Abstract
Ethanolysis of soybean oil was carried out over lime catalyst produced by calcination of scallop shells food wastes. The as prepared catalyst showed XRD lines belonging to lime, whereas post reaction samples presented XRD pattern belonging to Ca(OH)2 overlaid with lines of calcium diglyceroxide. The catalyst dynamics during ethanolysis was similar to that reported for methanolysis, being hydration and consequent diglyceroxide formation the main transformations of CaO catalyst during reaction. The influence of the reaction parameters, such as ethanol:oil molar ratio (10:1–14:1), catalyst loading (10–15%, based in oil weight) and time reaction (6–10 h), on the FAAE (fatty acids ethyl esters) yield was analyzed by response surface methodology. A polynomial model was fitted using Minitab software, showing a correlation between predicted and experimental FAEE yields of 0.921. The maximum FAEE yield of 99.2% was computed for optimal reaction parameters of 11:1 ethanol:oil molar ratio; 13.8% catalyst loading and 9.1 h of reaction time. The fitted model was verified for the optimal conditions, using three replicas, given 99.0% of FAEE yield instead of the 99.2% predicted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biofuels demand, as an alternative energy source, has been increasing in recent years. The environmental concerns not only to reduce the emissions of greenhouse gases, GHG, but also to improve the security of the energy supply took changes in the environmental policies [1]. Biodiesel, a mixture of fatty acids alkyl esters, it is pointed out as a promising renewable substitute for conventional fossil diesel [2].
Nowadays biodiesel is produced through catalyzed transesterification of vegetable oils and animal fats with short-chain alcohols, such as methanol or ethanol [3]. The mild reaction conditions needed, the fast alcoholysis rate and the easy phase separation, in parallel with its low price and industrial availability, make the methanol the most used alcohol in biodiesel production [4, 5]. However, the use of methanol has some drawbacks due to its toxicity and lack of sustainability. Methanol is majorly obtained from a fossil source such as natural gas reforming [6] thus, the biodiesel produced is not a fully renewable fuel. On the other hand, ethanol is made from agricultural products such as potatoes, grain, and corn, allowing the production of renewable fuel [7]. Ethanol has a higher oil dissolving capacity compared with methanol allowing one phase reaction mixture, whereas oils methanolysis proceeds in a two-phase reaction mixture with mass transfer limitations issues. Due to the extra carbon atom, fatty acid ethyl ester (FAEE) cloud and pour point are lower than that of fatty acid methyl esters (FAME) [8]. Also. the combustion heat and the cetane number are higher for FAEE than for FAME. The storage properties of FAEE fuel are also improved [4]. The main drawbacks of ethanolysis for biodiesel production are its lower reactivity compared with methanol as well as the more difficult separation of biodiesel from the coproduced glycerin [9].
Many studies have been carried out to compare the effect of methanol and ethanol on biodiesel production from different feedstocks [10,11,12,13].
Recently, Bolonio et al. [14] reported the production of fully renewable biodiesel using wastes from Spanish winery. The authors used bioethanol obtained by distillation of grapes leftovers and oil obtained by soxhlet extraction of grapeseeds. The ethanolysis was accomplished using a homogeneous sodium ethoxide catalyst.
All these studies reported that the yield obtained by ethanolysis is lower and more time is needed to complete the reaction than for methanolysis. The separation of FAEE from glycerin is more difficult too. However, it allows achieving completely renewable biodiesel. Garcia et al. [11] also studied the influence of alcohol on biodiesel production from animal fats. The results showed that although all biodiesel samples presented lower yield values when the ethanol is used, FAEE presented bigger resistance to oxidation than FAME.
Transesterification reactions are catalyzed by homogeneous basic catalysts although the interest in the use of heterogeneous basic catalysts is growing (Table 1).
The main advantages of heterogeneous catalysis are the easy catalyst separation from the reaction mixture and the ability to be reused [21]. As can be seen from the data presented in Table 1, calcium oxide is extensively used as a heterogeneous basic catalyst for the oils ethanolysis. Several attempts have been made to modify surface basicity by doping with an alkali metal and other elements. The conventional homogeneous basic catalyst (KOH) is always more active than heterogeneous CaO raw or modified catalysts.
Since the transesterification reaction is affected by several parameters, such as temperature, reaction time, Wcat/Woil, alcohol/oil ratio, among others, the research surface methodology (RSM) is often used to optimize the reaction conditions which allows to maximize the biodiesel production [22,23,24,25]. Neural networks, with genetic algorithms, are used less frequently for the same purpose [4].
In this work, the ethanolysis reaction of soybean oil was carried out over CaO heterogeneous catalyst obtained by calcination of calcium-rich alimentary wastes (scallop shells) and the reaction conditions were optimized using the research surface methodology.
Material and methods
Materials and catalyst preparation
Biodiesel (FAEE) was produced by soybean oil (alimentary grade) ethanolysis over heterogeneous Ca-based catalyst. The catalyst was prepared by calcination of alimentary wastes scallop shells. The as received shells were washed, dried, and coarsely crushed and then calcined in a muffle at 900 °C for 3 h. The heating step was performed at 5 °C/min. The calcined Ca material was ground in a ceramic mortar to obtain a fine powder (< 750 μm). More details on the catalyst preparation are given elsewhere [26].
Characterization of the catalyst and ethanolysis products
The catalysts, fresh and post-reaction, were characterized by X-ray diffraction (XRD) to identify the Ca crystalline phases and the degree of crystallinity of each phase. The diffractograms were recorded with a Rigaku Geigerflex diffractometer with Cu Kα radiation at 40 kV and 40 mA (2° min−1). ATR-FTIR spectra were collected for fresh and post-reaction catalysts to evaluate catalyst surface species, which can participate in the reaction mechanism. The FTIR spectra were recorded with a spectral resolution of 4 cm−1 on a Perkin Elmer Spectrum Two IR Spectrometer equipment with an ATR accessory from Pike using a ZnSe crystal. Liquid products from ethanolysis, oily phase containing FAEE and glycerin, were also characterized by ATR-FTIR.
Ethanolysis reaction tests
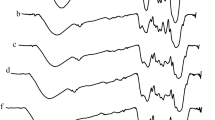
The ethanolysis tests were carried out in a 500 mL three-neck round-bottom pyrex flask equipped with a condenser and a magnetic stirrer. The reaction temperature was kept using a nest-shaped heating jacket with temperature control. The temperature sensor was in contact with the reaction mixture. All the reaction tests were performed at ethanol reflux temperature (75 °C) for ethanol:oil molar ratios of 10:1, 12:1, and 14:1. Tests using catalyst loading of 10.0, 12.5, and 15.0% (oil basis weight) were accomplished for three different reaction time (6, 8, and 10 h). For each reaction batch, the catalyst was previously contacted with methanol for 0.5 h and then the pre-heated oil (100 g, 60 °C) was added to the reaction flask. After the reaction period, the slurry was filtrated to remove the solid catalyst. The liquid effluent was transferred into a decantation funnel for glycerin phase separation. The oily phases were characterized, without further purification by ATR-FTIR to quantify the FAEE yield. The quantification was performed considering the reflectance bands in the range 1780–1690 cm−1 (Fig. 1) being the band centered at 1736 cm−1 attributed to biodiesel (FAEE) as reported by Zagonel et al. [27].
RSM analysis and optimization
Research surface methodology (RSM) was applied to evaluate the influence of reaction parameters on the FAEE yield. The Minitab® software (Version 18, Minitab Inc. USA) was used to evaluate the parameters influence, and interaction, as well as to optimize the reaction conditions (maximization of FAEE yield). Graphical analysis of the data was also obtained using Minitab software. The experimental design applied to this study was a full 33 factorial design consisting of 27 experiments. The coded and uncoded levels of the independent variables evaluated are presents in Table 2.
The experimental data achieved was analyzed by response surface regression to fit the following polynomial equation [28]:
Here Y is FAEE yield (response), Xi and Xj are the independent process variables (ethanol:oil molar ratio, catalyst loading, and reaction time). β0 is a constant, βi, βii, and βij are the regression coefficients, and k is the number of factors studied and optimized in the experiment. In this case, k is equal to 3. ε is the error term.
Results and discussion
Characterization of the catalyst
The fresh and post-reaction catalysts were characterized by XRD diffraction (Fig. 2). As expected, the scallop shells calcined at 900 °C present XRD patterns ascribable to lime (CaO) slightly contaminated with small crystallites (broad XRD lines) of calcium hydroxide (portlandite).
All the post-reaction catalysts presented XRD patterns similar to that depicted in Fig. 2, showing XRD lines belonging to calcium diglyceroxide and calcium hydroxide. Calcium diglyceroxide is reported to have enhanced catalytic activity in oil alcoholysis [29] but is also highly unstable leading to Ca leaching into reaction medium [30, 31]. For methanolysis the Ca(OH)2 phase is believed to be the catalyst active phase [30] and for oil ethanolysis Watcharathamrongkul et al. [8] reported an improved catalytic behavior for CaO catalyst loaded with Ca(OH)2. From XRD patterns it seems that CaO suffer deactivation during oil ethanolysis may be due to the long reaction periods used.
XRD data, in Fig. 2, show that the catalyst dynamics during ethanolysis is similar to that reported to occur during methanolysis [30]. Thus, catalyst reactivation can be achieved by calcination to remove hydroxide and diglyceroxide calcium species.
To evaluate the species, from the reaction medium, which remains adsorbed on the post-reaction catalysts ATR-FTIR spectra were acquired and compared with that from fresh catalyst [30]. IR spectra in Fig. 3 show for fresh catalyst reflectance bands to CaO surface with adsorbed carbonate species (1700–1100 cm−1 wavenumber) [32] whereas post-reaction catalysts show IR bands belonging to adsorbed esters species (1740 cm−1) and oil and glycerin species. The features in the range 3000–2800 cm−1 are attributable to alkyl species from adsorbed oils and esters.
The co-produced pale yellow, low viscosity glycerins were characterized by ATR-FTIR in order to assess the main functional groups of contaminants (MONG- organic matter non glycerin). The spectrum of a commercial glycerin (purum) was used as a reference. FTIR spectra, displayed in Fig. 4, show infrared features of glycerins slightly contaminated with ethanol and ethyl esters (1740 cm−1). As reported by Dias et al. [30], for oil methanolysis over calcium catalysts the purity of the obtained glycerin can be used as a measure of the extent of homogeneously catalyzed processes. The author stated that homogenous processes produce a brownish glycerin whereas pure heterogeneous processes allow to a clear glycerin phase. Spectral data and co-produced glycerin allowed to conclude that homogeneous catalyzed contribution can be discarded.
Ethanolysis reaction
The experimental and predicted values for biodiesel yield responses and all three variables coded form are given in Table 3. The maximum and minimum of FAEE yield achieved was 98.2% and 56.8% respectively. The RSM was applied to the experimental data of the FAEE yield to fit the polynomial equation, Eq. 1, using the coded levels of variables:
\({\text{Y}} = - 147 + 8.6A + 29.4{\text{B}} - 0.8{\text{C}} - 0.632A^{2} - 1.036B^{2} - 1.585C^{2} - 0.732{\text{AB}} + 1.685{\text{AC}} + 0.806{\text{BC}}\)
The graph between predicted and experimental biodiesel yield (%), Fig. 5, shows that predicted values are quite close to experimental ones. The correlation between the experimental and predicted FAEE yields was 0.921. This evidence validates the achieved equation.
The equation along with the Pareto chart, Fig. 6, shows that ethanol:oil molar ratio (A), the catalyst loading (B), the interaction of ethanol:oil molar ratio with time (AC) and catalyst loading with time (BC) have positive effects on the FAEE yield. Although the time, other interactions and square interactions influenced the FAEE yield negatively. In Fig. 6, the bars that represent variables C (time), AC, A (ethanol:oil molar ratio), BB, CC, and BC cross the reference line at 2.110. These parameters are statistically significant at the 0.05 level with the current model terms. Of all these parameters C (time) shows the most significant effect.
Influence of process variables on biodiesel yield
Interactions of process variables on the FAEE yield were also analyzed. They are exhibited graphically in response surface and contour plots, Fig. 7.
Fig. 7a shows the interaction between ethanol:oil ratio and amount of catalyst on FAEE yield achieved. The results show that independently of the amount of catalyst, yield decreases with the increase of ethanol:oil. On the other hand, an initial increase in the amount of catalyst increases the yield but after achieving the maximum value the yield decrease; except for 10:1 ethanol:oil ratio this decrease does not happen.
Response surface and contour plot of a ethanol:oil ratio (A) and catalyst loading (B) interactions impact on FAEE yield with 8 h of reaction time; b ethanol:oil ratio (A) and time (C) interactions impact on FAEE yield with 12.5% of catalyst loading; c catalyst loading (B) and time (C) interactions impact on FAEE yield with 12:1 of ethanol:oil ratio
Fig. 7b shows the interaction between ethanol:oil ratio and time on the FAEE yield achieved. The results show that for lower ethanol:oil ratio the increase of reaction time increases the yield but after reaching the maximum begins to decrease. However, for large ethanol:oil ratio the increase in time increases the yield.
The interaction between the amount of catalyst loading and time on FAEE yield is shown in Fig. 7c). For a small amount of catalyst, the increase of reaction time slight increases the yield but for a large amount, the increase of the reaction time increases significantly the FAEE yield.
Optimization of process variables
The optimization of independent variables was done to maximize de FAEE yield based on the polynomial equation obtained. The optimal reaction parameters achieved were: 11:1 ethanol: oil molar ratio; 13.8% catalyst loading and 9.1 h with 99.2% maximum FAEE yield predicted.
For a similar catalytic system, Avramović et al. [4] obtained slightly different optimal conditions (Table 4) because they used higher catalyst loading and higher ethanol/oil molar ratio which allowed them to decrease the reaction time.
Three replicas of the reaction test in the optimal conditions were performed to validate the above model (Eq. 1). The three replicas allowed an average FAEE yield of 99.0% with a variation of 0.19% between the predicted and experimental yield.
Conclusions
Fatty acids ethyl esters (FAEE) were successfully produced by oil ethanolysis using a basic heterogeneous Ca catalyst prepared by the calcination of scallop shells. During ethanolysis, the CaO catalyst was converted into Ca(OH)2 which reacted with produced glycerin forming the calcium diglyceroxide, thus showing a catalyst dynamics analogous to that reported for methanolysis.
The research surface methodology was successfully applied to the experimental data of the FAEE yield and the obtained analytic function was used to optimize the reaction conditions. The optimal parameters achieved were 11:1 ethanol:oil molar ratio; 13.8% catalyst loading with 9.1 h of reaction time having reached a maximum FAEE yield of 99.0%.
References
Directorate-General for Research and Innovation (European Commission) JRC (European C (2017) The Strategic Energy Technology (SET) Plan—EU Law and Publications
Moser BR (2009) Biodiesel production, properties, and feedstocks. Vitro Cell Dev Biol Plant 45:229–266
Huang D, Zhou H, Lin L (2011) Biodiesel: an alternative to conventional fuel. Energy Procedia 16:1874–1885. https://doi.org/10.1016/j.egypro.2012.01.287
Avramović JM, Veličković AV, Stamenković OS et al (2015) Optimization of sunflower oil ethanolysis catalyzed by calcium oxide: RSM versus ANN-GA. Energy Convers Manag 105:1149–1156. https://doi.org/10.1016/j.enconman.2015.08.072
Borges ME, Díaz L (2012) Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: a review. Renew Sustain Energy Rev 16:2839–2849. https://doi.org/10.1016/j.rser.2012.01.071
Dalena F, Senatore A, Marino A et al (2017) Methanol production and applications: an overview. In: Basile A (ed) Methanol: science and engineering. Elsevier B.V, London, pp 3–28
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37:19–27. https://doi.org/10.1016/j.renene.2011.06.045
Watcharathamrongkul K (2010) Calcium oxide based catalysts for ethanolysis of soybean oil. Songklanakarin J Sci Technol 32:627–634
Vujicic D, Comic D, Zarubica A et al (2010) Kinetics of biodiesel synthesis from sunflower oil over CaO heterogeneous catalyst. Fuel 89:2054–2061. https://doi.org/10.1016/j.fuel.2009.11.043
Verma P, Sharma MP (2016) Comparative analysis of effect of methanol and ethanol on Karanja biodiesel production and its optimisation. Fuel 180:164–174. https://doi.org/10.1016/j.fuel.2016.04.035
García M, Gonzalo A, Sánchez JL et al (2011) Metanolysis and ethanolysis of animal fats: a comparative study of the influence of alcohols. Chem Ind Chem Eng Q 17:91–97. https://doi.org/10.2298/CICEQ100224058G
Rashid U, Ibrahim M, Ali S et al (2012) Comparative study of the methanolysis and ethanolysis of Maize oil using alkaline catalysts. Grasas Aceites 63:35–43. https://doi.org/10.3989/gya.06891
Meneghetti SMP, Meneghetti MR, Wolf CR et al (2006) Biodiesel from castor oil: a comparison of ethanolysis versus methanolysis. Energy Fuels 20:2262–2265. https://doi.org/10.1021/ef060118m
Bolonio D, García-Martínez MJ, Ortega MF et al (2019) Fatty acid ethyl esters (FAEEs) obtained from grapeseed oil: a fully renewable biofuel. Renew Energy 132:278–283. https://doi.org/10.1016/j.renene.2018.08.010
Rubio-Caballero JM, Santamaría-González J, Mérida-Robles J et al (2013) Calcium zincate derived heterogeneous catalyst for biodiesel production by ethanolysis. Fuel 105:518–522. https://doi.org/10.1016/j.fuel.2012.09.054
Kaur N, Ali A (2014) Kinetics and reusability of Zr/CaO as heterogeneous catalyst for the ethanolysis and methanolysis of Jatropha crucas oil. Fuel Process Technol 119:173–184. https://doi.org/10.1016/j.fuproc.2013.11.002
Kaur N, Ali A (2015) Biodiesel production via ethanolysis of jatropha oil using molybdenum impregnated calcium oxide as solid catalyst. RSC Adv 5:13285–13295. https://doi.org/10.1039/c4ra14786c
Velickovic A, Avramovic J, Stamenkovic O, Veljkovic V (2016) Kinetics of the sunflower oil ethanolysis using CaO as catalyst. Chem Ind Chem Eng Q 22:409–418
Kaur M, Ali A (2014) Ethanolysis of waste cottonseed oil over lithium impregnated calcium oxide: kinetics and reusability studies. Renew Energy 63:272–279. https://doi.org/10.1016/j.renene.2013.09.024
Anastopoulos G, Dodos GS, Kalligeros S, Zannikos F (2013) Biodiesel production by ethanolysis of various vegetable oils using calcium ethoxide as a solid base catalyst. Int J Green Energy 10:468–481. https://doi.org/10.1080/15435075.2012.674081
Živković SB, Veljković MV, Banković-Ilić IB et al (2017) Technological, technical, economic, environmental, social, human health risk, toxicological and policy considerations of biodiesel production and use. Renew Sustain Energy Rev 79:222–247. https://doi.org/10.1016/j.rser.2017.05.048
Chumuang N, Punsuvon V (2017) Response surface methodology for biodiesel production using calcium methoxide catalyst assisted with tetrahydrofuran as cosolvent. J Chem. https://doi.org/10.1155/2017/4190818
Silva GF, Camargo FL, Ferreira ALO (2011) Application of response surface methodology for optimization of biodiesel production by transesterification of soybean oil with ethanol. Fuel Process Technol 92:407–413. https://doi.org/10.1016/j.fuproc.2010.10.002
Razack SA, Duraiarasan S (2016) Response surface methodology assisted biodiesel production from waste cooking oil using encapsulated mixed enzyme. Waste Manag 47:98–104. https://doi.org/10.1016/j.wasman.2015.07.036
Babaki M, Yousefi M, Habibi Z, Mohammadi M (2017) Process optimization for biodiesel production from waste cooking oil using multi-enzyme systems through response surface methodology. Renew Energy 105:465–472. https://doi.org/10.1016/j.renene.2016.12.086
Catarino M, Ramos M, Dias APS et al (2017) Calcium rich food wastes based catalysts for biodiesel production. Waste Biomass Valoriz 8:1699–1707. https://doi.org/10.1007/s12649-017-9988-8
Zagonel GF, Peralta-Zamora P, Ramos LP (2004) Multivariate monitoring of soybean oil ethanolysis by FTIR. Talanta 63:1021–1025. https://doi.org/10.1016/j.talanta.2004.01.008
Avhad MR, Sánchez M, Bouaid A et al (2016) Modeling chemical kinetics of avocado oil ethanolysis catalyzed by solid glycerol-enriched calcium oxide. Energy Convers Manag 126:1168–1177. https://doi.org/10.1016/j.enconman.2016.07.060
Soares Dias AP, Puna J, Gomes J et al (2016) Biodiesel production over lime. Catalytic contributions of bulk phases and surface Ca species formed during reaction. Renew Energy 99:622–630. https://doi.org/10.1016/J.RENENE.2016.07.033
Catarino M, Martins S, Soares Dias AP et al (2019) Calcium diglyceroxide as a catalyst for biodiesel production. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2019.103099
Fujimoto K (1992) FTIR spectroscopic study of C02 adsorption/desorption on MgO/CaO catalysts. J Phys Chem 9038:9035–9038. https://doi.org/10.1021/j100201a063
Acknowledgements
The authors acknowledge FCT (Fundacão para a Ciência e Tecnologia. Portugal) for funding project PTDC/EMS-ENE/4865/2014.This work was partially supported by FCT, through IDMEC, under LAETA, project FCT UID/EMS/50022/2019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramos, M., Soares Dias, A.P. & Teodoro, F. Soybean oil ethanolysis over Ca based catalyst. Statistical optimization of reaction conditions. Reac Kinet Mech Cat 130, 433–445 (2020). https://doi.org/10.1007/s11144-020-01791-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01791-y