Abstract
FePO4 was successfully synthesized via a simple method as a heterogeneous catalyst for photo degradation of methyl violet 2B in aqueous solution. The FePO4 was identified by the X-ray diffraction, Fourier transform infrared spectroscopy analyses and the scanning electron microscopy image The analysis was completed by the pHPZC and the band gap energy. The photocatalytic performance of FePO4 was investigated using reduction of methyl violet 2B as a model reaction. The results showed that FePO4 had converted nearly 99% of methyl violet 2B under UV light with optimum operating conditions of 1 g L−1 of FePO4, natural pH (pH 6.75), and 10 mg L−1 of methyl violet 2B, a kinetic study was also realized. The recyclable character of the catalyst under the optimum conditions for three cycles was successfully investigated without any structural modification of the FePO4.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Our environment continues to become more and more polluted, and the traditional chemical methods that deal with environmental pollution and especially water pollution have been unable to meet the demands of modern energy saving themes and environmental protection. Environmental problems caused by toxic substances and easily degradable organic pollutants (such as heavy metals, pharmaceutical wastes, pesticides, and dyes) have caused a serious threat to the well-being of humans and development in the 21st century [1].
The application of photocatalysis in the field of water treatment has received considerable attention in recent years. It is a highly promotive research area [2,3,4,5,6], which presents a technique of efficiency for the degradation of organic pollutants [7]. It is a method which requires accurate operating conditions. The increase of the temperature and the pressure is not necessary which reduces considerably its cost. By using a suitable photocatalyst, this technique allows the generation of hydroxyl radicals, a species with a very powerful oxidative character that has the capacity to degrade the majority of the polluting organic molecules [8].
During the last two decades, photocatalysis has undergone several changes with a very significant progress in the preparation of new types of nanostructured materials synthesized with a low cost, efficient and recyclable. Thus, the development of a photocatalyst that meets these criteria cannot only prevent the excessive use of photocatalysts, but also the recovery of deactivated photocatalysts, thereby reducing the total cost and reducing further the use of photocatalytic materials.
Iron-based photocatalysts are the aim of study by several researchers [9,10,11,12]. As photocatalysts, these types of materials have shown a high efficiency for the oxidation of organic pollutants due to their optical properties of UV–VIS absorption. Iron-based materials have many advantages; they are stable and compatible compounds with most organic pollutants. They meet all the criteria mentioned above [1].
FePO4 are materials that have been widely used in electrochemistry such as electrodes in lithium batteries [13,14,15,16] and electrical transport [17, 18]. In the present work, the use of this material will be deviated and will be used for the first time as photocatalyst. The FePO4 was synthesized via a simple and easy method using a couple of chemicals. To test the efficiency of this material as a photocatalyst, methyl violet 2B (MV2B) is chosen as the focus of this study because of its importance in textile industry and the fact that it is a very cheap dye. In addition to its use in the textile industry, the MV2B is also used in paper printing, ink making, and other fields [19, 20]. However, it is known to be irritating to the skin, eyes and respiratory system. Animal tests revealed a LC-50 level of 0.047 mg L−1 for Pimephalespromelas [21].
Materials
Methyl violet 2B (color index 42535) was obtained from Fluka whereas phosphoric acid, sulfuric acid, iron sulfate heptahydrate and sodium hydroxide were obtained from sigma Aldrich. In the experiments, all the solutions were prepared using ultrapure water.
Preparation of FePO4
Initially, 0.77 mol of phosphoric acid were dissolved in 50 mL of ultrapure water. The solution was vigorously stirred, and a solution of iron sulfate (0.5 mol L−1) was added, the mixture was refluxed for 2 h and filtered. The white precipitates were washed several times with distilled water and acetone and then dried in an electric oven overnight. Finally, the dried precipitates were annealed at 200 °C for 3 h.
Various characterization tools such as X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), the morphology of samples was observed using scanning electron microscopy.
Characterization
The FTIR spectrum was recorded with a Perkin-Elmer FTIR 1000 spectrometer at room temperature and in the frequency range of 400–4000 cm−1. The structure and crystallinity of the synthesized samples were characterized by powder XRD analysis using a RIGAKU MINIFLEX 600. The scanning electron microscope (SEM) images were obtained by a Coxem 30AX, and the solid UV–Visible spectra were obtained by an ANALATIKJENA SPECORD 200 PLUS.
Heterogeneous photo catalytic degradation of MV2B
The photocatalytic tests of FePO4 have been evaluated with a Pyrex glass reactor with a double wall (capacity 1000 mL). The experiments were performed with magnetic stirring under UV irradiation using a UVP Lamp pencil low mercury vapor pressure in argon, which was purchased from Pen-Ray Lamps Group Type 1115 (25 W, 18 mA, 254 nm). The lamp was inside a quartz tube Supracil immersed in a MV2B solution with initial concentration of 10 mg L−1 and a volume of 500 mL. Before the illumination, the solution was sufficiently stirred in the dark for 20 min to reach the equilibrium between the solid and the solution. At regular times of irradiation, 5 mL of MV2B solution was taken and centrifuged (5000 rpm) to remove the catalysts. The photodegradation of MV2B was monitored by measuring the maximum absorption peak at 580 nm using a Lambda 25 UV–Vis spectrometer.
The degradation rate was calculated from the following relation:
Here A0 and At are the initial absorbance of MV2B and the absorbance of MV2B after time t, respectively.
Results and discussion
Catalyst characterization
FTIR analyses
The structures of the FePO4 were further characterized in Fourier transform infrared spectra (FTIR). The sample spectra have shown a very broad band at 3272 cm−1 corresponding to the hydroxyl group [22]. The absorption band at 1610 is assigned to bending vibration of –OH groups attached to the adsorbed water interlayer [23].
The bands at 635 cm−1 can be attributed to the asymmetric vibration of the O–P–O [24], however the band at 1032 cm−1 can be attributed to the vibration Fe–O–P [25, 26]. Also, the bands located at the wavelength of 442 cm−1 are related to vibrations of phosphate groups [24, 27].
XRD analyses
Fig. 1 shows the XRD pattern of the FePO4, the appearance of the diffraction peaks shows the crystalline properties of the compound. The main diffraction peaks can be indexed using the Rietveld refinement to (200), (101), (210), (021), (020), (211), (220) corresponding to 2θ values of approximately 18.13º, 20.4º, 22.86º, 24.19º, 29.96º, 30.53º and 36.94º respectively (PDF2- ICDD 01-070-6685), it has also show that all the diffraction peaks can be indexed to orthorhombic lattice and this was in agreement with previous results of studies [16, 28, 29]. The cell parameters obtained from Rietveld refinement are: a = 9.74 Å, b = 5.72 Å, c = 4.89 Å, volume = 273.18 Å3.
SEM analysis
Fig. 2 shows the SEM image of the FePO4 on a scale of 100 μm. The SEM image in Fig. 2a shows the presence of the phosphate groups as a white spot [30] and the porosity of the materials. Fig. 2b presents the EDS pattern of the crystal in the SEM figures, the spectrum shows the presence of iron, phosphate and oxygen with percentages of 27.7, 16.8 and 55.3%.
The pHpzc measurement
One of the objectives of this study is to determine the influence of pH on the photocatalytic activity of the FePO4, and it is very important to know the pHPZC of the catalyst. It is the point where the total concentration of the surface anionic sites is equal to the total concentration of the surface cationic sites. Under this value (pH < pHPZC), the material surface is positively charged, which favors the adsorption of anions. Over this value (pH > pHPZc) the material surface is negatively charged, which favors the adsorption of cations [31, 32].
The pHPZC of the FePO4 catalyst is 7.2, this value was determined by the method cited by Muhammad Mushtaq and all [33].
The band gap energy
The gap energy corresponding to the FePO4 was calculated, and the results are shown in Fig. 3. The diffused reflectance spectrum is recorded in the range (300–1100 nm) the spectrum shows that the material has a maximum reflectance (81.06%) in the visible field at λ = 667 nm.
However, to know the type of transition and the gap energy, we have plotted the Tauc graph by studying the variation of the optical absorption coefficient as a function of the energy of the incident photons (hν) expressed by relation 2 [34, 35]
Here A is a constant, n = 2 for the direct transition, hν is the incident photon energy, and α is the absorption coefficient determined according to the relation 3 below [34, 36]
where \(t\) is the film thickness, R reflectance and T transmittance. The gap is determined by extrapolating the linear region of the plot to zero (αhν)2 = 0. Besides, Fig. 3 shows the direct, calculated gap energy which presents the values of 3.35 (ev), this confirms the efficiency of the catalysts under UV light.
Photocatalytic activity of FePO4
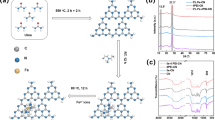
Fig. 4a shows the UV–Vis spectra of MV treated by FePO4 at different irradiation times and the effect of this photocatalyst on the MV2B degradation. The spectrum of the dye solution before irradiation had prominent peaks at 580, 299 and 247 nm corresponding to the conjugated triphenylmethane chromophore, diphenyl methyl type, and benzene moieties, respectively [37, 38]. It was observed that the absorption peaks diminished very fast and nearly completely disappeared after 90 min of irradiation time, indicating the breakdown of chromophore responsible for the characteristic color of MV2B.
In the other hand, Fig. 4b shows the effect of the photocatalyst concentrations on the MV2B elimination. The optimum concentration was obtained at 1 g L−1 with 99% of degradation after 180 min of treatment which can be explained by the increase in the specific photocatalyst surface in contact with the light UV which increases the amount of the hydroxyl radical formed during the illumination time. Fig. 4c shows the increasing of the hydrogen peroxide amount with the increasing of photocatalyst concentration. The H2O2 formed during the degradation was formed by recombination of hydroxyl radicals according to the Eq. 4. These results confirm the results showed in Fig. 4b [39].
Kinetics of the dye degradation by the FePO4 catalyst
The kinetics of the MV2B degradation were studied using the equation proposed by Gabor Lente [40].
Here X is termed the amplitude, k is the first order rate constant, and E is called the endpoint. The results are shown in Fig. 5.
Furthermore, the integration in time (in interval τ) is often a key question when the response time of the monitoring method is not much faster than the process studied. The integrated observation is described as [40]:
The calculated amplitude (X); the endpoint (E); the kinetic constants (k) and R2 value (coefficient of determination) are shown in the following table. (Table 1).
It was found from the Table 1 that the faster degradation was obtained with 1 g L−1 of the catalyst which corresponds to a value of k equal to 0.021 min−1.
Effect of pH on the MV2B elimination
The pH solution appears to play an important role in the photocatalytic process of various pollutants. So, to study the impact of the pH on the MV2B photocatalytic degradation using the synthesized material, various pH values have been used (acid, alkaline and natural pH) and the results are collected in Fig. 6. It could be inferred that both lower and higher initial pH had negative effect on the degradation of MV2B. The highest degradation rate was obtained under the natural initial pH (pH 6.75) reaching 99% of degradation after 180 min.
The degradation rate decreased at acidic pH, this is due to the fact that at this pH value there is a high production of hydroxyl radicals, a higher number of OH· species recombine to form H2O2 and do not react with MV2B, due to the recombination of OH· being faster than OH· attacked on MV2B [39], the hydrogen peroxide can also react with OH· radical (Eq. 7), producing an inhibitory effect on the decolorization [41], and as shown on the results, there is also a decrease in removal efficiency at pH 9. In one hand, it can be suggested that at high concentration of HO−, HO ·2 radicals may be formed. The reactivity of these radicals with organic pollutant is less than ·OH [41].
In the other hand, the photocatalytic degradation depends largely on the catalyst surface charge (pzc value) and the acid dissociation constant (pKa), the catalyst pzc = 7.2 and the dye pKa = 7.67 so, both the catalysts and the dye are positively charged in acidic pH, whereas they are negatively charged when the pH is alkaline. Consequently, this conducts into an electrostatic repulsion between the dye and the catalyst surface which leads to a decrease of the adsorption on the FePO4 surface. Generally, the deactivation of ·OH at high pH values has been reported [42, 43].
Chemical oxygen demand (COD)
In order to verify the performance of the photocatalyst on the MV2B degradation, the chemical oxygen demand the COD abatement range has been studied. The results are shown in Table 2; where an increase in COD abatement rate of 77% after 180 min of irradiation time is observed. This percentage indicates a degradation of the pollutant and intermediate products formed during the treatment process.
Photocatalytic activity of FePO4 recycling
Recycling photocatalytic activity tests were realized to evaluate the stability of the FePO4 sample under the same conditions, and their results are shown in Table 3. As shown in the figure, MV2B degradation slightly decreased after 3 h of three recycle tests. In order to determine why the photocatalytic activity decreased, the XRD characterization of the fresh and the used sample were conducted, and the results are shown in Fig. 7. It can be observed that there are no significant changes in characteristic diffraction peaks of the fresh and used FePO4 samples, indicating that the crystal structure was not altered after the third test. This means that the partial deactivation of this photocatalyst was not due to the change in crystal structure. Meanwhile, the slight decrease in MV2B degradation might also be due to the partial occupation of the active sites by the adsorption of intermediates and the partial loss of the photocatalyst during the recycling test processes.
Conclusion
The prepared photocatalyst composites have shown a higher photocatalytic activity and a great ability for the degradation of the selected dye. The results obtained have revealed that 99% of the pollutant were destroyed after 180 min of photodegradation under UV irradiation. Moreover, this study is enriched by the measurement of the hydrogen peroxide rate formed during the degradation process which has confirmed the previous results. Also, the COD measurement which showed a 77% decrease has validated the degradation of the dye as well as the intermediate products. All these factors classify the FePO4 as a good photocatalyst that can give a good degradation to the persistent products.
References
Wu W, Jiang C, Roy VAL (2015) Recent progress in magnetic iron oxide-semiconductor composite nanomaterials as promising photocatalysts. Nanoscale 7:38–58
Ahmed S, Rasul MG, Brown R, Hashib MA (2011) Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: a short review. J Environ Manag 92:311–330
Donal AK, McGuigan GK, Pilar IF et al (2014) Solar photocatalysis for water disinfection: materials and reactor design. Catal Sci Technol 4:1211–1226
Hoffmann MR, Scot TM, Wonyong C, Detlef WB (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:69–96
Agustina TE, Ang HM, Vareek VK (2005) A review of synergistic effect of photocatalysis and ozonation on wastewater treatment. J Photochem Photobiol C 6:264–273
Guo Q, Zhou C, Ma Z et al (2018) Elementary chemical reactions in surface photocatalysis. Annu Rev Phys Chem 69:451–472
Nagar R, Varrla E, Vinayan BP (2018) Photocatalysts for hydrogen generation and organic contaminants degradation. Multifunctional photocatalytic materials energy. Woodhead Publishing, Sawston, pp 215–236
Gligorovski S, Strekowski R, Barbati S, Vione D (2015) Environmental implications of hydroxyl radicals (·OH). Chem Rev 115:13051–13092
Zołtowska A, Agnieszka GZ, Hermann E, Teofil J (2018) Iron(III) phthalocyanine supported on a spongin scaffold as an advanced photocatalyst in a highly efficient removal process of halophenols and bisphenol A. J Hazard Mater 347:78–88
Telitel S, Dumur F, Campolo D et al (2016) Iron complexes as potential photocatalysts for controlled radical photopolymerizations: a tool for modifications and patterning of surfaces. J Polym Sci A 54:702–713
Jing Z, Dumur F et al (2016) Iron-based metal-organic frameworks (MOF) as photocatalysts for radical and cationic polymerizations under near uv and visible LEDs (385–405 nm). Macromol Chem Phys 217:2534–2540
Takahiro O, Hikaru M, Kei N (2018) Photocatalytic decomposition of gaseous methanol over anodized iron oxide nanotube arrays in high vacuum. Mater Res Bull 99:367–376
Saw LH, Somasundaram K, Ye K, Tay AAO (2014) Electro-thermal analysis of Lithium Iron Phosphate battery for electric vehicles. J Power Sources 249:231–238
Arpit M, Mihaela AD, Matteo D, Massimo S (2016) Inverse parameter determination in the development of an optimized lithium iron phosphate e graphite battery discharge model. J Power Sources 307:160–172
Hagberg J, Maples HA et al (2018) Lithium iron phosphate coated carbon fiber electrodes for structural lithium ion batteries. Comp Sci Technol 162:235–243
Paolella A, Cl Faure et al (2017) Light-assisted delithiation of lithium iron phosphate nanocrystals towards photo-rechargeable lithium ion batteries. Nat Commun 8:14643
Pavić L, Zl Skoko et al (2018) Electrical transport in iron phosphate glass-ceramics. J Non-Cryst Solids 502:44–53
Deng Y, Liao Q, Wang F, Zhu H (2018) Synthesis and characterization of cerium containing iron phosphate-based glass-ceramics. J Nucl Mater 499:410–418
Vinitha G, Ramalingam A (2018) Spectral characteristics and nonlinear studies of methyl violet 2B dye in liquid and solid media. Laser Phys 18:37–42
Zeyada HM, Habashi AA, Makhlouf MM et al (2016) Fabrication, electrical transport mechanisms and photovoltaic properties of methyl violet 2B/n-Si hybrid organic/inorganic solar cell. Microelectron Eng 163:134–139
Raziq M, Kooh R, Lim LBL, Lim LH, Owais O (2018) Phytoextraction potential of water fern (Azolla pinnata) in the removal of a hazardous dye, methyl violet 2B: artificial neural network modeling. Int J Phytorem 20:424–431
Baykan D, Oztas NA (2012) Synthesis and characterization of iron orthophosphate by solution combustion method. Mat Res Bul 47:4013–4016
Lu M, Wang F, Chen K et al (2015) The crystallization and structure features of barium-iron phosphate glasses. Spectrochim Acta A 148:1–6
Burba CM, Frech R (2006) Vibrational spectroscopic investigation of structurally-related LiFePO4, NaFePO4, and FePO4 compounds. Spectrochimica Acta A 65:44–50
Yin Y, Zhang H, Wu P, Zhou B, Cai C (2010) Iron phosphate nanostructures synthesized by microwave method and their applications in biosensing. Nanotechnology 21:425504–425514
Han S, Wang J, Li S, Wu D, Feng X (2014) Graphene aerogel supported Fe5(PO4)4(OH)3·2H2O microspheres as high-performance cathode for lithium ion batteries. J Mater Chem A 2:6174–6179
Al-Mutairi E, Narasimharao K, Mokhtar M (2015) Heteropolyacid generated on the surface of iron phosphate nanotubes: structure and catalytic activity studies. RSC Adv 5:63917–63929
Yang S, Song Y, Zavalij YP, Whittingham MS (2002) Reactivity, stability and electrochemical behavior of lithium iron phosphates. Electrochem Comm 4:239–244
Yamada A, Takei Y, Koizumi H, Sonoyama N, Kanno R (2005) Phase change in LixFePO4. Electrochem Solid-State Lett 8:409–413
Hsu J, Bai J, Kim CW et al (2018) The effects of crystallization and residual glass on the chemical durability of iron phosphate waste forms containing 40 wt% of a high MoO3 Collins-CLT waste. J Nucl Mater 500:373–380
Shorgoli AA, Shokri M (2017) Photocatalytic degradation of imidacloprid pesticide in aqueous solution by TiO2 nanoparticles immobilized on the glass plate. Chem Eng Commun 204:1061–1069
Douafer S, Lahmar H (2018) Physical and photoelectrochemical properties of the spinel LiMn2O4 and its application in photocatalysis. J Phys Chem Solids 118:62–67
Mushtaq M, Il Tan et al (2014) Influence of PZC (point of zero charge) on the static adsorption of anionic surfactants on Malaysian sandstone. J Dispers Sci Technol 35:343–349
Beke S, Sl Giorgio et al (2008) Structural and optical properties of pulsed laser deposited V2O5 thin films. Thin Solid Films 516:4659–4664
Kumar P, Malhotra L (2004) Electrochemical loading of hydrogen in palladium capped samarium thin film: structural, electrical, and optical properties. Electrochim Acta 49:3355–3360
Hassanien AS, Akl AA (2015) Influence of composition on optical and dispersion parameters of thermally evaporated non-crystalline Cd50S50_xSex thin films. J Alloys Compd 648:280–290
Li J, Dickon NLH, Song P, Kong C, Song Y (2015) Synthesis of SnO2 -activated carbon fiber hybrid catalyst for the removal of methyl violet from water. Mater Sci Eng B 194:1–8
Juretic H, Montalbo-Lomboy M, van Leeuwen JH et al (2015) Hydroxyl radical formation in batch and continuous flow ultrasonic systems. Ultrason Sonochem 22:600–606
Guettaia D, Boudjemaa A, Bachari K, Mokhtari M (2018) Enhanced performance of Fe-JUL-15 prepared by ultrasonic method through the photo-degradation of ibuprofen. Environ Prog Sustain Energy 37:738–745
Lente G (2015) Solving rate equations. deterministic kinetics in chemistry and systems biology: the dynamics of complex reaction networks. Springer International Publishing, Cham, pp 21–59
Aleboyeh A, Moussa Y, Aleboyeh H (2005) The effect of operational parameters on UV/H2O2 decolourisation of acid blue 74. Dyes Pigm 66:129–134
Kasiri MB, Aleboyeh H, Aleboyeh A (2008) Degradation of acid blue 74 using Fe-ZSM5 zeolite as a heterogeneous photo-Fenton catalyst. App Cat B 84:9–15
Ejhieh AN, Khorsandi M (2010) Photodecolorization of Eriochrome Black T using NiS–P zeolite as a heterogeneous catalyst. J Hazard Mater 176:629–637
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Benomara, A., Guenfoud, F. & Mokhtari, M. Removal of methyl violet 2B by FePO4 as photocatalyst. Reac Kinet Mech Cat 127, 1087–1099 (2019). https://doi.org/10.1007/s11144-019-01607-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-019-01607-8