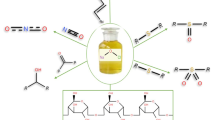
Abstract
The oxidation of thioanisole to its sulfone with hydrogen peroxide (H2O2) in the presence of acetic acid and Amberlyst 15 was investigated and found to be a simple and effective method. Oxidation experiments in the absence of acetic acid or Amberlyst 15 confirmed the essentiality of these components for the complete oxidation of thioanisole to its sulfone with H2O2. In the two-step oxidation process of sulfide, in the oxidation of sulfide to sulfoxide, H2O2 plays a major role, whereas in the oxidation of sulfoxide to sulfone, peracetic acid formed with H2O2 in the presence of acetic acid and Amberlyst 15 plays a major role. Sulfone formation increased with an increase in H2O2, temperature and Amberlyst 15 and decreased with acetic acid. However, with a very low amount of acetic acid, sulfone formation decreased due to water in H2O2 and released in the reaction. Reutilization of Amberlyst 15 for six cycles resulted in a 6.8 % decrease in sulfone yield and 3.4 % decrease in oxygenation. Dialkyl, dibenzyl, diphenyl, alkylaryl, arylbenzyl, alkylbenzyl sulfides are completely oxidized with this oxidation system to their corresponding sulfones. The reactivity of sulfides is in the order dialkyl > dibenzyl > diphenyl sulfides, which is in line with their order of nucleophilicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxidation of organic sulfides is the most utilized method to produce sulfoxides and sulfones, which is drawing continuous attention in chemical research and development due to the importance of these products as synthetic intermediates to produce biologically active molecules, including asymmetric oxidation products used in high value pharmaceuticals [1–7]. This method is also useful to remove sulfur compounds from petroleum fuels by oxidizing and separating (oxidative desulfurization) [8, 9], to make large scale solvents for metallurgy [10], to improve the electronic properties of oligothiophenes [11], to de-odor sulfur compounds from waste water streams [12] and oils [13], to study the oxygenation properties of oxidation systems [14, 15], etc.
The importance of the reaction forced the development of many oxidation methods for sulfides using a variety of reagents, mainly stoichiometric oxidants like permanganate [16], sodium bromate [17], periodic acid [18], HOF–CH3CN [11], perbenzoic acid [19] meta-chloroperbenzoic acid [20], sulfonic peracids [21], and many more reagents [22] have also been used which are not environment friendly. Increasing environmental concerns raised the interest to develop environmentally benign, simple, selective, economical methods using environment friendly and cheap oxidants like molecular oxygen (O2) and hydrogen peroxide (H2O2). O2 is one of the cheap and abundantly available environment friendly oxidants. However, it needs metal catalysts [23] like osmate [24] and ruthenium complexes [25] or needs to be used along with sacrificial agents like aldehyde [9, 26].
H2O2, which produces water as a byproduct is a green oxidant with effective oxygen content, low cost, safe use and storage. Even though H2O2 was used as such without the help of any activators for the oxidation of sulfides [10, 27], it needs longer reaction time and mostly forms sulfoxides. To overcome this limitation, H2O2 was used in the presence of metal catalysts or acids etc. for the effective oxidation of sulfides.
H2O2 was used along with transition metal catalysts like Ce(IV) triflate [28], tetra-(tetraalkylammonium)octamolybdate [29], for the selective oxidation of sulfides to sulfoxides, titania [30], Mo(VI) [31], tungstate complexes [32], for the oxidation of sulfides to sulfones, and tantalum [33], manganese complexes [34], zirconium chloride [35], methyltrioxorhenium [36], V, Bi doped heteropolyacids (H3PMo12O40) [37] for the oxidation of sulfides to sulfoxides or sulfones, depending on the conditions. However, the problem with the use of transition metal catalysts in oxidations with H2O2 is decomposition, which demands large excess of H2O2. Difficulty in the preparation and recovery, the high cost and toxic nature of the metal complexes further prompted metal free oxidation using H2O2 in organic synthesis. Acids like acetic acid [38] and Amberlite IR120 H [39] were used along with H2O2 as metal free catalysts to oxidize sulfides to sulfoxides.
H2O2 can be used with lower carboxylic acids for in situ generation of percarboxylic acids, which are effective oxidants. Formic acid with H2O2 constitutes an effective oxidation system, which generates performic acid very fast [40]. Since formic acid is more corrosive and poses problems in oxidation as well in recovery equipment, it is advantageous to use acetic acid instead of formic acid. The generation of peracetic acid by reacting acetic acid and H2O2 is usually done in the presence of sulfuric acid as a homogeneous catalyst [41], and this methodology was also used for sulfide oxidation [42, 43]. Disadvantages like corrosion in the use of sulfuric acid in the processes led to the use of heterogeneous solid acid catalysts [44, 45] for peracetic acid generation. Yazu et al. [43] used Amberlyst 15 with H2O2 and acetic acid to oxidize sulfides in petroleum fuels. Amberlyst 15 is a commercially available, macro-porous resin acid catalyst with strong sulfonic acid groups and is suitable for both aqueous and non-aqueous media and can be removed from the solvent simply by filtration.
Herein, we describe the complete conversion of sulfides to sulfones with H2O2 in the presence of acetic acid and Amberlyst 15 as an eco-friendly, metal free and reusable catalytic oxidation system.
Experimental
Materials
Thioanisole (99 %) and benzylphenyl sulfide (99 %) were purchased from Sigma Aldrich, Amberlyst 15 (acidity, 4.7 meq/g) was purchased from Fluka, H2O2 (50 wt%) was purchased from RFCL Limited, India. Acetic acid (99.8 %) was purchased from Merck Limited, India.
Typical method for the oxidation of sulfides
The oxidation reaction was carried out in a 25 ml round bottom flask taking 5.7 ml (98 mmol) acetic acid, 1 mmol thioanisole, and 56 mg Amberlyst 15 and kept on a magnetic stirrer with water bath at 50 °C. After the desired temperature was attained, 3.53 mmol (0.24 ml) of H2O2 was added while stirring (850 rpm). The progress of the reaction was monitored by analyzing the sulfide and its reaction products using gas chromatography (GC, Chemito 1000) connected with flame ionization detector (FID) using a 15 m long RESTEK MXT-1 capillary column with 0.25 mm inner diameter. The GC oven was operated with 60 °C initial temperature, 2 min of holding time and an increase in temperature of 10 °C/min till 250 °C. After the completion of the reaction, the reaction mixture was cooled and filtered to remove the solid Amberlyst 15. The sulfone was extracted twice with chloroform by adding some water using a separating funnel. The chloroform layer was washed with water, dried on sodium sulfate and the solvent was evaporated on a rotary evaporator to obtain the sulfone, which was identified by GC–MS (HP 5972 MS detector coupled with HP 5890 series II) at 70 eV. Similarly, the oxidation of different sulfides was carried out under the above mentioned conditions.
Reutilization of Amberlyst 15 catalyst
The oxidation of thioanisole was carried out as mentioned in “Typical method for the oxidation of sulfides”. After observing the completion of the reaction, liquid contents were removed from the flask and Amberlyst 15 was washed with acetic acid, acetone and dried. This dried catalyst was reused in a second cycle for the oxidation of thioanisole and a similar procedure was continued for six cycles.
Results and discussion
Oxidation of thioanisole
Oxidation with H2O2 in the presence of acetic acid and Amberlyst 15
The oxidation of thioanisole was carried out with H2O2 in the presence of acetic acid and Amberlyst 15 at 50 °C and the concentration of sulfide, and its oxidation products (sulfoxide, sulfone) with time are shown in Fig. 1. These results show that the concentration of thioanisole decreased to 17 % within 10 min (83 % conversion) forming 65.8 % sulfoxide and 17.2 % sulfone. Later, sulfide and sulfoxide slowly disappeared and completely converted to sulfone in 70 min. In the two-step oxidation process of sulfide, initially it becomes sulfoxide (intermediate) and then in a consecutive step gets converted to sulfone (Scheme 1). Keeping the above fact in view, it is understood that the decrease in sulfide concentration is directly proportional to sulfoxide formation. As seen from Fig. 1, the decrease in thioanisole is not equal to the increase in its sulfone with time, the difference is due to the build-up of thioanisole sulfoxide, which is the intermediate compound. It indicates that thioanisole conversion to its sulfoxide is faster than that of sulfoxide conversion to sulfone.
It is well known that peracetic acid is prepared using acetic acid and H2O2 in the presence of strong acid catalysts like sulfuric acid to accelerate this equilibrium reaction [46]. The liquid acid catalyst can be replaced by solid acid catalysts to get advantages like easy separation, less corrosion and reusability [44, 45]. Here, Amberlyst 15 acts as a solid acid catalyst to facilitate the in situ generation of peracetic acid from acetic acid and H2O2. Peracetic acid is a strong oxidant (oxidation potential 1.81 eV) and transfers one oxygen atom to substrate. In sulfide oxidation, one molecule of peracetic acid oxidizes sulfide to sulfoxide and becomes acetic acid and similarly another molecule of peracetic acid oxidizes sulfoxide to sulfone (Scheme 1).
Thioanisole, acetic acid and aqueous H2O2 exist as one liquid phase and Amberlyst 15 as the solid phase in the reaction mixture. The reaction was conducted by varying the speed of magnetic stirrer from 100 to 850 rpm to check the mass transfer limitation for the formation of sulfone at 55 min. It is found that the maximum formation of sulfone reached at 250 rpm. The mass transfer limitation below this rpm may be due to poor interaction of phases.
Oxidation with H2O2 in the absence of acetic acid or Amberlyst 15
To find out the essential role of acetic acid and Amberlyst 15, which are participating in catalytic cycle in the oxidation of thioanisole to its sulfone, the reaction was carried out with H2O2 in acetic acid without Amberlyst 15 and also in methanol in the presence of Amberlyst 15. Data in Table 1 reveal that oxidation of thioanisole with H2O2 in acetic acid (in the absence of Amberlyst 15) lead to the fast conversion of sulfide (99.7 %) within 10 min with marginal (3.7 %) selectivity to sulfone. Even after a long reaction time of 50 min, it is increased to only 5 %. Golchoubian et al. [38] reported the oxidation of sulfides to sulfoxides with excess H2O2 in acetic acid without a catalyst. They also reported that dry H2O2 in glacial acetic acid does not generate peracetic acid on standing. Similarly, here also it is observed that conversion of sulfide to sulfoxide is higher with H2O2 in acetic acid.
The oxidation of thioanisole with H2O2 in methanol in the presence of Amberlyst 15 (in the absence of acetic acid) gave 91.5 % sulfide conversion in 50 min and a major portion remained as sulfoxide (89.8 % selectivity). Maggi et al. [39] reported the oxidation of thioanisole to its sulfoxide using one equivalent H2O2 in the presence of acid catalyst, Amberlite IR120 H, in methanol. In our studies with excess H2O2, it is expected to achieve more oxidation to sulfone but the observed conversion to sulfone is marginal.
In both the above cases, in the absence of peracetic acid, only H2O2 is acting as oxidant and conversion of sulfide to sulfoxide is quite high. As mentioned by Golchoubian et al. [38], H2O2 may not be protonated under these conditions but polarized and act as an electrophile to react with the electron rich nucleophilic sulfide and form the sulfoxide. Further oxidation of the sulfoxide to sulfone is not favored due to the decreased nucleophilicity of sulfoxide compared to that of sulfide [34] due to which the reaction becomes slow and marginal sulfone formation is observed. These experiments show that both acetic acid and Amberlyst 15 are required along with H2O2 to oxidize sulfide completely to sulfone within shorter reaction time (Fig. 1). It indicates that acetic acid is essential to achieve complete conversion of thioanisole to its sulfone by participating in the formation of peracetic acid.
The outer peroxidic oxygen of peracetic acid has non-bonding electrons and it can also react with polarizable softer nucleophiles [47]. Sulfoxides having less nucleophilicity than sulfides can be attacked to transfer oxygen and leaving acetic acid. The interaction under acidic conditions with sulfides and sulfoxides may be through outer peroxidic oxygen in a cyclic transition state and transferring oxygen to sulfur by braking outer peroxidic oxygen bonds with hydrogen and oxygen [48].
In the oxidation of thioanisole with H2O2 in the presence of acetic acid and Amberlyst 15 (Fig. 1), the initial fast conversion of sulfide to sulfoxide was observed when the expected availability of H2O2 is higher than that of peracetic acid. However, with time, the formation of peracetic acid increases and its participation in the oxidation of sulfide and sulfoxide becomes more significant. Due to this, a rapid decrease in sulfoxide and increase in sulfone is seen after 10 min of reaction time.
Overall, in the oxidation of thioanisole to its sulfone with H2O2 in the presence of acetic acid and Amberlyst 15, H2O2 and peracetic acid are involved as oxidants in both steps of sulfide oxidation. Under these reaction conditions, in the oxidation of sulfide to sulfoxide, a major role is played by H2O2 and in the oxidation of sulfoxide to sulfone, a major role is played by peracetic acid.
In order to optimize reaction conditions as well as to understand the effect of the components in this oxidation system, thioanisole oxidation was done by varying the quantities of Amberlyst 15, H2O2, acetic acid, and changing the temperature of the reaction.
Effect of Amberlyst 15
The effect of Amberlyst 15 on the oxidation of thioanisole was studied by varying its quantity and results are presented in Fig. 2. It shows the sulfide (Fig. 2a) and sulfone (Fig. 2b) present (in %) in the reaction mixture with time for different quantities of Amberlyst 15. It is evident from Fig. 2 that the absence of Amberlyst 15 leads to maximum sulfide conversion but with marginal sulfone formation whereas its presence in the reaction system leads to quantitative sulfone formation with increasing reaction time. It is also observed that an increase in the quantity of Amberlyst 15 from 0 to 224 mg resulted in increased sulfone formation.
Fig. 3 shows sulfide conversion, sulfone formation and oxygenation (total oxygen transfer for sulfoxide and sulfone at that time as % of estimated oxygen transfer for complete sulfone formation) in 10 min reaction time with varying quantities of Amberlyst 15. The increase in sulfone formation with an increasing amount of Amberlyst 15 is attributed to an increase in peracetic acid formation. Unlike in the oxidative desulfurization of fuel using H2O2 and acetic acid, where it forms two liquid phases [43] (non polar fuel and polar acetic acid), here, in acetic acid, sulfide and H2O2 are miscible and form one liquid phase, and Amberlyst 15 remains as a solid phase. The Amberlyst 15 has a macro reticular pore structure, which makes the hydrogen ion sites (strongly acidic sulfonic acid groups) located throughout the bead more accessible for liquid reactants. Increasing its quantity further increases its interaction with reactants to form more peracetic acid, thus enhancing its performance.
Sulfide conversion is even decreased with an increase of Amberlyst 15 from 0 to 56 mg and increased with a further increase of Amberlyst 15. Since sulfide conversion mainly depends on H2O2, the initial decrease in sulfide conversion may be due to a decrease in its availability as it is also used in peracetic acid formation. However, higher amounts of Amberlyst 15 cause the rapid formation of peracetic acid and further promote the rapid oxidation of sulfide as well as sulfoxide. It is also seen that % oxygenation is not increased while increasing the amount of Amberlyst 15 from 0 to 0.56 mg, but the increase in % oxygenation is observed to be consistent with a further increase of Amberlyst 15.
Effect of H2O2
To study the effect of H2O2 on the oxidation of thioanisole, it was varied from 0.88 mmol (0.06 ml) to 5.29 mmol (0.36 ml) (sulfide to H2O2 molar ratios 1:0.88, 1:1.76, 1:3.53 and 1:5.29) in the reaction and results are shown in Fig. 4. The results clearly show that when H2O2 is taken in less than equimolar quantity (0.88 mmol), only sulfoxide was formed and a very high efficiency of H2O2 utilization, 95 % at 160 min and 83.9 % at 70 min was observed. An increase in H2O2 resulted in an increase in sulfide conversion (Fig. 4a) as well as sulfone formation (Fig. 4b). For the complete oxidation of sulfide to sulfone in 70 min, it is observed that a minimum of 3.53 mmol H2O2 is required (56.8 % efficiency of H2O2 utilization).
Sulfide conversion, sulfone, oxygenation and efficiency of H2O2 utilization are shown in % at 40 min reaction time in Fig. 5. It also shows that with an increase in H2O2 sulfide conversion, sulfone formation and oxygenation increase continuously. Unlike Amberlyst 15, H2O2 is required in both the steps of sulfide oxidation as shown in Scheme 2. Hence, it affected sulfide conversion, sulfone formation and oxygenation similarly. However, using a very high ratio of H2O2 did not yield higher results in the same proportion. It can be seen from the H2O2 utilization efficiency in Fig. 5, which indicates that an increase in H2O2 addition decreases its efficiency.
Effect of acetic acid and water
Since acetic acid is acting as the solvent as well as participating in the catalytic cycle in the oxidation of thioanisole, the effect of its quantity was studied by using 11.4 ml (196 mmol), 5.7 ml (98 mmol) and 2.85 ml (49 mmol) acetic acid. Results in Fig. 6 show that upon a decrease of acetic acid quantity from 11.4 to 2.85 ml, sulfide conversion is increased (Fig. 6a). It is due to decrease in acetic acid quantity for the same quantity of other contents in the reaction flask, which increases the concentration of reactants, resulting in an increase in the rate of the reaction. The same effect is also expected to increase sulfone formation (Fig. 6b). Results show that a decrease in acetic acid from 11.4 to 5.7 ml increased sulfone formation but a further decrease to 2.85 ml resulted in a little bit of decrease in sulfone formation. Since it is known that the dilution of acetic acid by water leads to the hydrolysis of peracetic acid [46], the observed decrease in sulfone may be attributed to the dilution effect of water present in H2O2 and released from it during the reaction. Even though the same quantity of water may be present in all three experiments, its dilution effect is expected to be more in the presence of a small amount of acetic acid. In this quantity of acetic acid (2.85 ml), the effect of water may be overriding the effect of increase in the concentrations due to the decrease in acetic acid and resulting in a decrease in the formation of sulfone. Hence 5.7 ml acetic acid may be the optimum amount for maximum sulfone formation.
To further confirm whether water decreases sulfone formation, the reaction was conducted by adding 0.24 ml water to 2.85 ml of acetic acid. It shows that dilution by water did not decrease the sulfide conversion, whereas it has drastically decreased the sulfone formation. Dilution by water decreases peracetic acid, which is playing a major role in the oxidation of sulfoxide to sulfone, hence it decreased the sulfone formation. It also indicates that, as said before, the oxidation of sulfide to sulfoxide is occurring with species other than the peracid, i.e. H2O2. So, the addition of a small amount of water did not decrease the sulfide conversion, moreover, it was increased a little bit. This small positive effect on sulfide conversion may be due to the higher polarity of reaction media after addition of water.
Oxygenation with time for experiments using 2.85 ml, 11.4 ml acetic acid and 0.24 ml water in 2.85 ml acetic acid is shown in Fig. 7. A decrease in acetic acid from 11.4 to 2.85 ml increased oxygenation more initially due to the increase of sulfide conversion to sulfoxide. Then the difference between these plots is decreased where sulfoxide oxidation to sulfone mostly occurs with peracetic acid. Even though the increase in the concentrations of contents contributes positively in both the experiments, the negative effect of water may be the reason for the decrease in oxygenation at lower volumes of acetic acid. When 0.24 ml of water was added to 2.85 ml acetic acid, the oxygenation plot followed almost same trend as that of acetic acid without water till 10 min, where sulfide to sulfoxide conversion occurs. It even increased a bit due to little more sulfide conversion as shown in Fig. 6, which may be due to the increased polarity of reaction media because of added water. After 10 min of reaction time, oxygenation decreased with the addition of water, where sulfone formation is occurring. It is clearly seen that water slows down the oxidation of sulfoxide to sulfone by slowing down the formation of peracetic acid.
Effect of temperature
To study the effect of temperature, the reaction was carried out at 40, 50 and 60 °C and results are shown in Fig. 8. The sulfide oxidation (Fig. 8a) as well as sulfone formation (Fig. 8b) is increased drastically with an increase in temperature from 40 to 50 °C but less of an increase is observed when the temperature is further increased from 50 to 60 °C. The complete conversion of sulfide to sulfone could be achieved even at 50 °C in reasonable time (70 min), which may be considered as the suitable temperature for the reaction. The plot of ln(1 − XA) versus reaction time is shown in Fig. 9 for different temperatures and the rate constant (k) values for sulfide conversion at temperatures 40, 50 and 60 °C are 0.031, 0.070 and 0.125 min−1. The reaction seems to be pseudo-first order and the activation energy from the Arrhenius plot based on the obtained k values is found to be 60.49 kJ/mol (Fig. 10).
Reutilization of Amberlyst 15 catalyst
The oxidation of thioanisole was carried out with H2O2 in the presence of acetic acid and Amberlyst 15 at 50 °C. After the completion of the reaction, the catalyst was washed, dried and reused for the oxidation of thioanisole. This procedure was continued till six cycles, since sulfide is completely converted in all cases, sulfone present and oxygenation in % at 70 min reaction time are shown in Fig. 11. It shows that there is 100 % sulfone formation till the second cycle, which indicates 100 % oxygenation and thereafter it slowly decreased as some sulfoxide remains at 70 min reaction time. At the end of the sixth cycle, 93.2 % sulfone (6.8 % sulfoxide) was present, which indicates that 96.6 % oxygenation occurred with a quantitative recovery of catalyst. It shows that the activity decrease due to leaching is not considerable.
Oxidation of organic sulfides
Apart from thioanisole (alkylaryl sulfide), other organic sulfides such as dialkyl, diaryl, dibenzyl, alkylbenzyl, arylbenzyl sulfides were oxidized to their sulfones using H2O2 in the presence of acetic acid and Amberlyst 15 at 50 °C. Results are presented in Table 2. Results show that all the sulfides were converted to their sulfones in the mentioned reaction times and established that H2O2 in the presence of acetic acid and Amberlyst 15 is an effective and simple oxidation system for the oxidation of organic sulfides to their sulfones. After the completion of the reaction, Amberlyst 15 was filtered out and sulfone was extracted twice with chloroform by adding some water. The chloroform layer was washed with water and dried on sodium sulfate. The solvent was evaporated on a rotary evaporator to obtain the sulfones, which were identified by GC–MS.
Dibutyl sulfide was converted to sulfone quantitatively within 40 min. Similarly, other symmetrical substrates like dibezyl sulfide were converted in 70 min and diphenyl sulfide took a long time, i.e. 90 min. This shows that the general reactivity order of sulfides is dialkyl > dibenzyl > diaryl. This order is in line with the electron donating nature of the substituents, the sulfide with more electron donating groups attains more nuclephilicity and is more prone to oxidation. Similarly, the reactivities of unsymmetrical sulfides with a combination of substituents like benzylalkyl, benzylaryl, alkylaryl etc. are intermediate of those of symmetrical sulfides.
Conclusions
The oxidation of thioanisole to its sulfone was carried out with H2O2 in the presence of acetic acid and Amberlyst 15 and found to be a simple and effective method. It is evident that H2O2 plays a major role in sulfide conversion to its sulfoxide, and peracetic acid formed with acetic acid and H2O2 in the presence of acid catalyst Amberlyst 15 plays a major role in sulfoxide conversion to sulfone. Sulfone formation increases with H2O2, temperature and Amberlyst 15 and decreases with acetic acid. However, with very low amounts of acetic acid, sulfone formation decreased due to the decrease in peracetic acid by water in H2O2 and released in the reaction. Reutilization of the catalyst for six cycles resulted in 6.8 % decrease in sulfone formation and 3.4 % decrease in oxygenation. Other organic sulfides like dialkyl, dibenzyl, diphenyl alkylaryl, arylbenzyl, alkylbenzyl sulfides are completely oxidized to their sulfones with this system. The reactivity of sulfides is in the order dialkyl > dibenzyl > diphenyl sulfides, which is in line with their nucleophilicity order.
References
Patai S, Rappoport Z, Stirling CJM (eds) (1988) The chemistry of sulphones and sulphoxides. Wiley, New York
Patai S, Rappoport Z (1994) The synthesis of sulphones, sulphoxides and cyclic sulfides. Wiley, New York
Page PCB (1995) Organosulfur chemistry: synthetic aspects. Academic Press, London
Kagan HB, Luukas T (1998) In: Beller M, Bolm C (eds) Transition metals for organic synthesis, 2nd edn. Wiley, Weinheim, pp 361–373
Jackson DA, Rawlinson H, Barba O (2001) R Soc Chem 260:47–53
Simpkins NS (1993) Sulphones in organic synthesis. Pergamon, Oxford
Legros J, Dehli JR, Bolm C (2005) Adv Synth Catal 347:19–31
Otsuki S, Nonaka T, Takashima N, Qian W, Ishihara A, Imai T, Kabe T (2000) Energy Fuels 14:1232–1239
Rao TV, Sain B, Kafola S, Nautiyal BR, Sharma YK, Nanoti SM, Garg MO (2007) Energy Fuels 21:3420–3424
Sharipov AKh (1997) Chem Techol Fuels Oils 33:125–137
Shefer N, Rozen S (2010) J Org Chem 75:4623–4625
Sharma A, Singh B, Saxena A (2009) Carbon 47:1911–1915
Sahle-Demessie E, Devulapelli V (2008) Appl Catal B Environ 84:408–419
Ricoux R, Allard, Dubuc M, Dupont C, Marechal JD, Mahy JP (2009) Org Biomol Chem 7:3208–3211
Franke A, Fertinger C, Van Eldik R (2008) Angew Chem Int Ed 47:5238–5242
Gokel GW, Gerdes HM, Dishong DM (1980) J Org Chem 45:3634–3639
Shaabani A, Behnam M, Rezayan AH (2009) Catal Commun 10:1074–1078
Xu L, Cheng J, Trudell ML (2003) J Org Chem 68:5388–5391
Paybarah A, Bone RL, Corcoran WH (1982) Ind Eng Chem Process Des Dev 21:426–431
Kubota A, Takeuchi H (2004) Org Process Res Dev 8:1076–1078
Kluge R, Schulz M, Liebsch S (1996) Tetrahedron 52:5773–5782
Hudlicky M (1990) Oxidations in organic chemistry, ACS monograph 186. ACS, Washington DC, pp 252–259
Mashkina AV (1990) Catal Rev Sci Eng 32:105–161
Choudary BM, Reddy CV, Prakash BV, Kantam ML, Sreedhar B (2003) Chem Commun 6:754–755
Lee HB, Ren T (2009) Inorg Chim Acta 362:1467–1470
Rao TV, Sain B, Kumar K, Murthy PS, Prasada Rao TSR, Joshi GC (1998) Synth Commun 28:319–326
Liu F, Fu Z, Liu Y, Lu C, Wu Y, Xie F, Ye Z, Zhou X, Yin D (2010) Ind Eng Chem Res 49:2533–2536
Raju BR, Sarkar S, Reddy UC, Saikia AK (2009) J Mol Catal A Chem 308:169–173
Yang C, Jin Q, Zhang H, Liao J, Zhu J, Yu B, Deng J (2009) Green Chem 11:1401–1405
Al-Maksoud W, Daniele S, Sorokin AB (2008) Green Chem 10:447–451
Jeyakumar K, Chakravarthy RD, Chand DK (2009) Catal Commun 10:1948–1951
Noyori R, Aoki M, Sato K (2003) Chem Commun 16:1977–1986
Kirihara M, Yamamoto J, Noguchi T, Hirai Y (2009) Tetrahedron Lett 50:1180–1183
Lindsay Smith JR, Murray J, Walton PH, Lowdon TR (2006) Tetrahedron Lett 47:2005–2008
Bahrami K (2006) Tetrahedron Lett 47:2009–2012
Yamazaki S (1996) Bull Chem Soc Jpn 69:2955–2959
Palermo V, Sathicq AG, Vazquez PG, Thomas HJ, Romanelli GP (2011) React Kinet Mech Cat 104:181–195
Golchoubian H, Hosseinpoor F (2007) Molecules 12:304–311
Maggi R, Chitsaz S, Loebbecke S, Piscopo CG, Sartori G, Schwarzer M (2011) Green Chem 13:1121–1123
Greenspan FP (1946) J Am Chem Soc 68(5):907
John JA, Weymouth FJ (1962) Chem Ind 2:62–69
Ukkirapandian V, Sadasivam V, Sivasankar B (2008) Pet Sci Technol 26:423–435
Yazu K, Makino M, Ukegawa K (2004) Chem Lett 33:1306–1307
Palani A, Pandurangan A (2006) Catal Commun 7:875–878
Saha MS, Nishiki Y, Furuta T, Denggerile A, Ohsaka T (2003) Tetrahedron Lett 44:5535–5537
Zhao X, Zhang T, Zhou Y, Liu D (2007) J Mol Catal A Chem 271:246–252
Fortnum DH, Battaglia CJ, Cohen SR, Edwards JO (1960) J Am Chem Soc 82:778–782
Furia FDi, Modena G (1982) Pure Appl Chem 54(10):1853–1866
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tumula, V.R., Bondwal, S., Bisht, P. et al. Oxidation of sulfides to sulfones with hydrogen peroxide in the presence of acetic acid and Amberlyst 15. Reac Kinet Mech Cat 107, 449–466 (2012). https://doi.org/10.1007/s11144-012-0491-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-012-0491-8