Abstract
In this paper, an efficient method for the synthesis of 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromene derivatives is described. The method uses Fe3O4 magnetic nanoparticles coated with poly (ε-caprolactone)/poly (ethylene glycol)/poly (ε-caprolactone) (MNPs-Fe3O4/PCL–PEG–PCL) as a biodegradable and green catalyst under solvent-free conditions. The catalyst was prepared from PCL–PEG–PCL copolymer and MNPs-Fe3O4 and characterized by FT-IR, X-ray diffraction and SEM spectroscopy. Most aromatic aldehydes bearing electron-withdrawing or electron donating groups reacted successfully with malononitrile and dimedone under solvent-free conditions at 80 °C. The corresponding chromenes were isolated in good to excellent yields and the structures of them were confirmed by 1H NMR and IR spectroscopy. The catalyst could be recovered using an external magnet without a considerable loss in its catalytic activity. Moreover, some advantages of this method are simplicity of procedure, easy separation of the catalyst, high yields, efficiency, stability and non-toxicity of the catalyst, short reaction times, and environmentally benign conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromene and its derivatives are an important class of heterocyclic compounds that have been shown various biological properties. Many of these compounds act as anti-viral [1], anti-inflammatory [2], antiproliferative [3], anti-tumor [4], anti-cancer [5], anti-estrogenic [6], antibacterial [7], anti-helminthic [8], anticonvulsant [9], sex pheromone [3], and antimalarial agents [10]. The synthesis of chromene derivatives has been attracted much attention of many researchers due to their biological properties. In recent years, different homogeneous or heterogeneous catalysts have been developed for the preparation of these compounds such as Fe3O4/SiO2 [11], K2CO3 [12], Ca(OH)2 [13], MgO [14], nano Ag/kaolin [15], Fe3O4-chitosan nanoparticles [16], hetero-polyacid [17], etc. Most of these methods have several drawbacks such as low yields of products, long reaction times, harsh reaction conditions, expensive reagents, the use of toxic catalysts, and cumbersome work-up. Therefore, it is necessary to introduce an efficient, safe, and green method for the synthesis of these compounds.
Recently, because of their interesting properties, magnetic nanoparticles have been used in many reactions as catalysts. However, these magnetic nanomaterials usually do not have thermal and chemical stability, and they would be aggregated and deformed during the chemical reactions. Therefore, the development of effective strategies for improving the chemical stability of magnetic nanoparticles is necessary. One way to overcome these challenges is the surface modification of magnetic nanoparticles using surface stabilizers and polymers [16, 18,19,20,21].
Magnetic nanoparticles (MNPs-Fe3O4)/poly (ε-caprolactone) [PCL]-co-poly ethylene glycol (PEG) [22] is magnetic nanocomposite including a biodegradable copolymer [PCL–PEG–PCL] which is covered by Fe3O4 nanoparticles. It has been found that these nanocomposites can play a significant role in antitumor drug delivery to fight lung tumors [23]. In the present study, we wish to investigate the catalytic activity of this new magnetic nanocomposite [MNPs-Fe3O4/PCL–PEG–PCL] in the synthesis of 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromene derivatives from aldehydes, malononitrile, and dimedone under solvent-free conditions. It must be noted that it is the first application of this nanocomposite as a catalyst in organic transformations.
Experimental
General
All reagents used for this study were the products of Merck and Aldrich, which were used with no further purification. All yields are the isolated product yields which had been purified. Melting points were determined with Barnstead Electrothermal. To record IR spectra, a Shimadzu IR-470 spectrometer was utilized, and 1H NMR data were obtained using a Bruker 300 MHz spectrometer in CDCl3. The structures of the products were confirmed by their melting points, 1HNMR, IR spectroscopy and compared with the literature. Besides, X-ray powder diffraction analysis was conducted on a Bruker AXS D8 ADVANCE X-ray diffractometer using a Cu tube in the range of 2θ = 10–80°. The size and shape of the particles were determined using scanning electron microscopy (TESCAN, VEGA SEM).
Preparation of copolymer PCL–PEG–PCL
The PCL–PEG–PCL was synthesized by the ring opening polymerization of 3-caprolactone with Sn(Oct) as the catalyst [34]. The polyethylene glycol was dried under a nitrogen atmosphere at 80 °C for 2 h. A mixture of 3-caprolactone (4 g), PEG (2 g), and Sn(Oct) (0.01 mmol) was stirred under a nitrogen atmosphere at 120 °C for 12 h. The product was cooled to room temperature and dissolved in dichloromethane. Then cold diethyl ether was added, and the precipitate was filtered, and the purification process was repeated twice more. The obtained product was dried under vacuum at room temperature for 24 h. The structure of PCL–PEG–PCL copolymer was confirmed by IR and 1H NMR spectroscopy and compared with the literature [24].
Synthesis of Fe3O4 nanoparticles (MNPs)
Magnetic nanoparticles were synthesized by a chemical precipitation method. According to this method, the FeCl3·6H2O (34.6 mmol), FeCl2·4H2O (17.30 mmol) and 160 ml double distilled water were stirred under N2 gas. Then, 20 ml NH4OH (25%) was added dropwise to the mixture and stirred at 80 °C for 30 min. The reaction mixture was centrifuged at 5000 rpm for 20 min. The precipitated particles were washed twice with deionized water and collected by a strong permanent magnet [25].
Preparation of MNPs-Fe3O4/PCL–PEG–PCL
Two hundred mg of PCL–PEG–PCL copolymer was dissolved in methylene chloride (3 ml) and added to an aqueous solution of MNPs-Fe3O4 (10 mg in 1 ml distilled water). The mixture was homogenized under ultrasound irradiation for 3 min. Then, 20 ml solution of polyvinyl alcohol (1%) was added and homogenized again for 2 min. The solvent was concentrated, and the mixture was centrifuged at 6000 rpm for 15 min. The resulting solid was filtered and then dried to give the pure MNPs-Fe3O4/PCL–PEG–PCL.
General procedure for the preparation of 2-amino-4H-chromene derivatives
A mixture of the aldehyde (1 mmol), malononitrile (1 mmol), dimedone (1 mmol), and catalyst (20 mg) was stirred at 80 °C under solvent-free conditions. The progress of the reaction was followed by TLC (n-hexane/ethyl acetate, 2/1). After the completion of the reaction, the catalyst was separated magnetically from the reaction mixture. The crude product was recrystallized from ethanol to give the pure compound.
Results and discussion
In continuation of our previous works on the development of new, green, and efficient reagents for the synthesis of biologically active heterocycles [26,27,28,29,30] herein, we wish to report the simple synthesis of 2-amino-4H-chromene derivatives using MNPs-Fe3O4/PCL–PEG–PCL as a reusable heterogeneous catalyst under solvent-free conditions (Scheme 1).
First, the catalyst was prepared from PCL–PEG–PCL copolymer and MNPs-Fe3O4 as explained in the experimental section and characterized by FT-IR, X-ray diffraction and SEM spectroscopy.
The FT-IR spectra of MNPs-Fe3O4, PCL–PEG–PCL copolymer, and synthesized MNPs-Fe3O4/PCL–PEG–PCL nanocomposite are given in Fig. 1. The absorption bands at 570 cm−1 presented in Fig. 1a was attributed to Fe–O bond. In Fig. 1c, the main bands were observed at 3439 (O–H) 2945 and 2867 cm-1 (C–H), 1725 (C=O), 1245 (C–O, ester group) and 1193 (C–O ether group). The FT-IR spectra of MNPs-Fe3O4/PCL–PEG–PCL is presented in Fig. 1b. The observed band at 1725 was attributed to ester carbonyl group of copolymer.
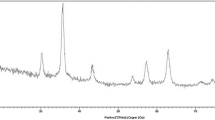
The XRD patterns of Fe3O4, PCL–PEG–PCL, and Fe3O4/PCL–PEG–PCL illustrate the crystalline structures of them (Fig. 2). The comparison of Fig. 2a–c demonstrates that the characteristic peaks of Fe3O4 did not change after coating with PCL–PEG–PCL.
The average diameter of MNPs-Fe3O4 was estimated approximately 55.8 nm by SEM micrograph (Fig. 3a). Fig. 3b clearly shows that MNPs-Fe3O4/PCL–PEG–PCL were successfully synthesized in nanoscale dimensions.
In order to consider the catalytic activity of MNPs-Fe3O4/PCL–PEG–PCL, the reaction of 4-nitrobenzaldehyde (1 mmol), malononitrile (1 mmol), and dimedone (1 mmol) was selected as the model reaction and an optimization was done to identify the optimum amount of catalyst, the best solvent, and the suitable temperature (Table 1). The best result was obtained in the presence of 20 mg of the catalyst at 80 °C under solvent-free conditions (Table 1, entry 3). No product was observed in the absence of the catalyst within 20 min (Table 1, entry 7). Furthermore, the model reaction was performed in the presence of PCL–PEG–PCL and MNPs-Fe3O4 as the catalyst under optimized reaction conditions which led to no product formation in the case of copolymer and the reaction was performed very slowly in the presence of MNPs-Fe3O4 (Table 1, entries 8, 9). Therefore, it can be concluded that modified Fe3O4 NPs with PCL–PEG–PCL is more effective than pure Fe3O4 NPs for the synthesis of 4H-chromene derivatives.
The results in Table 1 indicate that using 20 mg MNPs-Fe3O4/PCL–PEG–PCL as the catalyst at 80 °C under solvent-free conditions is the most suitable condition for the synthesis of 2-amino-4H-chromene derivatives. After obtaining the optimum conditions, the generality of this method was studied by the reaction of various aromatic aldehydes with malononitrile and dimedone at 80 °C under solvent-free conditions (Table 2).
Most aromatic aldehydes bearing electron-withdrawing or electron donating groups reacted successfully under these conditions and the corresponding chromenes were isolated in good to excellent yields. Furthermore, isatin produced 2-amino-7,7-dimethyl-2’,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3’-indoline]-3-carbonitrile in good yield (Table 2, entry 9).
A plausible mechanism for the synthesis of 2-amino-4H-chromene derivatives using MNPs-Fe3O4/PCL–PEG–PCL as a heterogeneous catalyst is shown in Scheme 2. First, a Knoevenagel condensation reaction between aldehyde and malononitrile is performed. In this mechanism, the role of Fe3O4/PCL–PEG–PCL is to activate carbonyl and nitrile groups for nucleophilic attacks.
In addition, the reusability of the catalyst was examined in the model reaction. After the completion of the reaction, the catalyst was separated by an external magnet from the reaction mixture, washed three times with 5 ml of ethanol, dried, and reused in the synthesis of 4e for five times without a considerable loss in its catalytic activity (Fig. 4).
In order to show the importance of this study, the catalyst’s efficiency was compared with some other catalysts in the synthesis of 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromene derivatives (Table 3). The results obviously demonstrate that the preference of MNPs-Fe3O4/PCL–PEG–PCL over the previously used catalysts with respect to the yields and times of reactions.
Conclusion
In this study, a new, green, and efficient procedure is reported for the synthesis of 4H-chromene derivatives using MNPs-Fe3O4/PCL–PEG–PCL. The simplicity of procedure, easy separation of the catalyst, short reaction times, reusability, stability, and non-toxicity of the catalyst in addition to the high yields of products and the absence of solvent are the advantages of this methodology.
References
Martínez-Grau A, Marco J (1997) Bioorg Med Chem Lett 7:3165–3170
Moon DO, Choi YH (2007) Kim ND, Park YM, Kim GY. Int Immunopharmacol 7:506–514
Bianchi G, Tava A (1987) Agric Biol Chem 51:2001–2002
Anderson DR, Hegde S, Reinhard E, Gomez L, Vernier WF, Lee L, Liu S, Sambandam A, Snider PA, Masih L (2005) Bioorg Med Chem Lett 15:1587–1590
Ough M, Lewis A, Bey EA, Gao J, Ritchie JM, Bornmann W, Boothman DA, Oberley LW, Cullen JJ (2005) Cancer Biol Ther 4:102–109
Jain N, Xu J, Kanojia RM, Du F, Jian-Zhong G, Pacia E, Lai M-T, Musto A, Allan G, Reuman M (2009) J Med Chem 52:7544–7569
Kumar RR, Perumal S, Senthilkumar P, Yogeeswari P, Sriram D (2007) Bioorg Med Chem Lett 17:6459–6462
Tripathi R, Tripathi R, Bhaduri A, Singh S, Chatterjee R, Murthy P (2000) Acta Trop 76:101–106
Bhat MA, Siddiqui N, Khan SA (2008) Acta Pol Pharm 65:235–239
de Andrade-Neto VF, Goulart MO, da Silva Filho JF, da Silva MJ, Maria do Carmo F, Pinto AV, Zali MG, Carvalho L, Krettli AU (2004) Bioorg Med Chem Lett 14:1145–1149
Alizadeh A, Khodaei MM, Beygzadeh M, Kordestani D, Feyzi M (2012) Bull Korean Chem Soc 33:2546–2552
Pourmohammad M, Mokhtary M (2015) Chimie CR 18:554–557
Kolla SR, Lee YR (2011) Tetrahedron 67:8271–8275
Kumar D, Reddy VB, Sharad S, Dube U, Kapur S (2009) Eur J Med Chem 44:3805–3809
Sadeghi B, Lasemi Z, Azimi R (2015) Orient J Chem 31:1175–1179
Safari J, Javadian L (2015) Ultrason Sonochem 22:341–348
Heravi MM, Bakhtiari K, Zadsirjan V, Bamoharram FF, Heravi OM (2007) Bioorg Med Chem Lett 17:4262–4265
Hong R, Fischer NO, Emrick T, Rotello VM (2005) Chem Mater 17:4617–4621
Arundhathi R, Damodara D, Likhar PR, Kantam ML, Saravanan P, Magdaleno T, Kwon SH (2011) Adv Synth Catal 353:1591–1600
Liu X, Guan Y, Ma Z, Liu H (2004) Langmuir 20:10278–10282
Kim M, Chen Y, Liu Y, Peng X (2005) Adv Mater 17:1429–1432
Zhang J, Misra R (2007) Acta Biomater 3:838–850
Askari S, Salehi R, Zarghami N, Akbarzadeh A, Rahmati-yamchi M (2016) Artif Cell Nanomed B 44:1753–1763
Manjili HK, Sharafi A, Danafar H, Hosseini M, Ramazani A, Ghasemi MH (2016) RSC Adv 6:14403–14415
Mehdinia A, Kayyal TB, Jabbari A, Aziz Zanjani MO, Ziaei E (2013) J Chromatogr A 1283:82–88
Siadatifard SH, Abdoli-Senejani M, Bodaghifard MA (2016) Cogent Chem 2:1188435
Azizi N, Abdoli-Senejani M, Abbasi F (2016) Tetrahedron Lett 57:5009–5011
Hojati SF, Amiri AH, Raouf H (2017) Appl Organomet Chem. https://doi.org/10.1002/aoc.3595
Hojati S, Raouf H (2016) Org Prep Proced Int 48:474–480
Keshavarz M, Abdoli-Senejani M, Hojati SF, Moosavifar M (2017) Org Prep Proced Int, In press
Sarrafi Y, Mehrasbi E, Vahid A, Tajbakhsh M (2012) Chin J Catal 33:1486–1494
Zavar S (2017) Arab J Chem 10:S67–S70
Safari J, Zarnegar Z (2014) J Mol Struct 2014(1072):53–60
Makvandi M, Dil FA, Malekzadeh A, Baghernejad M, Niknam K (2013) Iran J Catal 3:221–228
Joshi VM, Magar RL, Throat PB, Tekale SU, Patil BR, Kale MP, Pawar RP (2014) Chin Chem Lett 25:455–458
Acknowledgements
We are thankful to the Islamic Azad University Arak-Branch for its financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Keshavarz, M., Abdoli-Senejani, M., Hojati, S.F. et al. Fe3O4 magnetic nanoparticles coated with a copolymer: a novel reusable catalyst for one-pot three-component synthesis of 2-amino-4H-chromene. Reac Kinet Mech Cat 124, 757–766 (2018). https://doi.org/10.1007/s11144-018-1361-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-018-1361-9