Abstract
For a better understanding of the devolatilization characteristics of lignite, a highly volatile lignite was devolatilized at four different heating rates in a thermogravimetric analyzer in this study. The results showed that the lignite pyrolysis process was strongly affected by the heating rate. As the heating rate increased, the differential thermogravimetry peak shifted toward higher temperature. Based on thermal analysis and kinetic equations, the effects of the heating rate on the pyrolysis characteristics has been studied and the information about the linkage between the activation energy (E), pre-exponential factor (A) and weight loss of each heating rate has been analyzed simultaneously. In the pyrolysis of lignite, the activation energies of the samples were found to increase with decreasing heating rate for each temperature stages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pyrolysis is the first step in all thermochemical coal conversion processes such as coal gasification, combustion, and liquefaction [1–3] and also considered as a simple and effective way for coal cleaning conversion [4].
Studying the behavior of fossil fuels by thermal analysis during pyrolysis has gained much interest [5–8]. Thermogravimetric analysis (TGA) is one of the most important techniques used to study the thermal events and the kinetics of the pyrolysis of coal [9–14]. It provides a measurement of weight loss of the sample as a function of time and temperature [15]. Application of chemometrics to TGA data at different steps of the devolatilization is useful to design or to select optimal experimental procedures. The TGA data can also provide maximum relevant chemical information by analyzing chemical data and obtain knowledge about the chemical systems [4, 16]. Characteristic thermal analysis properties, which depend on the temperature at a defined heating rate or on the time at a constant temperature (static measurements), were studied [17] in order to evaluate the kinetic data and to establish reliable models for the complex reaction scheme of coal pyrolysis.
Studying the kinetics of coal pyrolysis is a useful method for estimating the reactivity of fossil fuels during their utilization and conversion processes and it is necessary way to develop predictive models for coal conversion processes [18]. Kinetic parameters such as activation energy and pre-exponential factor have been shown to vary with temperature, heating rate and coal type when the reaction orders were assumed to be n = 1 [15, 19]. The heating rate has been assumed to be the controlling factor when the same coal was used under same temperature [20]. The recorded ranges of the activation energy and pre-exponential factor was 0–900 kJ/mol and 0–1038 min−1, respectively [4, 15–20].
The largest lignite reservoir (77 %) of China is in Neimen province [21]. The investigations were performed on a lignite sample with a geological age of Jurassic from the deposit of Neimen province, China, which was suitable for conversion processes like liquefaction and coke making used for TGA. The kinetic parameters were extracted from TGA with differing heating rate by the application of the Arrhenius equation corresponding to the mass degradation and the kinetic parameters were obtained on the basis of pyrolysis curves and kinetic equations.
Experimental
Coal samples
Freshly mined lignite samples from China with high volatile content were used in this study. The air-dried samples were ground to 0.45–1 mm before use. The physical and chemical characteristics of coal used in this study are listed in Table 1.
TGA and DTG
A STA 409C TGA apparatus was used to analyses the TG and differential thermogravimetry (DTG). TGA experiments generate mass loss versus temperature, reflecting the thermal behavior and composition of the initial sample. About 10 mg sample was used in each experiment. The TGA system was purged firstly with high purity nitrogen. The sample was heated up from 110 to 900 °C at a constant heating rate of 10, 20, 30 and 50 °C/min. The carrier gas was N2 with a flow rate of 60 ml/min. The weight changes of the sample were recorded continuously as a function of time or temperature and the TG and DTG curves were obtained.
The rate of mass loss was recorded as a function of temperature or time. The results obtained at different heating rates were examined.
Calculation of kinetic parameters
The basic task of chemical kinetics is to study the factors that influence the reaction rate [22]. There are a number of possible approaches for modeling the complex devolatilization process. To determine the reaction rate, it is necessary to determine the change of reactant or product concentration with the reaction time. In this study, a non-isothermal method, the Costs–Redfern integral equation [19, 20] was used to obtain the kinetic information. This was done for several heating rates to derive the preexponential factor A and the activation energy E.
The pre-exponential factor, A, is assumed to be constant and independent of temperature in this model. This approach is advantageous and can be easily applied to study the kinetics of a temperature-programmed pyrolysis.
First, the lignite pyrolysis process was assumed as follows:
-
(a)
High-purity nitrogen (99.999 %) was used as protective gas and it had no influences to lignite pyrolysis;
-
(b)
It had no temperature gradient in the lignite sample interior during the pyrolysis heating process; in an infinitesimal time period, the pyrolysis non-isothermal method reaction can be seen as am isothermal method and heterogeneous reaction;
-
(c)
The Arrhenius expression was suitable for describing the lignite pyrolysis reaction:
$$ \frac{{{\text{d}}\alpha }}{{{\text{d}}t}} = kf(\alpha ) $$
where α is the pyrolysis conversion of initial material, which derived from TG curves, t is the reaction time (min), f(α) is the is the function of unreacted initial sample, k is the rate constant and it was typically correlated with temperature by an Arrhenius expression:
where A is the pre-exponential factor (min−1), E is the apparent activation energy (kJ/mol), R is the gas constant (8.314 J/mol/K), and T is the absolute temperature (K).
If the mass of sample was changed from w 0 (g) to w t (g) with the reaction time t, then the Eq. 1 can be expressed as follows:
where w 0, w t and w ∞ are the original mass (g) of the test sample, the mass (g) at time t or T and the final mass (g) at the end of pyrolysis, in order.
If the sample is heated at a constant heating rate, \( \beta = \frac{{{\text{d}}T}}{{{\text{d}}t}} \), then Eq. 1 can be expressed as follows:
Integrating the above equation gives:
According to Costs–Redfern integral equation, it can be assumed that lignite pyrolysis reaction followed the nth order reaction:
when \( \frac{E}{RT} \gg 1,\; \left( {1 - \frac{2RT}{E}} \right) \approx 1 \), then the first term of the right side in the above Eqs. 2 and 3 were almost a constant, then the plot of \( { \ln }\left[ {\frac{{{\text{d}}\alpha /{\text{d}}T}}{f(\alpha )}} \right] \) versus \( \frac{1}{T} \) gives a straight line, where E can be determined from the slope, A from the intercept and the lignite pyrolysis reaction may be obtained with reaction order of n. Thus, n can be checked by a linear correlation, the values of E and A can be acquired simultaneously.
Results and discussion
Thermal behavior
To analyze the pyrolysis characteristics, the lignite was pyrolyzed and analyzed with the TG system. The weight loss and weight loss rate curves under different heating rate are presented in Figs. 1 and 2.
It can be seen from Fig. 1 that the profiles of the TG curves of different heating rates were found to be quite different. The depth of the curves reduces with an increase in the heating rate. Gradient of the TG curves lower heating rate was faster than that in higher heating rate.
Fig. 2 shows that as the heating rate decreased, the slope of the weight loss rate curve increased before the temperature of maximum rate of weight loss, indicating that the decomposition process was a faster process while the slope of the weight loss rate curve increased with the increasing heating rate after the temperature of maximum rate of weight loss, indicating that the decomposition process was faster in higher temperature region.
From the curves shown in Figs. 1 and 2, some characteristic parameters were obtained, including the following parameters: temperature of initial weight loss (T i), temperature at the end of the reaction (T f), the maximum weight loss rates (R max) and the corresponding peak temperatures (T max) at which the T i is defined as temperature at α = 5 %, and T f is calculated at α = 85 % similarly to T i. The thermogravimetric properties of different heating rate are given in Table 2.
It can be seen that the characteristic temperatures, T max, presented higher values as heating rate increased. This effect indicates that:
-
(a)
The pyrolysis process may feature a temperature delay with the higher heating rate because it takes some time to transmit heat from the surface to the interior of the sample and release volatiles from the interior to the sample surface;
-
(b)
Some time is needed for releasing volatiles from the interior to the sample surface during the pyrolysis process. There is not enough time for releasing volatiles completely at high heating rates;
-
(c)
The reaction temperature would move to a higher value too rapidly under high heating rate, because volatiles could not released totally in such a short time.
As a consequence, high heating rate, in a manner, may hinder the pyrolysis reaction. The characteristic temperatures T i, T f and the T max shifted to higher values with increasing heating rate. The lowest heating rate, 10 °C/min, presented a maximum weight loss at approximately 25 °C below the highest heating rate, 50 °C/min, and presented a maximum weight loss in the 350–570 °C temperature range simultaneously.
The 350–570 °C temperature range has higher weight loss than the other two temperature ranges, which is close to 60 % of total weight loss in 110–900 °C. This indicates that it is adapted to obtain more pyrolysis products in low temperature lignite pyrolysis.
Kinetic analysis
It was necessary to investigate the pyrolysis kinetics to understand the thermal behavior in lignite pyrolysis.
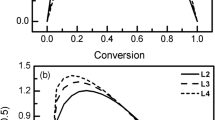
Based on the above mentioned method of “calculation of kinetic parameters” at different heating rates of 10, 20, 30 and 50 °C/min, curves between \( { \ln }\;\left[ {\frac{{ - { \ln }\;(1 - \alpha )}}{{T^{2} }}} \right]\;\left( {n\; = \;1} \right),\;{ \ln }\left[ {\frac{{1 - \left( {1 - \alpha } \right)^{1 - n} }}{{T^{2} \left( {1 - n} \right)}}} \right],\;\,\left( {n \ne \, 1} \right) \) versus \( \frac{1}{T} \) were constructed and are shown in Figs. 3, 4, 5 and 6 at the different orders of n = 1, 2 and 3.
It can be seen from the above Figs. that the kinetic curves had three different ranges and this was probably in accord with three different stages of the continuous chemical reaction in the lignite pyrolysis process. The kinetic curves gave a better correlation with the reaction orders of n = 1 and 2 than 3 and the heating rate had important influence on the kinetic curves probably due to it can change the thermo-chemical reaction route and reaction mechanism in a degree.
The apparent activation energy (E) and pre-exponential factor (A) were calculated and shown in Table 3 with reaction order of n = 2.
According to basic kinetic concepts, a variation of kinetic parameters may imply a variation in the mechanism that controls the pyrolysis process [20]. Table 3 showed that different heating rates may produce a great difference in the kinetic parameters. Moreover, it gives a variation in activation energy at different heating rates. In the lower temperature range of 150–350 °C, the value of activation energy increased with the increasing heating rate while it decreased in the higher temperature stages of 350–850 °C. This was probably because the effect of the heating rate on secondary reactions of the primary pyrolysis products [23].
The activation energy increased with the increasing temperature in every heating rate; the largest activation energy appeared in the temperature range of 570–850 °C. This means that the reaction needs more energy from the surroundings in this temperature range [15].
The activation energy is the lowest at the heating rate of 20 °C/min in the main reacting temperature range of 350–570 °C, this indicates that there was a suitable heating rate in lignite pyrolysis when we pay more attention to obtain high yield of products. Heating rate of 20 °C/min may give more products in low temperature lignite pyrolysis.
Conclusions
From the TG, DTG and kinetics analyses of the lignite pyrolysis, the following conclusions may be drawn:
The heating rate has a large influence on lignite pyrolysis, both the initial, final and the maximum value of weight loss rate temperatures shift to higher temperatures with the increase of heating rate and the most sharp devolatilization occurred at 350–570 °C and this indicates that it is adapted to obtain more pyrolysis products in low temperature lignite pyrolysis.
The kinetics depended on the heating rate in lignite pyrolysis. The apparent activation energy increased with the increasing temperature in every heating rate and the highest activation energy appears in the temperature of 570–850 °C. The activation energy is the lowest at the heating rate of 20 °C/min in the main reacting temperature range of 350–570 °C, this means that in this temperature range, it is an more easier pyrolysis process with the heating rate of 20 °C/min than the other three heating rates for low temperature lignite pyrolysis.
References
Singh S, Wu C, Williams PT (2012) J Anal Appl Pyrolysis 94:99
Park SS, Seo DK, Lee SH, Yu T-U, Hwang J (2012) J Anal Appl Pyrolysis 97:29
Jiménez F, Mondragón F, López D (2012) J Anal Appl Pyrolysis 95:164
Puja Khare P, Baruah BP, Rao PG (2011) Fuel 90:3299
Vejahati F, Xu Z, Gupta R (2010) Fuel 89:904
Kok MV (2001) Thermochim Acta 369:149
Iordanidis A, Georgakopoulos A, Markova K (2001) Thermochim Acta 371:137
Alonso MJG, Borrego AG, Alvarez D, Kalkreuth W, Menéndez R (2001) Fuel 80:1857
Pan YG, Enrique V, Luis PJ (1996) Fuel 75:412
Garcia-Perez M, Chaala A, Yang J, Roy C (2001) Fuel 80:1245
Mansaray KG, Ghaly AE (1999) Energy Sources 21:773
Teng HS, Lin HC, Ho JA (1997) Ind Eng Chem Res 36:3974
Enrico B, Federica L, Luigi P, Leonardo T (2002) Fuel 81:1041
Vuthaluru HB (2003) Fuel Process Technol 85:141
Cai JQ, Wang YP, Zhou LM, Huang QW (2008) Fuel Process Technol 89:21
Esteban M, Ariño C, Cruz JM (2006) Trends Anal Chem 25:86
Kök MV (2008) J Therm Anal Calorim 91:763
Du Z, Sarofim AF, Longwell JP (1990) Energy Fuels 4:296
Zhou L, Luo T, Huang Q (2009) Eng Convers Manag 50:705
Zhang C, Jiang X, Wei L, Wang H (2007) Eng Convers Manag 48:797
Shao J (2009) Shenhua Technol 7:17
Xiu J, Chu Z, Che Y (2002) Fuel 81:793
Arenillas A, Rubiera F, Pevida C, Pis JJ (2001) J Anal Appl Pyrolysis 58–59:685
Acknowledgments
This work was supported by the National Basic Research Program of China (Grant No. 2012CB723105), National Science & Technology Pillar Program (Grant No. 2012BAA04B03), Natural Science Foundation of China (Grant No. 21006066, 51274147) and Shanxi Provincial Natural Science Foundation (Grant No. 2011021009-2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Y., Zhang, Y., Wang, Y. et al. Thermogravimetric study of the kinetics and characteristics of the pyrolysis of lignite. Reac Kinet Mech Cat 110, 225–235 (2013). https://doi.org/10.1007/s11144-013-0586-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-013-0586-x