Abstract
In order to improve the catalytic performance in the Baeyer–Villiger oxidation of cyclohexanone by molecular oxygen, ordered mesoporous Sn–TiO2 catalysts with high Lewis acidity were successfully designed and synthesized via a facile one-pot evaporation induced self-assembly method. Their physical and chemical properties were characterized by different techniques including XRD, N2 adsorption–desorption, UV–Vis spectra, ICP, Py-IR, SEM and TEM. The XRD, TEM and N2 adsorption–desorption results showed that the ordered mesoporous structure can be preserved after tin incorporation. The UV–Vis, SEM and Py-IR results indicated that tin species can be homogeneously tetrahedrally incorporated in the crystalline framework of mesoporous anatase TiO2 and create the Lewis acidity. The ordered mesoporous 15Sn–TiO2 catalyst showed highest catalytic performance where cyclohexanone conversion of 91.4 % and ε-caprolactone selectivity of 93.2 %. Moreover, catalytic recycling tests demonstrated that the Sn–TiO2 catalysts exhibited high potential reusability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Baeyer–Villiger (B–V) oxidation reaction provides a method for converting ketones to their corresponding lactones or esters, which inspired great interests due to their extensive potential applications for fine chemicals, pharmaceuticals and polyesters etc. [1–4]. In the traditional industrial process, different types of explosive and corrosive peroxyacids are usually applied, which would cause a huge amount of toxic by-products resulting in the difficult separation and environmental pollution [5–7]. To meet green chemistry requirements, many efforts have been made to synthesize ε-caprolactone by B–V oxidation of cyclohexanone with hydrogen peroxide and molecular oxygen. However, molecular oxygen can act as a more suitable oxidant because it was safe and cheap as well as less corrosion to equipment [8–10].
Mesoporous materials with regular mesopores have been regarded as promising supports or catalysts because of their high surface areas and large pore volumes [11–13], and the catalytic performance in many catalytic processes, including oxidation, alkylation and esterification etc., can be promoted by the incorporation of different heteroatoms into the framework of the mesoporous materials [14]. Sn-doped microporous and mesoporous materials, such as Sn-β and Mg/Al/Sn hydrotalcite-like oxides etc. [15, 16], can create the Lewis sites, which can activate the carbonyl group of cyclohexanone [17, 18] and would act as efficient and stable heterogeneous catalyst for the Baeyer–Villiger oxidation. However, the catalytic performance would be dropped because the mesoporous framework with higher loading of tin species would be partially collapsed [19]. Therefore, it is essential to seek a suitable mesoporous material for higher incorporation of tin species, which can act as a promising catalyst in the B–V oxidation of cyclohexanone by molecular oxygen.
Mesoporous TiO2, as a typical mesoporous material, has been widely used in various catalytic reactions owing to its excellent chemical and thermal stability [20–22], and many transition metal species (Cu, Fe, Cr, etc.) can be easily and successfully incorporated because of its mesoporous framework with reducible active oxygen species [20, 23–25]. At the same time, the small influence by incorporation of tetravalent tin (Sn4+) on the mesoporous framework of TiO2 would be probably formed owing to the similar ionic radius of Sn4+ (0.069 nm) and Ti4+ (0.061 nm) as well as their equal charges. In addition, the mesoporous TiO2 can accept the lone-pair electron by its 3d empty orbitals, which can also activate the carbonyl group with nucleophilic attack [26]. Based on the above-mentioned opinions, the multicomponent mesoporous Sn–TiO2 materials via the evaporation induced self-assembly (EISA) method raised by Grosso, which has been acknowledged as the most simple and facile method for the preparation of ordered mesoporous metal oxides [27, 28], would be hopefully as a promising catalyst with higher catalytic performance in the B–V oxidation of cyclohexanone by their synergistic effect. To the best of our knowledge, this is the first report for such a one-pot synthesis of ordered mesoporous Sn–TiO2 catalyst with amount of Lewis acidity for the B–V oxidation of cyclohexanone by molecular oxygen.
Herein, a series of ordered mesoporous Sn–TiO2 catalysts are prepared by a facile one-pot EISA strategy, and the physical and chemical properties are characterized by XRD, N2 adsorption–desorption, ICP, Py-IR, UV–Vis spectra, SEM and TEM techniques. In addition, these synthesized catalysts are employed in the B–V oxidation of cyclohexanone by molecular oxygen, and the catalytic reusability is also investigated.
Experimental
Catalysts preparation
Mesoporous Sn–TiO2 catalysts are synthesized according to the literature [29]. In a typical synthesis process, 1.6 g of F127, 0.6 g of citric acid and 1.4 g of hydrochloric acid were dissolved into 30 g anhydrous ethanol. After that, 3 g of titanium isopropoxide and a demanded amount of tin tetrachloride (0, 0.18, 0.23, 0.29 and 0.35 g) were simultaneously added into the above solution and vigorously stirred at 303 K for 24 h. The solution was transferred to a Petri dish to evaporate at 318 K for 48 h and then thermally treated at 373 K for another 24 h. The catalysts was collected by calcination in air at 623 K for 5 h to remove the organic template and defined as xSn–TiO2, where x stands for the weight percent of tin species in the catalysts.
Catalysts characterization
The low-angle and wide-angle XRD patterns of the catalysts in the 2θ range from 0.75º to 5º and from 10º to 80º were obtained on a Bruker D8 instrument with Ni-filtered Cu K α radiation (λ = 0.154 nm) and operated at 40 kV, 40 mA when the scanning rate was 0.05º/s. Nitrogen adsorption–desorption isotherms were obtained using Bel Japan Inc.. The surface area was calculated by the BET method and the mesoporous size distribution was obtained by the BJH adsorption model. The weight percent of tin species was analyzed by ICP (Optima 2100 DV, PerkinElmer, USA) after the catalysts had been dissolved in HF solution. UV–Vis DRS for the catalysts were measured on Lambda 950 spectrophotometer. The wavelength range was from 200 to 800 nm and the BaSO4 was as a reference compound. FT-IR spectra of pyridine (Py-IR) were obtained on the Thermo Nicolet Nexus spectrometer in KBr pellets. The catalysts was pretreated in 10−2 Pa at 573 K for 3 h and then cooled down to room temperature. After that, pyridine was adsorbed for 2 h and the temperature was raise to 473 K for 1 h to remove the physisorbed pyridine. IR spectra were measured when the temperature of the catalyst was room temperature. The morphology of the catalysts was visualized using a JEOL JEM 2100 TEM operated at 120 kV. The catalysts were dispersed in ethanol assisted by an ultrasonic technique. The dispersion of the semi-quantitative elemental composition (Sn, Ti) was verified by energy dispersive X-ray (EDX) spectrometer in the Oxford INCA EDAX Detecting Unit.
Catalytic activity test
The catalytic performance of the synthesized catalysts were investigated in the B–V oxidation of cyclohexanone by molecular oxygen, which was carried out in a three-neck flat bottom flask equipped with a reflux condenser at atmospheric pressure. In a typical process, 0.12 g catalyst and 1.2 g benzaldehyde as pro-oxygenic agent were simultaneously added into the solution containing of 0.5 g cyclohexanone and 15 g acetonitrile as solvent. Subsequently, molecular oxygen was introduced to the reaction system at a rate of 10 mL/min. The solution was raised up to 343 K and kept for 5 h with continuous stirring. After that, the flask was cooled down to room temperature and the reaction mixture was analyzed on a SP-6890 gas chromatograph equipped with a SE-30 column (0.25 μm × 50 m) and a flame ionization detector (FID). The cyclohexanone conversion and ε-caprolactone selectivity were calculated when dodecane was the internal standard. The reuse ability of the catalyst was investigated by filtration without any treatment in the recycling tests.
Results and discussion
Catalysts characterization
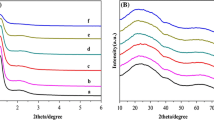
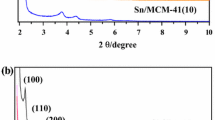
The XRD patterns for different xSn–TiO2 catalysts are shown in Fig. 1. It can be seen from Fig. 1a that compared to pure mesoporous TiO2 catalyst, one obvious diffraction peak centered at around 1.2 ° can be obviously observed for mesoporous xSn–TiO2 catalysts, suggesting their short-range ordered mesoporous characters [30]. In addition, the peak intensity for xSn–TiO2 catalysts decreased slightly with the weight percent of tin species increasing from 9 to 15 and the peak was shifted to lower angles indicating that the mesoporous structure can be kept well and the interplanar spacing increased on the basis of the Bragg equation [31]. However, the peak intensity for 18Sn–TiO2 catalyst severely decreased because of the partial destruction of ordered mesoporous structure by excess tin species probably. As can be seen from Fig. 1b, the pure crystalline anatase TiO2 was observed and the intensities of these diffraction peaks decreased by the introduction of tin species [32]. It is interesting to note that no significant characteristic peak corresponding to tin-containing compounds such as SnO2 or Sn x TiO y can be found, which may be owing to the highly dispersion of tin species or their homogeneous incorporation into the framework of mesoporous anatase TiO2 [33].
The physical properties for the synthesized xSn–TiO2 catalysts were characterized by N2 adsorption–desorption. Fig. 2 shows the N2 adsorption–desorption isotherms and pore size distribution. It can be clearly seen from Fig. 2 that a typical IV-type isotherms with well-defined H2 hysteresis loops and sharp capillary condensation steps at a relative pressure ranging from 0.40 to 0.70 for all the catalysts, which indicated that they have a uniform mesoporous system [34], whose pore sizes were narrowly around at 4–5 nm. Some physical data are listed in Table 1. The surface area, pore size and pore volume increased with the weight percent of tin species increasing from 0 to 15 because of its larger interplanar spacing proposed by the small angle XRD patterns. The drop of specific surface area for 18Sn–TiO2 catalyst may be owing to the partial destruction of the structure, which is coincident with the results of XRD patterns. In addition, the actual loading of tin species was found to be almost agreed with the targeted values based on the ICP results.
Fig. 3 is the TEM images for 15Sn–TiO2 catalyst. As can be seen from Fig. 3a, the typical regular mesoporous system was obviously observed, which was in good agreement with the small-angle XRD patterns. The mesoporous framework of anatase TiO2 is crystallized with lattices in the d-spacing of 0.346 nm from the HR-TEM image shown in Fig. 3b, which is well-matched to the 101 reflection [20]. Meanwhile, the lattice fringes 0.211 nm assigned to the Sn species can be observed [35], which can be concluded that tin species can be homogeneously incorporated into the framework of mesoporous anatase TiO2 based on the results of wide-angle XRD patterns.
In order to clarify the type or coordination state of tin species in the xSn–TiO2 catalysts, the typical UV–Vis DRS technique was employed and the results are shown in Fig. 4. It can be seen that two obvious absorption bands centered at around 220 and 310 nm for TiO2 catalyst can be observed [36]. Compared to TiO2, a red shift of absorption bands for xSn–TiO2 catalysts can be observed, which is probably owing to the reduced crystallinity by XRD analysis [37]. A new band centered at around 210 nm and no obvious absorption band centered at 400 nm corresponding to bulk SnO2 particles can be observed, suggesting that tin species would be homogeneously tetrahedral incorporated into the framework of mesoporous anatase TiO2 supports [38].
Fig. 5 is the SEM photo and election mapping of particles for 15Sn–TiO2 catalyst. It can be seen that a conchoidal morphology was obtained. The element mapping of the 15Sn–TiO2 catalyst confirmed that Sn species are homogeneously dispersed on the catalyst surface, which agreed to the XRD analysis and UV–Vis DRS spectra.
Py-IR spectra for different xSn–TiO2 catalysts are shown in Fig. 6. The bands at 1455 and 1545 cm−1 could be assigned to the effects between pyridine and Lewis and Brønsted acid sites, respectively [39]. It can be seen that all the xSn–TiO2 catalysts mainly have Lewis acid sites and it would be promoted by the tetrahedrally incorporated tin species based on the UV–Vis spectra. At the same time, the Lewis acidity increases with the weight percent of tin species increasing, which would be beneficial to their catalytic performances in the oxidation of cyclohexanone by molecular oxygen.
Catalyst performance
Table 2 shows the summary of catalytic performances of different xSn–TiO2 catalysts in the B–V oxidation of cyclohexanone by molecular oxygen. It can be seen that the ε-caprolactone cannot be found when the reaction was conducted in the absence of benzaldehyde, suggesting its extremely low oxidation capacity of molecular oxygen [40]. However, a cyclohexanone conversion of 36.2 % and ε-caprolactone selectivity of 81.9 % can be obtained when the benzaldehyde was added into the reaction system even though it was carried out without catalyst, which indicates that the benzaldehyde is essential for the B–V oxidation of cyclohexanone by molecular oxygen [41]. Further, cyclohexanone conversion of 78.3 % and ε-caprolactone selectivity of 84.2 % can be obtained for pure mesoporous TiO2 catalyst, owing to its 3d empty orbital, which can activate the carbonyl group with nucleophilic attack [26]. Both cyclohexanone conversion and ε-caprolactone selectivity would be promoted after the incorporation of tin species, which attributes to increased Lewis acid between the tin species and the TiO2 supports probably. And cyclohexanone conversion can reach maximum 91.4 %, which is higher than that of carbon materials [40], mesoporous Mg–Al mixed oxides [10], when the 15Sn–TiO2 sample was catalyst, owing to its appropriate BET surface area, pore volume and Lewis acidity probably. With the weight percent of tin species further increasing to 18 %, cyclohexanone conversion and ε-caprolactone selectivity decreased because of its loss of surface area probably even though it has excess Lewis acid amount.
Table 3 is the reusable ability for 15Sn–TiO2 catalyst in the B–V oxidation of cyclohexanone by molecular oxygen. As seen from Table 3, the conversion of cyclohexanone and selectivity of ε-caprolactone declines slightly with the reaction number increasing. Conversion of cyclohexanone 88.7 % and selectivity of ε-caprolactone 90.8 % can be obtained after repeated reaction for 5 times, indicating that the 15Sn–TiO2 catalyst has good reusable ability because it was only collected by filtration without any treatment in the next reaction number.
The possible reaction mechanism
Reaction mechanism for B–V oxidation of cyclohexanone by molecular oxygen has been widely studied and a two-step mechanism was proposed in the previous literatures [41–44]. In order to clarify the effect of benzaldehyde in the reaction system, its oxidation by molecular oxygen was carried out. The yield of benzoic acid was almost the same regardless of Sn–TiO2 catalyst (not shown here), suggesting that the negligible effect on the oxidation of benzaldehyde over Sn–TiO2 catalyst can be obtained. Therefore, based on the catalytic performance of Sn–TiO2 catalyst above, a possible mechanism was proposed and shown in Scheme 1. First, the free carbonyl radical generated from benzaldehyde, which would be attached with molecular oxygen to created perbenzoic acid intermediate. Subsequently, the peroxide intermediate reacted with the activated cyclohexanone by the Lewis acidic site on the Sn–TiO2 catalyst [17, 18] to form the ‘Criegee’ type intermediate [5], followed by rearrangement to ε-caprolactone along with benzoic acid as by-product.
Conclusions
Ordered mesoporous xSn–TiO2 catalysts with high tin species loading are designed and prepared by a one-pot EISA method and applied in the B–V oxidation of cyclohexanone by molecular oxygen in this paper. Tin species can be homogeneously tetrahedrally incorporated into the structure of ordered mesoporous TiO2 supports and it can increase the Lewis acid amount. The 15Sn–TiO2 catalyst shows highest catalytic performances, whose cyclohexanone conversion and ε-caprolactone selectivity is 91.4 and 93.2 %, respectively, and it has good reusable ability because the catalytic performance decreases slightly even after repeated reaction for 5 times. The possible mechanism was also proposed. This method provides a valuable reference of a promising catalyst for the B–V oxidation of cyclohexanone by molecular oxygen.
References
Rahman S, Enjamuri N, Gomes R, Bhaumik A, Sen D, Pandey JK, Enjamuri S, Chowdhury B (2015) Appl Catal A 505:515–523
Alegria EC, Martins LM, Kirillova MV, Pombeiro AJ (2012) Appl Catal A 443:27–32
Jiménez-Sanchidrián C, Ruiz JR (2008) Tetrahedron Lett 64:2011–2026
Zhang G, Ren X, Zhang H, Peng Y, Gui S (2015) Catal Commun 58:59–63
Renz M, Meunier B (1999) Eur J Org Chem 4:737–750
Dutta B, Jana S, Bhunia S, Honda H, Koner S (2010) Appl Catal A 382:90–98
Jeong EY, Ansari MB, Park SE (2011) Acs Catal 1:855–863
Zang J, Ding Y, Yan L, Wang T, Lu Y, Gong L (2014) Catal Commun 51:24–28
Ma Y, Liang Z, Feng S, Zhang Y (2015) Appl Organomet Chem 29:450–455
Paul M, Pal N, Mondal J, Sasidharan M, Bhaumik A (2012) Chem Eng Sci 71:564–572
Geng L, Zhang X, Zhang W, Jia M, Liu G (2014) Chem Commun 50:2965–2967
Liu J, Qiao S, Hu Q (2011) Small 7:425–443
Teng W, Wu Z, Fan J, Chen H, Feng D, Lv Y, Wang J, Asiri AM, Zhao D (2013) Energy Environ Sci 6:2765–2776
Jeenpadiphat S, Björk EM, Odén M, Tungasmita DN (2015) J Mol Catal A 410:253–259
Corma A, Nemeth LT, Renz M, Valencia S (2001) Nature 412:423–425
Jiménez-Sanchidrián C, Hidalgo JM, Llamas R, Ruiz JR (2006) Appl Catal A 312:86–94
Corma A, Navarro MT, Renz M (2003) J Catal 219:242–246
Sasidharan M, Kiyozumi Y, Mal NK, Paul M, Rajamohanan PR, Bhaumik A (2009) Micropor Mesopor Mater 126:234–244
Taralkar US, Kalita P, Kumar R, Joshi PN (2009) Appl Catal A 358:88–94
Min H, Ran X, Fan J, Su Y, Yang J, Teng W, Zhang W, Li G, Zhao D (2015) J Mater Chem A 3:7399–7405
Zhou Z, Yu P, Qin J, Wu W, Xu L, Gu Z, Liu X (2016) J Porous Mater 23:239–245
Yang Y, Tian C (2015) Res Chem Intermed 41:5271–5281
Wang T, Yang G, Liu J, Yang B, Ding S, Yan Z, Xiao T (2014) Appl Surf Sci 311:314–323
Hamdy MS (2014) J Mol Catal A 393:39–46
Wang Y, Li B, Zhang C, Cui L, Kang S, Li X, Zhou L (2013) Appl Catal B 130:277–284
Xia C, Ju L, Zhao Y, Xu H, Zhu B, Gao F, Lin M, Dai Z, Zou X, Shu X (2015) Chin J Catal 36:845–854
Grosso D, Cagnol F, Soler-Illia G, Crepaldi EL, Amenitsch H, Bruneau AB, Bourgeois A, Sanchez C (2004) Adv Funct Mater 14:309–322
Li W, Zhao D (2013) Chem Commun 49:943–946
Pan D, Guo M, He M, Chen S, Wang X, Yu F, Li R (2014) J Mater Res 29:811–819
Zhang X, Zhang F, Chan K (2005) Appl Catal A 284:193–198
Miao Z, Zhao H, Yang J, Zhao J, Song H, Chou L (2014) Micropor Mesopor Mater 198:271–280
Li W, Bai Y, Liu C, Yang Z, Feng X, Lu X, Laak NK, Chan KY (2009) Environ Sci Technol 43:5423–5428
Yu M, Li C, Zeng G, Zhou Y, Zhang X, Xie Y (2015) Appl Surf Sci 342:174–182
Morris SM, Fulvio PF, Jaroniec M (2008) J Am Chem Soc 130:15210–15216
Chen M, Yang J, Liu Y, Li W, Fan J, Ran X, Teng W, Sun Y, Zhang W, Li G, Dou S, Zhao D (2015) J Mater Chem A 3:1405–1409
Kumar N, Hazarika SN, Limbu S, Boruah R, Deb P, Namsa ND, Das SK (2015) Micropor Mesopor Mater 213:181–187
Chen G, Ji S, Sang Y, Chang S, Wang Y, Hao P, Claverie J, Liu H, Yu G (2015) Nanoscale 7:3117–3125
Pachamuthu MP, Shanthi K, Luque R, Ramanathan A (2013) Green Chem 15:2158–2166
Zhang Q, Yang H, Yan W (2014) RSC Adv 4:56938–56944
Nabae Y, Rokubuichi H, Mikuni M, Kuang Y, Hayakawa T, Kakimoto M (2013) ACS Catal 3:230–236
Kaneda K, Ueno S, Imanaka T (1994) J Chem Soc, Chem Commun 25:797–798
Murahashi SI, Oda Y, Naota T (1992) Tetrahedron Lett 33:7557–7560
Ueno S, Ebitani K, Ookubo A, Kaneda K (1997) Appl Surf Sci 121:366–371
Llamas R, Jiménez-Sanchidrián C, Ruiz JR (2007) React Kinet Catal Lett 90:309–313
Acknowledgments
This work was supported by Jiangsu Planned Projects for Post-doctoral Research Funds (1302121C); Open Project of Beijing Key Laboratory for Enze Biomass and Fine Chemicals; Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Z., Yu, Y., Yu, P. et al. Ordered mesoporous Sn–TiO2 catalysts via an evaporation induced self-assembly method for the Baeyer–Villiger oxidation of cyclohexanone by molecular oxygen. Reac Kinet Mech Cat 120, 295–305 (2017). https://doi.org/10.1007/s11144-016-1094-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-1094-6