Abstract
In this work, we described the synthesis of a nanocatalyst, which consists of polyaniline (PANI)/multiwall carbon nanotubes (MWCNTs) and the deposited Pd nanoparticles. FT-IR, X-ray diffraction, X-ray photoelectron spectroscopy, thermogravimetric analysis (TG) and transmission electron microscopy were used to characterize the nanocatalyst. The interaction between PANI and MWCNTs have been explained according to the results of the FT-IR and XRD analysis. This complex was an efficient catalyst for the Heck reactions of acrylic acid with aryl iodides in air at low temperature (60 °C) using 0.9 mol% Pd of the catalyst. Furthermore, it also exhibited catalytic properties for the bromide and activated chlorobenzene. The yield of cinnamic acid was 71 % for the Heck reaction of acrylic acid with iodobenzene even though the complex was used nine times.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon nanotubes (CNTs), discovered by Iijima [1], have attracted considerable attention due to their unique structure, excellent mechanical and thermal properties [2–4]. Owing to their unique structure and interesting properties, CNTs are the excellent candidates for numerous applications in chemical sensors, drug delivery and catalysis [5–7]. The high surface-to-volume ratio makes CNTs ideal supporting materials for catalysts, especially in heterogeneous catalysis [8, 9]. However, there are some difficulties in dispersing metal nanoparticles on the surface of pristine CNTs because of their hydrophobic nature and poor dispensability in organic/aqueous solvents. Hence, strategies were evolved for the modification of CNTs with ionomers [10], conducting polymers [11] and non-conducting polymers [9, 12].
The palladium-catalyzed Heck reaction (Scheme 1) has received increasing attention in the last decades, as it is an excellent method for the synthesis of new carbon–carbon bonds [13, 14]. The Heck reaction based on the use of homogeneous catalysts is well understood, in which phosphine ligands are used to stabilize and activate the catalytically active Pd(0) and to prevent formation of catalytically inactive “palladium black” [15]. However, these traditional homogeneous catalyst systems have several difficulties in recovery and recycling the expensive homogeneous catalyst, which have so far limited their widespread industrial application to a large extent [16, 17]. More recently, “ligand free” catalysts using stabilized palladium nanoparticles such as palladium nanoclusters immobilized on a solid support have been widely reported [18–22] because of their excellent catalytic performance, good stability and satisfactory reusability compared to traditional homogeneous Pd(OAC)2, PdCl2 catalysts.
Based on their well-tuned physico-chemical properties, MWCNTs could be a promising support for the Pd nanoparticles. However, used as a support for metals, PANI also functions as a reducing agent and stabilizer, robust enough towards a variety of conditions. Here, we focus on the synthesis of a novel palladium nanocatalyst supported on PANI functionalized multi-walled carbon nanotubes and its efficiency in the promotion of the Heck reaction.
Experimental
Materials and equipment
IR spectra were obtained using an Avatar360 Fourier Transform Infrared FT-IR spectrometer (Nicolet Company, American). X-Ray diffraction (XRD) patterns were measured on a Philips X’Pert Pro X-ray diffractometer (Holland) (Cu Kα radiation, 2θ range 10–80°, scan step size 0.04°, time per step 0.5 s, generator voltage 40 kV, tube current 40 mA). X-ray photoelectron spectra (XPS) were measured on an AXISULTRA spectrometer (Kratos, England) using mono-Al Kα radiation. The C 1s photoelectron line was used for energy calibration and the C 1s binding energy was taken to be 284.8 eV. The thermal analysis was performed on an EXSTAR6000 (Seiko Company, Japan) thermal analysis system at a heating rate of 10 °C/min, and in nitrogen atmosphere. Transmission electron microscope (TEM) films were obtained on JEM-2010 (JEOL. Ltd, Japan, accelerating voltage 200 kV).
Acrylic acid, styrene and DMF were distilled before use. Aryl halides were obtained from Lancaster and were used as received. All other reagents were obtained from commercial sources and were used as received.
Preparation of MWCNTs–PANI
MWCNTs–COOH (c–MWCNT) was synthesized according to a previously reported method [23]. In a 1000 mL flask equipped with a condenser, pristine MWCNTs (1 g) were refluxed under stirring in the mixture of 1:3 (v/v) HNO3 and H2SO4 at 110 °C for 1 h, which was followed by dilution with deionized water and then vacuum-filtration through an organic filter paper. The filter cake was washed with deionized water several times until the pH of the filtrate reached 7. The filtered solid was then washed with acetone and THF to remove most of the water from sample and dried under vacuum for 24 h at 60 °C.
The composite of MWCNTs–PANI was synthesized via in situ chemical oxidation polymerization. c–MWCNTs were dispersed in 1.0 M HCl solutions with sonication for 1 h. Aniline monomer (the mass ratio of aniline to c-MWCNTs is 1:1) also dissolved in 1.0 M HCl solution was added to the above c-MWCNTs suspension and sonication continued for 30 min. A certain volume of 1.0 M HCl solution containing 0.125 M ammonium persulfate (APS) (the molar ratio of APS to Aniline is 1:1) was slowly added dropwise into the suspension with constant stirring at a reaction temperature of 0–5 °C for 4 h. Then the green suspension, indicating the formation of insoluble polyaniline, was filtered and rinsed several times with distilled water, methanol and acetone. The obtained powder was vacuum dried at 60 °C for 24 h.
Preparation of the Pd–MWCNTs–PANI nanocatalyst
Ethanol (25 mL) and 0.04 g PdCl2 were added to a 100 mL round-bottomed flask and stirred for 20 min. MWCNTs–PANI (0.2 g) was added to the solution, and stirred for 72 h at 60 °C. The mixture was filtered and washed with ethanol and acetone, and then dried under vacuum at 60 °C for 12 h. About 0.2 g green MWCNTs–PANI supported palladium chloride was obtained and was used as a novel catalyst for the Heck reaction.
The Pd content was 0.64 mmol/g as determined by Inductively Coupled Plasma (ICP) spectrometry. The chemical modification of MWCNTs and subsequent loading of palladium is outlined in Scheme 2.
Preparation of the Pd–MWCNTs nanocatalyst
The Pd–MWCNTs nanocatalyst was prepared similarly to the procedure mentioned above for the Pd–MWCNTs–PANI nanocatalyst using MWCNTs–COOH instead of MWCNTs–PANI.
Preparation of the Pd–PANI nanocatalyst
The Pd–PANI nanocatalyst was prepared similarly to the procedure mentioned above for Pd–MWCNTs–PANI nanocatalyst using PANI instead of MWCNTs–PANI.
Heck reaction
Typically, the Heck reaction was conducted as follows: aryl halide (1 mmol), acrylic acid or styrene (1.5 mmol), tri-n-butylamine (3 mmol, acrylic acid as reaction substrate; 1.5 mmol, styrene as reaction substrate) and 0.9 mol% Pd of Pd–MWCNTs–PANI mixed with DMF (0.2 mL) were introduced into a three-necked flask (100 mL) equipped with a reflux condenser. The reaction mixture was immersed into an oil bath at a set temperature. On completion of the reaction between the aryl halide and acrylic acid, the reactant mixture was cooled to room temperature; 25 mL H2O and 0.6 g Na2CO3 were added into the reaction system, which was stirred at room temperature for 20 min. The Pd–MWCNTs–PANI complex was recovered from the mixture by filtration, and was washed with distilled water (2 × 15 mL), ethanol (2 × 15 mL) and diethyl ether (2 × 15 mL). The pH of the filtrate was adjusted to 1–2 with dilute aqueous HCl solution. The white precipitate appeared and was filtered, washed with water several times and dried in air. Then the product was obtained and the yield of the product was calculated based on the aryl halide. At the end of the reaction between the aryl halide and styrene, the reactant mixture was cooled to room temperature and dissolved in diethyl ether (30 mL), stirred at room temperature for 20 min and subjected to filtration. Then the filtrate was treated with 3 M HCl (2 × 15 mL), brine (3 × 15 mL) and dried over MgSO4. The solid product was obtained by recrystallization from diethyl ether.
Results and discussion
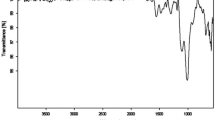
Fig. 1 shows the FT-IR spectra of MWCNTs–PANI and PANI. The spectrum of MWCNTs–PANI (a) displayed all the important characteristic bands associated with PANI (b), the most obvious change is the displacement of position. For example, the C=C stretching deformation of the quinoid (1562/cm) and benzenoid ring (1481/cm), the C–N stretching vibration (1299/cm), the C–H in plane bending vibration (1112/cm), and the C–H out of plane bending vibration (800/cm) can be observed in the spectrum of PANI [24]. Meanwhile, the MWCNTs–PANI shows characteristic peaks at 1555/cm, 1473/cm, 1293/cm, 1121cm and 804/cm, indicating the presence of interaction between MWCNTs and PANI.
Fig. 2 shows the XRD patterns of MWCNTs, MWCNTs–PANI and Pd–MWCNTs–PANI. As shown in Fig. 2a, MWCNTs exhibits a sharp, high intensity peak at 26.3° and a lower intensity peak at 42.6°, which are attributed to (002) and (100) planes of hexagonal graphite and appearance of these peaks with high intensity suggests that MWCNTs have a high graphitic structure [25]. The MWCNTs–PANI (Fig. 2b) shows peaks at 25.4 and 43.6°. A minor blue shift and lower intensity in the bands was observed for MWCNTs–PANI, which indicated that a site–selective interaction between PANI and MWCNTs occurs as a consequence of the in suit polymerization. The XRD pattern of the nanocatalyst is shown in Fig. 2c, three major diffraction peaks at 2θ of 40.6, 46.9 and 68.5° in the range of 10–80° were observed, which can be indexed to the diffraction from (111), (200) and (220) crystallographic planes of face-centered cubic palladium based on the date of JCPDS file (NO. 89-4897). The additional peak in Fig. 2c at 26.2° was attributed to MWCNTs. The result shows that the Pd nanoparticles were readily deposited on MWCNTs–PANI.
XPS analysis of the Pd–MWCNTs–PANI was performed to further characterize the catalyst. The wide XPS scan from Pd–MWCNTs–PANI (Fig. 3) shows the presence of palladium (Pd 3d) together with carbon (C 1s) derived from MWCNTs–PANI. The nitrogen (N 1s) and oxygen (O 1s) signals were due to PANI produced during the synthesis of the Pd–MWCNTs–PANI nanocomposite. Thus, the XPS date also confirmed the presence of Pd in the nanocomposite. As shown in Fig. 4, the characteristic peaks corresponding to the Pd 3d5/2 and 3d3/2 appeared at 334.1 and 339.5 eV, respectively. Both peaks originating from Pd nanoparticles exhibit perfect match with literature reported states (3d5/2 334.9 eV, 3d3/2 340.4 eV) for Pd(0)[26].
The thermal stability is necessary for the catalytic and recyclability of a catalyst, because the Heck reaction is usually carried out at high temperature. TG and DTA analyses were performed in N2 and the profiles obtained are shown in Fig. 5. TG (a) analysis shows that the complex was stable up to 250 °C. Then the complex began to lose weight due to the decomposition of the polymer layer. Thermal analysis indicates that the novel catalyst can meet the basic thermal qualifications as an efficient supported catalyst for the Heck reaction.
The surface morphologies of MWCNTs–COOH, MWCNTs–PANI and Pd–MWCNTs–PANI nanocatalyst were characterized through TEM. The morphology of MWCNTs–PANI (Fig. 6b) was obviously different from MWCNTs (Fig. 6a). The diameter of MWCNTs–PANI nanotubes was about 80–140 nm but that of MWCNTs was about 40–80 nm. In the image of Pd–MWCNTs–PANI (Fig. 6c), a core shell structure of MWCNTs at the center can be seen clearly, which indicates that the MWCNTs were wrapped by PANI chains. Fig. 6c and d also show that Pd nanoparticles were randomly dispersed on MWCNTs–PANI and some of them appeared in clusters. The average size of palladium nanoparticles is in the range of 4–9 nm.
In order to evaluate the catalytic performance of Pd–MWCNTs–PANI, the Heck reactions of aryl halides and olefins were studied. The influences of reaction temperature, reaction time and amount of catalyst on the catalytic properties of Pd–MWCNTs–PANI were systematically investigated by using the coupling of iodobenzene and acrylic acid as a model reaction.
The reaction temperature has a significant effect on the Pd–MWCNTs–PANI activity. As shown in Table 1, with the temperature increase from 60 to 100 °C, the yield increased (entries 1–5). The catalyst remained active at as low a temperature as 60 °C, an acceptable yield of 63 % was obtained. However, the yield did not increase any more above 90 °C. The coupling yield was found to increase with reaction time (entries 6, 7, 4, 8). The influence of the amount of catalyst was studied in the next step (Table 2). It was found that the optimum amount of catalyst was 0.9 mol% Pd.
These optimized reaction conditions were applied in the Heck reactions of different types of aryl halides with acrylic acid and styrene. The experimental results are summarized in Table 3. As shown by entries 1–8 in Table 3, aryl iodides with either electron-withdrawing or electron-donating substituents reacted with acrylic acid and styrene at 90 °C and resulted in the corresponding coupling products in excellent yields after 7 h.
Furthermore, more challenging couplings with aryl bromides and aryl chlorides were investigated. The coupling reactions of aryl bromides with acrylic acid or styrene required higher temperatures and nitrogen atmosphere because of the relatively high bond energy of the C–Br bond. It can be seen from Table 4 that the Heck arylations of acrylic acid or styrene with aryl bromides can be efficiently carried out at 120 °C in nitrogen atmosphere to afford the product in high yield (Table 4, entries 1, 3–8) except for 4-bromotoluene with acrylic acid (Table 4, entry 2).
The ideal substrates for coupling reactions are aryl chlorides, since they tend to be cheaper and more widely available than their bromide or iodide counterparts. Few supported Pd catalysts have sufficient activity for the coupling reactions of aryl chlorides [27, 28]. However, Pd–MWCNTs–PANI can catalyze the reactions of aryl chlorides with electron-withdrawing groups (Table 4, entry 11, 12). This catalyst shows no activity with chlorobenzene substrates (Table 4, entry 9, 10).
The possibility of recycling and reusing the catalyst was a very important theme and made them useful for commercial applications. Pd–MWCNTs–PANI complex was recovered from the mixture by filtration, and was washed with distilled H2O (2 × 15 mL), ethanol (2 × 15 mL) and diethyl ether (2 × 15 mL). Thus, we investigated the recovery and reusability of the Pd–MWCNTs–PANI using the model reaction of iodobenzene with acrylic acid. It can be seen from Table 5 that the catalytic activity of Pd–MWCNTs–PANI was almost unchanged after seven times of recovery. The Pd content of the catalyst after the first cycle reuse is 0.61 mmol/g as determined by ICP spectrometry. This suggested that a slight amount of Pd leached from the catalyst as the reaction proceeded. In the subsequent cycles, the catalytic activity of this catalyst decreased and the catalyst weight began to decrease observably, which could probably be attributed to the leaching of palladium and the decomposition of the PANI layer in the reaction process. This shows that Pd–MWCNTs–PANI may have practical utility.
Finally, the performance of the Pd–MWCNTs–PANI catalyst was compared with Pd–MWCNTs and Pd–PANI catalysts by the model reaction under identical conditions. The yield of trans-cinnamic acid in the presence of Pd–MWCNTs–PANI was 94 % when the reaction was carried out for 7 h, while the yield was only 71 and 81 % in the presence of Pd–PANI and Pd–MWCNTs under the same conditions. There was no trans-cinnamic acid produced in the presence of Pd–MWCNTs when bromobenzene and acrylic acid were used as model substrates. Consequently, the combination of PANI and MWCNTs provides multiple binding sites, which are ideal platforms for the better immobilization of Pd nanoparticles and results in an enhanced performance.
Conclusion
We have prepared a nanocomposite of MWCNTs–PANI via in situ polymerization. There was a strong interaction between PANI and MWCNTs, which was demonstrated by FT-IR and XRD. The MWCNTs–PANI was effectively employed as a substrate for the deposition of Pd nanoparticles. The resulting Pd–MWCNTs–PANI was used for the Heck coupling reactions of aryl halides with alkenes and found to be effective. Moreover, the catalyst can be easily separated and recovered from the reaction mixture by filtration and reused many times.
References
Iijima S (1991) Nature 354:56
Dai HJ, Hafner JH, Rinzler AG, Colbert DT, Smalley RE (1996) Nature 384:147
Baughman RH, Zakhidov AA, de Heer WA (2002) Science 297:787
Odon TW, Huang J, Kim P, Lieber CM (1998) Nature 391:62
Kong J, Franklin NR, Zhou C, Chaplines MG, Peng S, Cho K (2000) Science 287:622
Miyako E, Nagata H, Hirano K, Makita Y, Hirotsu T (2007) Nanotechnology 18:475103
Dieckmann G, Dalton A, Johnson P, Razal J, Chen J, Giordano G, Munoz E, Musselman I, Baughman R, Draper R (2003) J Am Chem Soc 125:1770
Song S, Yang H, Rao R, Liu H, Zhang A (2010) Appl Catal A 375:265
Neelgund GM, Oki A (2011) Appl Catal A 399:154
Qiu B, Lin Z, Wang J, Chen Z, Chen J, Chen G (2009) Talanta 78:76
Sheng Q, Zheng J (2009) Bioelectron 24:1621
Ragupathy D, Gopalan AI, Lee KP (2009) Electrochem Commun 11:397
Beletskaya IP, Cheprakov AV (2000) Chem Rev 100:3009
Polshettiwar V, Molnar A (2007) Tetrahedron 63:6949
C. Amatore, A. Jutand, Acc (2000) Chem Res 33: 314
Clark JH, Macquarries DJ (2000) Green Chem 2:53
Yamada MA, Takeda K, Takahashi H (2004) Tetrahedron 60:4097
Kalbasi RJ, Mosaddegh N (2012) Mater Res Bull 47:160
Islam RU, Witcomb MJ, Mallick K (2010) Catal Commun 12:116
Mondal J, Modak A, Bhaumik A (2011) J Mol Catal A Chem 350:40
Zhu J, Zhou JH, Zhao TJ (2009) Appl Catal A Gen 352:243
Xu Y, Xue M, Li JJ, Zhang LJ, Cui YC (2010) Reac Kinet Mech Cat 100:347
Gao C, Vo CD, Jin YZ, Li WW (2005) Macromolecules 38:8634
Mi HY, Zhang XG (2007) Electrochem Commun 9:2859
Neelgund GM, Oki A (2011) J Nanosci Nanotechnol 11:3621
Sun DP, Yang JZ, Li J, Xu X, Yang X (2010) Appl Surf Sci 256:2241
Selvakumar K, Zapf A, Beller M (2002) Org Lett 4:3031
Prockl S, Kleist W, Gruber MA, Kohler K (2004) Angew Chem Int Ed 43:1917
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nie, G., Zhang, L. & Cui, Y. Preparation of Pd nanoparticles deposited on a polyaniline/multiwall carbon nanotubes nanocomposite and their application in the Heck reaction. Reac Kinet Mech Cat 108, 193–204 (2013). https://doi.org/10.1007/s11144-012-0506-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-012-0506-5