature
In general, the process temperature effect for the conversion of biodiesel was varied from 45 °C to 65 °C. The response was conceded out for 150 min with a 4 wt% of catalyst amount and 15:1 methanol to oil of molar ratio. Therefore, it was perceived that the conversion yield of biodiesel was less as 60% at a lower temperature of 45 °C and extended 97% at 65 °C. After this temperature, the methanol gets evaporated and the reaction could not proceed with lesser alcohol or in absence of alcohol. Reaction temperature versus conversion yield was shown in Fig. 5c. While the temperature was increased over 65 °C lessening in biodiesel yield was perceived which might be due to the inaccessibility of the needed quantity of methanol in the reaction combination for transesterification. Consequently, the maximum instability of methanol decreasing the temperature and its interface with the catalyst therefore the reaction rate gets vary. Parallel detections were also described by other researchers previously. Hence 65 °C was considered as optimum reaction temperature. Moreover, based on the statistical parameter value and the 0.008 of p-value were illustrated in Table 5, it shows that the reaction of temperature was noteworthy for the production of biodiesel from Musa balbisiana.
Effect of reaction time
The results showed that the biodiesel yield was low in the first 1 h, since at low reaction rate, the heterogeneous catalysts were not completely activated and then increases gradually at the next hours. The reaction time effect versus conversion yield was illustrated in Fig. 5d. Moreover, all the reactions of transesterification were accompanied on the state 4 wt% of catalyst amount, 15:1 methanol to oil of molar ratio, amplitude 75%, and the temperature of the reaction was 65 °C, respectively. However, the further rise of reaction time in the range of 60 min to 150 min, the biodiesel conversion yield was increased progressively and achieved an utmost yield of 97.8% at 150 min. After 150 min, the biodiesel changes were perceived to be decreased. This reduction in changes may be due to the converse transesterssification activated between glycerol and FAME at the extension of reaction time. Therefore, 150 min reaction time was examined as finest time for making biodiesel. Subsequently, the p-value was shown in Table 5 proven by its value 0.041, thus, the reaction time was an essential parameter in the production of biodiesel from Musa balbisiana.
Effect of Amplitude
It has been observed that the amplitude was varied from 20% to 75%. The yield was increased when reached its maximum at the 75% of amplitude. Furthermore, the significant gain was not perceived with the further increase of Amplitude. The effect of ultrasonic amplitude on FAME yield was illustrated in Fig. 6. It shows that the amplitude of acoustic waves was increased. Therefore, the droplets obtained as lesser in size and the amount of flowing bubbles was enhanced the mixtures of oil and methanol. Thus, a finest mixture rate was generated at the 75% of amplitude.
Consequently, the p-value from Table 5 shows that the amplitude was a significant parameter of biodiesel from Musa balbisiana.
Effect of reusability
In this research, the reusability test was performed on 7 cycles under the optimized reaction condition. The reusability test was represented as the reuses of catalyst repeatedly provide sufficiently good biodiesel conversion levels after the transesterification reaction. Sequentially, the catalyst used for the reusability test was recovered by filtration technique and hereafter, the catalyst was cleaned with methanol to filter the adsorbed particles. For this time, the filtered particles were dehydrated in an oven at 120 °C and it was utilized for the subsequent cycle. The effect of reusability of heterogeneous catalyst was shown in Fig. 7.
The collaboration result of catalyst amount and molar ratio on conversion yield for biodiesel production contour plot is shown in Fig. 8. From the graph, the pure peak point indicated the finest state and the utmost conversion yield in Musa balbisiana root biodiesel production. Therefore, the finest point of the method contains methanol to oil of molar ratio was 15:1 and the catalyst amount was attained 4 wt% at 65 °C for 150 min of reaction time.
Consequently, the finest state tends to the utmost predicted value of biodiesel conversion yield was 97.8%. While the molar ratio was increased, then the conversion yield was also enhanced. Moreover, the less amount of catalyst improves the conversion yield. Depending on the test of the nanocatalyst, the additional quantity of methanol tends to increase the reaction conversion yield.
Kinetic studies on transesterification process
The action of catalyst mostly based on the arrangement, characteristics of chemical and the surface feature constraints, which means, the particular surface area, acidic or basic sites surface density of the catalyst. Therefore, the basic information about fundamental belongings of catalysts that associates their minute assemblies with kinetics reaction was necessary for the progress and optimization of the higher behavior of catalysts. Moreover, if the optimum temperature exceeds then the biodiesel yield will be diminished. Since the result of the temperature and response time may suggest the measure of vitality that was a contribution to the response, this research explored that the joined impact of temperature and response time on biodiesel transformation.
Through this methodology, here could investigate the chance of bringing down vitality contribution without it influencing biodiesel transformation. The dissimilar temperature such as 45 °C, 55 °C, and 65 °C for time were investigated for the kinetic studies. Moreover, catalyst weight of 4wt% and oil to methanol ratio of 1:15 parameters were kept constant attained from the HRSM-ABO method. The finest conversion yield attained at the maximum temperature of 65 °C. Thus, the linear relationship of \(- \ln \,(1 - R_{ME} )\) versus \(t^{\prime}\) at various temperature was illustrated in Fig. 9a and pseudo first order reaction are supported by the attained strong correlation by Eq. 7.
Moreover, the reaction rate value was estimated at the temperature of 45 °C, 55 °C, and 65 °C. Consequently, \(R^{2}\) = 0.9981 was attained correlation between the \(1/T\) versus \(\ln \,c\) over the provided series of reaction temperature was shown in Fig. 9b. The frequency factor \((c_{0} )\) of \(4.4 \times 10^{ - 12} \min^{ - 1}\) was estimated from the intercept and activation energy \((E^{\prime}_{a} )\) of 43.5 kJ/mol was obtained from the slope of the graph. According to literature, there were studies in [19, 24] the activation energy was estimated as 47.67 kJ/mol and 57.68 kJ/mol under the equilibrium reaction time. Nevertheless, the activation energy of the proposed work was considerably less compared with the conventional works of literature on biodiesel formulation due to the obtained reaction temperature and the nanocatalyst.
Properties of the produced biodiesel
The physicochemical properties of the obtained biodiesel using Li–CaO catalyst from root ash of Musa with 15: molar ratio was presented in Table 6. Here, the kinematic viscosity, density, water, ash content, cloud and flash point were estimated. In the fuel system, density is considered as one of the significant property because it can affect the atomization fuel productivity. In pure condition, the density of biodiesel was 880 kg m−3, which enhanced the percentage of biodiesel. A similar outcome has been obtained in the investigation of biodiesel production using Euonymus Maackii Seed oil [19]. Sequentially, the kinematic viscosity is an additional significant property for biodiesel, which shows the ability of material flow and optimal quality of biodiesel atomization. The feature of injection and combustion performance of diesel can impact by the kinematic viscosity and density parameters. The kinematic viscosity of the proposed methyl ester was 5.85 mm2 s−1 and the measured density to be 880 kg m−3 for prepared biodiesel, which was approximately within the limit of ASTM standard. Hence, the kinematic viscosity of produced biodiesel attained from Musa balbisiana is very proximate to the standard limit, using Li–CaO based nanocatalyst. The results of the proposed study are reliable with conventional literature Chicken fat [14] and Rice Bran Oil [16].
Additionally, the flashpoint of biodiesel was assisting to safe and store biodiesel. The produced biodiesel was nontoxic at temperatures beyond the flashpoint. The cloud point was utilized to estimate the function of biodiesel at a cold temperature. Moreover, the flash and Cloud point of the produced yield was detailed to be 170 °C and negative of 11.6 °C, respectively. The flashpoint and density value were less than the agreed value, which was also compared with the other conventional works [14, 16, 19]. Nevertheless, compared to conventional diesel, these higher values are the optimum advantages of proposed biodiesel. From the physical properties of the established that the Musa balbisiana root along with Lesquerella fendleri and Borage seed oil can be utilized to significant high quality biodiesel production. The measured flashpoint, kinematic viscosity, and water content were in the agreed limit of ASTM D6751 standard.
Comparison of yield production
The finest state of Li–CaO catalyst for Musa balbisiana root biodiesel production was examined in this research. In Musa balbisiana root biodiesel production, the Li–CaO catalysts produced more yields. The proposed technique attained the 97.8% yield using the heterogeneous catalyst and two types of mixed oil. By using these oils and catalyst, the highest yield was obtained at an optimal temperature at 65 °C compared with other materials. The comparison of the proposed finest condition with existing works for biodiesel production was detailed in Table 7.
From the comparison illustrated in Table 6 shows that the performance of proposed Li–CaO from Musa balbisiana with Lesquerella fendleri and Borage seed oil produced 97.8% of Yield at considerably significant temperature and time compared with the various materials from literature. The proposed Borage and Lesquerella fendleri oil based biodiesel formulation attained 97.8% of yield under the methanol to oil of molar ratio 15:1 at 65 °C for 150 min of process time and 4 wt% catalyst amount, effectively. Moreover, the produced biodiesel using proposed catalyst was taken less time while compared with other catalysts and also the amount of catalyst used in the method was significantly less in the range of 0.5 to 4 wt%. After the 7 cycles, the catalyst was maintained for reusability at a suitable level. Hence, this is the reason the Li–CaO Musa balbisiana was selected for biodiesel.
Conclusion
In this research, biodiesel production using probe-type ultrasonic reactor has been modelled and the experimentally validated outcomes were implemented in Matlab Simulink software. It has been demonstrated that the Li–CaO nanocatalyst from root ash of Musa balbisiana was prepared for biodiesel production. The transesterification reaction was taken place successfully with the novel combination of non-edible oils were Lesquerella fendleri and Borage seed oil in the occurrence of the Li–CaO. Consequently, characteristics of this catalyst were analyzed by various spectroscopic methods such as SEM, XPS, FTIR, EDX, TEM, BET, and XRD. After the transesterification reaction, the optimized conversion yield of biodiesel was achieved due to utilize of novel HRSM-ABO technique. The findings of this research demonstrated that Li–CaO sourced from Musa balbisiana root is an effective catalyst and an extreme conversion yield of 97.8% was attained at the 15:1 methanol to oil of molar ratio, 4wt% of catalyst quantity at 65 °C for the reaction time of 150 min and the amplitude of 75%. Moreover, the HRSM-ABO technique has been effectively utilized in this work to expand the conversion yield of produced biodiesel while predicted by RSM. Besides, the kinetics study of transesterification for the produced biodiesel was validated. Thus, the outcome of the proposed method of biodiesel production is compared with the other existing works and proven the efficiency of the proposed biodiesel production approach.
References
Martins F, Felgueiras C, Smitkova M, Caetano N (2019) Analysis of fossil fuel energy consumption and environmental impacts in European countries. Energies 12(6):964. https://doi.org/10.3360/en12060964
Bhuiya MMK, Rasul MG, Khan MMK, Ashwath N (2014) Second generation biodiesel: potential alternative to-edible oil-derived biodiesel. Energy Procedia 61:1969–1972. https://doi.org/10.1016/j.egypro.2014.12.054
Boonyuen S, Smith SM, Malaithong M, Prokaew A, Cherdhirunkorn B, Luengnaruemitchai A (2018) Biodiesel production by a renewable catalyst from calcined Turbo jourdani (Gastropoda: Turbinidae) shells. J Clean Prod 177:925–929. https://doi.org/10.1016/j.jclepro.2017.10.137
Demirbas A (2006) Biodiesel production via non-catalytic SCF method and biodiesel fuel characteristics. Energ Convers Manage 47(15–16):2271–2282. https://doi.org/10.1016/j.enconman.2005.11.019
Meher LC, VidyaSagar D, Naik SN (2006) Technical aspects of biodiesel production by transesterification - a review. Renew Sust Energ Rev 10:248–268. https://doi.org/10.1016/j.rser.2004.09.002
Georgogianni KG, Katsoulidis AK, Pomonis PJ (2009) Transesterification of rapeseed oil for the production of biodiesel using homogeneous and heterogeneous catalysis. Fuel Process Technol 60(7–8):1016–1022. https://doi.org/10.1016/j.fuproc.2009.03.002
Idrissou Y, Mazari T, Benadji S, Hamdi M (2016) Homogeneous and heterogeneous sunflower oil methanolysis over 12-tungstophosphoric, sulfuric and boric acids. React Kinet Mech Cat 119(1):291–304. https://doi.org/10.1007/s11144-016-1042-5
Jamal Y, Rabie A, Boulanger BO (2015) Determination of methanolysis rate constants for low and high fatty acid oils using heterogeneous surface reaction kinetic models. React Kinet Mech Cat 114(1):63–74. https://doi.org/10.1007/s11144-014-0780-5
Navas MB, Lick ID, Bolla PA, Casella ML, Ruggera JF (2018) Transesterification of soybean and castor oil with methanol and butanol using heterogeneous basic catalysts to obtain biodiesel. Chem Eng Sci 187:444–454. https://doi.org/10.1016/j.ces.2018.04.068
Puna JF, Correia MJN, Dias APS, Gomes J (2013) Biodiesel production from waste frying oils over lime catalysts. React Kinet Mech Cat 109(2):405–415. https://doi.org/10.1007/s11144-013-0557-2
Ilgen O (2011) Dolomite as a heterogeneous catalyst for transesterification of canola oil. Fuel Process Technol 92(3):452–455. https://doi.org/10.1016/j.fuproc.2010.10.009
Sirisomboonchai S, Abuduwayiti M, Guan G (2015) Biodiesel production from waste cooking oil using calcined scallop shell as catalyst. Energ Convers Manage 95:242–247. https://doi.org/10.1016/j.enconman.2015.02.044
Kumar A (2018) Global warming, climate change and greenhouse gas mitigation. Biofuels: Greenhouse Gas Mitigation and Global Warming, New Delhi: Springer, pp. 1–16
Seffati K, Honarvar B, Esmaeili H, Esfandiari N (2019) Enhanced biodiesel production from chicken fat using CaO/CuFe2O4nanocatalyst and its combination with diesel to improve fuel properties. Fuel 235:1238–1244. https://doi.org/10.1016/j.fuel.2018.08.118
Ala'aH, JamilF, Al-HajL, MyintMTZ, Mahmoud E. (2018). Biodiesel production over a catalyst prepared from biomass-derived waste date pits. Biotechnol Rep, 20: e00284
Mazaheri H, Ong HC, Masjuki HH, Amini Z (2018) Rice bran oil based biodiesel production using calcium oxide catalyst derived from Chicoreusbrunneus shell. Energy 144:10–19. https://doi.org/10.1016/j.energy.2017.11.073
Arul E, Raja K, Krishnan S, Sivaji K, Das SJ (2018) Bio-directed synthesis of calcium oxide (CaO) nanoparticles extracted from limestone using honey. J Nanosci Nanotechno 18:5760–5793. https://doi.org/10.1166/jnn.2018.15386
Hoseini SS, Najafi G, Ghobadian B, Mamat R (2018) Ailanthus altissima (tree of heaven) seed oil: characterisation and optimisation of ultrasonication-assisted biodiesel production. Fuel 220:621–630. https://doi.org/10.1016/j.fuel.2018.01.094
Liu JZ, Cui Q, Kang YF, Meng Y, Gao MZ, Efferth T (2019) Euonymus maackii rupr. seed oil as a new potential non-edible feedstock for biodiesel. Renew Energy 133:261–267. https://doi.org/10.1016/j.renene.2018.10.035
Jeon Y, Chi WS, Hwang J, Kim JH, Shul YG (2019) Core-shell nanostructured heteropoly acid-functionalized metal-organic frameworks: Bifunctional heterogeneous catalyst for efficient biodiesel production. Appl Catal B-Environ 242:51–59. https://doi.org/10.1016/j.apcatb.2018.09.071
Teo SH, Islam A, Chan ES, Choong SYT (2019) Efficient biodiesel production from Jatrophacurcus using CaSO4/Fe2O3-SiO2 core-shell magnetic nanoparticles. J Clean Prod 208:816–826. https://doi.org/10.1016/j.jclepro.2018.10.107
Deng X, Fang Z, Liu Yh, Yu CLL (2011) Production of biodiesel from Jatropha oil catalyzed by nanosized solid basic catalyst. ENERGY 36(2):777–784. https://doi.org/10.1016/j.energy.2010.12.043
Fard RGZ, Jafari D, Palizian M, Esfandyari M (2019) Biodiesel production from beef tallow using the barium oxide catalyst. React Kinet Mech Cat 128(2):723–738. https://doi.org/10.1007/s11144-019-01672-z
Kesserwan F, Ahmad MN, Khalil M (2020) Hybrid CaO/Al2O3 aerogel as heterogeneous catalyst for biodiesel production. Chem Eng J 385:123834. https://doi.org/10.1016/j.cej.2019.123834
Wong WY, Lim S, Pang YL, Shuit SH, Chen WH (2020) Synthesis of renewable heterogeneous acid catalyst from oil palm empty fruit bunch for glycerol-free biodiesel production. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.138534
Hossain SMZ, Taher S, Khan A, Sultana N, Irfan MF Experimental study and modeling approach of response surface methodology coupled with crow search algorithm for optimizing the extraction conditions of papaya seed waste oil. Arab J Sci Eng, 1–13
Kalanakoppal Venkatesh Y, Mahadevaiah R, Haraluru Shankaraiah L, Ramappa S, Sannagoudar Basanagouda A (2018) Preparation of a CaO nanocatalyst and its application for biodiesel production using butea monosperma oil: an optimization study. JAOCS, J Am Oil Chem Soc 95:635–649. https://doi.org/10.1002/aocs.12079
Ismail S, Ahmed AS, Anr R, Hamdan S (2016) Biodiesel production from castor oil by using calcium oxide derived from mud clam shell. J Renew Energ 2013:1–8. https://doi.org/10.1155/2016/5274917
Konwar LJ, Boro J, Deka D (2018) Activated carbon supported cao from waste shells as a catalyst for biodiesel production. Energy Sources, Part A: Recovery, Util Environ Effects 40:601–607. https://doi.org/10.1080/15567036.2012.733483
Vardast N, Haghighi M, Dehghani S (2019) Sono-dispersion of calcium over Al-MCM-41used as a nanocatalyst for biodiesel production from sunflower oil: influence of ultrasound irradiation and calcium content on catalytic properties and performance. Renew Energ 132:979–988. https://doi.org/10.1016/j.renene.2018.08.046
Wayne LL, Gachotte DJ, Walsh TA (2019) Transgenic and genome editing approaches for modifying plant oils. Transgenic Plants, New York: Humana Press, pp. 367–394
Mohadesi M, Moradi G, Ghanbari M, Moradi MJ (2019) Investigating the effect of n-hexane as solvent on waste cooking oil conversion to biodiesel using CaO on a new support as catalyst. Meas: J Int Meas Confed 135:606–612
Boey PL, Maniam GP, Hamid SA (2011) Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: a review. Chem Eng J 168(1):15–22. https://doi.org/10.1016/j.cej.2011.01.009
Kelarijani AF, Zanjani NG, Pirzaman AK (2019) Ultrasonic assisted transesterification of rapeseed oil to biodiesel using nano magnetic catalysts. Waste Biomass Valori. https://doi.org/10.1007/s12649-019-00593-1
Sanford SD, White JM, Shah PS, Wee C (2009) Feedstock and biodiesel characteristics report. Renew Energy Group 413:1–136
Kumar D, Ali A (2010) Nanocrystalline lithium ion impregnated calcium oxide as heterogeneous catalyst for transesterification of high moisture containing cotton seed oil. Energy Fuels 24:2091–2097. https://doi.org/10.1021/ef601318s
Narula V, Khan MF, Negi A, Kalra S, Thakur A, Jain S (2017) Low temperature optimization of biodiesel production from algal oil using CaO and CaO/Al2O3 as catalyst by the application of response surface methodology. Energy 140:879–884. https://doi.org/10.1016/j.energy.2017.09.028
Odili JB, Kahar MNM, Anwar S (2015) African buffalo optimization: a swarm-intelligence technique. Procedia Comput Sci 76:443–448. https://doi.org/10.1016/j.procs.2015.12.291
Galvan D, Cremasco H, Mantovani ACG, Bona E (2020) Kinetic study of the transesterification reaction by artificial neural networks and parametric particle swarm optimization. Fuel 267:117221. https://doi.org/10.1016/j.fuel.2020.117221
Ming C, Rizwanul Fattah IM, Chan QN, Pham PX, Medwell PR, Kook S, Yeoh GH, Hawkes ER, Masri AR (2018) Combustion characterization of waste cooking oil and canola oil based biodiesels under simulated engine conditions. Fuel 224:167–177. https://doi.org/10.1016/j.fuel.2018.03.053
Sarma AK, Kumar P, Aslam M, Chouhan APS (2014) Preparation and characterization of Musa balbisiana colla underground stem nano-material for biodiesel production under elevated conditions. Catal Lett 144(7):1344–1353. https://doi.org/10.1007/s10562-014-1206-8
Da Silva CL, Barañano AG, Pinheiro CJG, Menini L, Pinheiro PF (2019) Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures. Green Process Synth 8:235–244. https://doi.org/10.1515/gps-2018-0076
Rezaei R, Mohadesi M, Moradi GR (2013) Optimization of biodiesel production using waste mussel shell catalyst. Fuel 109:534–541. https://doi.org/10.1016/j.fuel.2013.03.004
Acknowledgement
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors proclaim that they have no potential conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Informed consent
For this type of investigation formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, U., Gupta, P. Modeling and optimization of novel biodiesel production from non-edible oil with musa balbisiana root using hybrid response surface methodology along with african buffalo optimization. Reac Kinet Mech Cat 130, 875–901 (2020). https://doi.org/10.1007/s11144-020-01807-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01807-7