Abstract
This work deals with the photocatalytic oxidation of toluene at room temperature and atmospheric pressure in the gas phase. The differential equations of the reactor model are solved numerically with simultaneous estimation of the model parameters. Estimation of the kinetic data is performed using a modified differential method of data analysis and a Nelder–Mead method of nonlinear optimization for parameter estimation. The reaction is performed in an annular photoreactor using UVA black light blue fluorescent lamp. The experiments are carried out at different total flow rates of the reaction feed (20–160 cm3 min−1), two different inlet concentrations of toluene (2.67 and 5.24 g m−3) and at constant relative humidity (25%). A good agreement between the experimental data and theoretical predictions is obtained, supporting the applicability of the proposed models to describe the investigated process performed in laboratory annular photoreactor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photocatalytic oxidation using semiconductors as photocatalysts represents an excellent technology for environmental remediation. In particular, photocatalytic oxidation based on TiO2 has been demonstrated to be highly effective in the treatment of contaminated water and air [1–11]. Photooxidation on TiO2 surface irradiated with suitable UV radiation leads to the degradation and complete mineralization of volatile organic compounds (VOCs) into environmentally friendly species without significant energy input. The process is simple and requires only standard room conditions and stable and nontoxic catalyst. Degussa TiO2 catalyst (P-25) has been, and continues to be the preferred choice in many studies in this field due to its high activity. Other photocatalyst include ZnO, ZnS, CdS, etc., but these are less active [1].
Although many studies have investigated the photocatalytic oxidation of gas-phase volatile organic compounds by using TiO2, most of them focused on reaction rate and reactor performance, as well as on the identification of reaction by-products [12–16]. Different photoreactor configurations are reported in the references [8, 17–22]. Generally, the photocatalytic reactors in use for air treatment can be categorized according to: (a) gas flow geometry (tubular, annular, flat plate), (b) type of their light sources or (c) the way by which the photocatalyst is introduced into the system [23]. However, there is still a lack of mathematical models that can be applied to photocatalytic reactor design and scale-up. Different mathematical models are applied depending on photoreactor configuration, such as fixed bed annular reactor [24–28], batch reactor [18, 29–31], plane plate reactor [28, 32–34], honeycomb monolith reactor [17], microchannel reactor [35], annular Venturi reactor [18], etc.
The objective of the present research was to investigate the sensitivity of a gas-phase photocatalytic reaction to reaction conditions with respect to air pollutant such as toluene and to propose appropriate mathematical models. The proposed models can be used as a tool for designing and simulating the behavior of a photocatalytic annular reactor with titanium dioxide based materials as oxidation catalyst.
Experimental
Preparation and characterization of photocatalyst
The commercial TiO2 powder catalyst supplied by Degussa (P25) was used to prepare the catalyst layer. The sodium silicate-modified TiO2 was coated on the internal glass surface of the outer tube of an annular reactor and used for conducting the photoreactivity experiments. The interior tube of the annular reactor was made of quartz, due to its UV-conducting nature. Catalyst characterization was performed by several techniques, such as X-ray powder diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy and surface measurements (adsorption/desorption of nitrogen at 77 K). A detailed description of catalyst preparation and characterization can be found elsewhere [25].
Reactor and experimental set-up
The reaction was performed at room temperature in an annular photocatalytic reactor designed to study the treatment of different organic pollutants in the gas phase. Toluene, as one of the typical organic compounds indoors, was used as a model VOC in this study. The reacting gaseous mixture consisted of toluene, air and water in various ratios. The reactant mixture was generated by bubbling air through saturators containing bidistilled water and toluene (LiChrosolv®, Merck) at room temperature. High purity synthetic air was used as a carrier gas. Before switching on the UV-lamp, the gas mixture was fed into the reactor in order to achieve the equilibration of the catalyst with the reagents. The toluene concentration at the end of this period was taken as the initial value (C A,0 ). Illumination of the catalyst was provided by an 8 W fluorescent black light blue lamp (Sylvania ®). Space time in the reactor was varied by changing total flow rate of gas mixture at the constant reactor volume or reactor length. Conversion, X A , was calculated with respect to toluene concentration.
Sampling and analysis
Samples of 0.5 ml were withdrawn periodically using a gas sampling valve, and the toluene concentration was measured on-line by a gas chromatograph (Varian 3300) equipped with Carbowax 20 M packed column (Restek) and FID detector. The reaction was performed under steady-state conditions. Toluene degradation was not detected in the dark or in the absence of catalyst. Thus, all variations in toluene concentration were ascribed to the photocatalytic activity of the catalyst. More details about the experimental apparatus and procedures are given elsewhere [25].
Results and discussion
Modelling of an annular photocatalytic reactor
Assumptions and governing equations
This study deals with specific aspects associated with mathematical modelling of the annular photocatalytic reactor. The approach to modelling an annular reactor is similar to modelling other similar reactor configurations. Generally, mathematical models can range from very simple to some very complex ones. The required degree of sophistication depends on the process and the reaction mechanism, and on its sensitivity to changing in the operating conditions. The degree of accuracy with which the kinetic and transport parameters are known is of equal importance. Avoiding unnecessary complexity is recognized as a general rule.
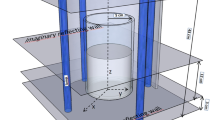
In this work, three different heterogeneous reactor models, accounting for a 1D (Model I) and 2D description of gas phase (Model II and III), were applied for modelling and simulating the annular photocatalytic reactor. Fig. 1 shows the scheme of the channel inside the annular reactor with the basic features of a 2D heterogeneous model (Model III). The main assumptions applied to all three models were: steady-state conditions, negligible pressure drop along the reactor, and the reaction was assumed to occur on the surface of the catalyst.
According to the 1D model, the mass transfer of reactant (toluene) from the main stream (bulk) to the catalyst surface is located in the thin boundary layer, in which only concentration gradient exists. This means that the whole transfer resistance is defined by the convective mass transfer coefficient, depending on gas properties and hydrodynamic conditions. It is natural to assume that the transfer of toluene molecules from the gas phase is due to diffusion across the whole diameter of free space between inner and outer tube. 2D models consider this fact and suppose the mass transfer to the catalyst layer by diffusion. The most important difference between proposed 2D models (Model II and Model III) is in the regime of fluid flow. Model II considers ideal flow conditions, and Model III takes laminar flow into consideration. More realistic, of course, is the assumption of the laminar flow, which can be confirmed by calculating the Reynolds number. For both 2D models, the molecular coefficient of toluene diffusion in the reaction mixture is required as a parameter in model equations. The value of this parameter can be calculated from the data in the references. Reaction rate constants and mass transfer coefficients are the estimated parameters in 1D model. The mass transfer coefficient can be calculated from empirical correlations, and the obtained value is a measure of goodness of fit with experimental results. In the 2D models, the only fitting parameter is the reaction rate constant, because the molecular diffusion coefficient is taken as a known value. The other assumptions used to develop the models were steady-state and isothermal conditions. A gas mixture flows through the reactor under constant volume velocity and concentration of all present substances. Ideal flow was proposed for Model I and II, and laminar flow was proposed for Model III. Axial diffusion in the gas phase was neglected. The catalyst was deposited on the inner wall of the outer tube in a very thin layer and intraphase diffusion in the reaction path was negligible. Deactivation of the catalyst was neglected because of the rapidly achieved stationary conversion of toluene and frequently changing catalyst layer. Model equations are summarized in Table 1. The simple pseudo first-order kinetic model was used to describe the kinetics of toluene photooxidation.
Numerical approach for the model solution
The derived heterogeneous models are given by mass balances of toluene, as shown in Table 2. Computer algorithms were developed in the program package Matlab ®. Model I was solved by Runge–Kutta method for ordinary differential equations (ODE). Models II and III, represented with partial differential equations (PDE), were solved using the method of lines [36]. By using this method, model equations were discretized along the axial and radial coordinates by backward differences. The inlet conditions for the Models II and III were the same as for Model I (Eq. 3). The mass transfer coefficient (k g ) was calculated using Eq. 5, taking into account the correlation with the corresponding Sh number, while the value of the molecular diffusion coefficient of toluene in air, D A, was taken from the literature (0.0804 cm2 s−1) [37]. All computations were performed only with one estimated parameter, e.g. reaction rate constant, k A , in order to avoid uncertainty due to a great number of parameters. Parameter estimation was performed using a modified differential method of data analysis and the Nelder–Mead method of non-linear optimization. The correlation criterion was the root mean square deviation between the experimentally obtained and theoretically predicted values, SD:
Average (i.e. “mixing cup”) toluene concentration in the fluid phase was calculated using Eq. 16,
Model results and analysis
The photocatalytic oxidation of toluene in an annular reactor is an example of a complex reaction system. Therefore, it is a challenge to develop the appropriate mathematical model in order to describe this reaction system. Computer simulation of an annular photocatalytic reactor needs a basic choice between one-dimensional and two-dimensional model. A pragmatic choice should be based on the compared evaluation of the reliability and the complexity of mathematical models. It is well known that the 2D model with the proper boundary conditions can describe real transport phenomena inside the annular reactor, while the one-dimensional model requires a prior estimation of the mass transfer coefficient.
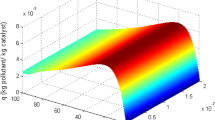
Fig. 2 shows a set of typical oxidation results predicted by the heterogeneous 1D model obtained at inlet toluene concentration of 2.67 and 5.24 g m−3, respectively. As expected, the conversion of toluene increases by increasing space time in the reactor, and obviously, at the end, leads to final equilibrium conversion. Conversion of toluene decreases with increased inlet toluene concentration in the whole range of the total flow rate. A similar dependence of the degradation rate on inlet contaminant concentrations is reported in the literature references [38]. These results can be attributed to a limited number of active sites on TiO2 surface available for toluene adsorption. A satisfactory degree of correlation between the experimental values and values predicted by 1D heterogeneous model was established.
Similar results are obtained using more complex 2D heterogeneous models. The estimated values of the rate constant (k A ) and the root mean square deviation obtained using 1D and 2D models are given in Table 2. As it can be seen, the lower values of the root mean square deviation are obtained using 2D models, indicating that 2D models can probably be used with higher accuracy in order to describe the behavior of the annular reactor. Obviously, a more realistic picture of the annular reactor performance can be obtained using more complex 2D heterogeneous models, especially taking into consideration concentration gradients in the fluid phase (the place between interior and outer tube of the reactor) due to mass transfer by diffusion. In this case, the imaginary boundary layer (film), which is usually close to the interior wall of the outer reactor tube, is broadened over the whole space between the reactor’s two concentric tubes.
The advantage of the 2D in comparison to the 1D heterogeneous model is its ability to predict conversion profiles along the reactor length (Fig. 3), as well as across the radius of the annular reactor (Fig. 4). According to the shape of these profiles, it is possible to draw some conclusions about the relationship between the rate of the interphase mass transfer and the reaction rate at the surface of TiO2 layer. Apparently, the reaction rate is faster in comparison with the interphase diffusion. This can be supported by the high increase of toluene conversion near the surface of the catalyst layer (r = 100%), especially at the entry of the reactor (see Fig. 3). However, along the reactor length, the conversion increases slower due to the fact that mean concentration in the fluid phase decreases along the reactor length not only due to the chemical reaction at the catalyst surface, but also due to the developing radial diffusion profile in the fluid phase. Fig. 5 shows the comparison of results obtained using Model II and Model III. As it can be seen from Fig. 5, almost the same results are obtained using both 2D models. Although the 2D models are rather complex, the benefit of their use is a better and more reliable prediction of reactor performance under a wide range of operating conditions.
Axial profiles of the toluene conversion in the gas phase of the annular reactor for different fractions of the overall reactor radius, where curve at 100% represents the values of conversion at the surface of TiO2 layer along the reactor length (Model III). Reaction conditions: inlet toluene concentration: 5.24 g m−3
Conclusions
This paper reports the results of experimental and theoretical investigation of the photocatalytic oxidation of toluene in the gas phase. Different mathematical models, such as 1D and 2D heterogeneous models based on ideal flow and laminar flow conditions, were used to simulate the behavior of a photocatalytic annular reactor with titanium dioxide as the oxidation catalyst. The proposed models were based on the physical picture, especially regarding the hydrodynamics of the gas phase inside the reactor and the mode of toluene transfer from gas phase to the catalytic surface. The 2D heterogeneous models were developed in order to predict the axial profile of conversion along the reactor length and the radial profile across the reactor radius. Differential equations of the reactor model were solved numerically with simultaneous estimation of the model parameter. All computations were performed only with one estimated parameter, e.g. reaction rate constant, k A , in order to avoid uncertainty due to a great number of parameters. The proposed models were verified by comparing computer simulation data with the experimental laboratory results. Taking into account the root mean square deviation as a correlation criterion, better estimation results are obtained with 2D heterogeneous models. Generally, a good agreement between the experimental data and theoretical predictions was obtained, supporting the applicability of the proposed models to describe investigated process performed in the annular photocatalytic reactor.
Abbreviations
- a :
-
Geometric surface area (m2)
- A R :
-
Surface area of reactor (annulus) cross-section (m2)
- C A :
-
Toluene concentration (g m−3)
- C A,0 :
-
Inlet toluene concentration (g m−3)
- CA,g :
-
Toluene concentration in the fluid phase (g m−3)
- CA,s :
-
Toluene concentration at the solid phase (g m−3)
- \( \overline{C}_{A ,t} \) :
-
Average toluene concentration in the fluid phase (g m−3)
- D A :
-
Molecular diffusion coefficient of toluene in air (m2 s−1)
- k A :
-
Reaction rate constant (min−1)
- k g :
-
Interphase mass transfer coefficient (m h−1)
- N :
-
Number of the experimental points (dimensionless)
- r :
-
Radial reactor coordinate (m)
- r A :
-
Reaction rate (mol h−1 kg−1)
- R :
-
Diameter of reactor annulus (m)
- SD:
-
Root of the mean square deviation (dimensionless)
- u :
-
Superficial fluid velocity (m h−1)
- um :
-
Mean superficial fluid velocity (m h−1)
- v 0 :
-
Volume flow rate (m3 h−1)
- V :
-
Reactor volume (m3)
- X A :
-
Toluene conversion (dimensionless)
- z :
-
Axial reactor coordinate (m)
- Z:
-
Length of the reactor (m)
- κ :
-
Ratio of inner and outer reactor diameter (dimensionless)
- τ:
-
Space time (min)
- Re:
-
Reynolds number (dimensionless)
- Sc:
-
Schmidt number (dimensionless)
- Sh:
-
Sherwood number (dimensionless)
References
De Lasa H, Serrano B, Salaices M (2005) Photocatalytic reaction engineering. Springer, New York
Michael STM, Hoffmann R, Choi W, Banhemann DW (1995) Chem Rev 95:69
Zhao J, Yang X (2003) Build Environ 38:645
Bhatkhande DS, Pangarkar VG, Beenackers AACM (2001) J Chem Technol Biotechnol 77:102
Ollis DF (2000) CR Acad Sci Paris, Série Iic. Chimie/Chem 3:405
Fox MA, Dulay MT (1993) Chem Rev 93:341
Mills A, Lee SK (1992) J Photochem Photobiolog A Chem 152:233
Paz Y (2010) Appl Cat B Environ. doi:10.1016/j.apcatb.2010.05.011
Cao L, Gao Z, Suib SL, Obee TN, Hay SO, Freihaut JD (2000) J Catal 196:253
Mo J, Zhang Y, Xuz Q, Zhu Y, Lamson JJ, Zhao R (2009) Appl Catal B Environ 89:570
d’Hennezel O, Pichat P, Ollis DF (1998) J Photochem Photobiolog A Chem 118:197
Jeong J, Sekiguchi K, Lee W, Sakamoto K (2005) J Photochem Photobiolog A Chem 169:279
Mendez-Roman R, Cardona-Martinez N (1998) Catal Today 40:353
Xie C, Xu Z, Yang Q, Li N, Zhao D, Wang D, Du Y (1994) J Mol Catal A Chem 217:193
Alberici RM, Jardim WF (1997) Appl Catal B Environ 14:55
Nicollella C, Rovatti M (1998) Chem Eng J 69:119
Romero-Vargas Castrillón S, Ibrahim H, De Lasa H (2006) Chem Eng Sci 61:3343
Ibhadon AO, Arabatzis IM, Falaras P, Tsoukleris D (2007) Chem Eng J 133:317
Imoberdorf GE, Cassano AE, Irazoqui HA, Alfano OM (2007) Catal Today 129:118
Imoberdorf GE, Cassano AE, Irazoqui HA, Alfano OM (2007) Chem Eng Sci 64:1138
Furman M, Corbel S, Wild G, Zahraa O (2010) Chem Eng Proc Proc Intensification 49:35
Paz Y (2009) Adv Chem Eng 36:289
Wu JF, Hung CH, Yuan CS (2005) J Photochem Photobiol A Chem 170:299
Obuchi E, Sakamoto T, Nakano K (1999) Chem Eng Sci 54:1525
Tomašić V, Jović F, Gomzi Z (2008) Catal Today 137:350
Taghipour F, Mohseni M (2005) AIChE J 51(11):3039
Imoberdorf GE, Irazoqui HA, Alfano OM, Cassano AE (2007) Chem Eng Sci 62:793
Palmisano G, Addamo M, Augugliaro V, Caronna T, Paola AD, Lopez EG, Loddo V, Marcı G, Palmisano L, Schiavello M (2007) Catal Today 122:118
Kim SB, Hwang HT, Hong SC (2002) Chemosphere 48:437
Zalazar CS, Martin CA, Cassano AE (2005) Chem Eng Sci 60:4311
Demeestere K, Visscher AD, Dewulf J, Leeuwen MV, Langenhove HV (2004) Appl Catal B Environ 54:261
Obee TN, Hay SO (1997) Environ Sci Techol 31:2034
Mehrdad Keshmiri TT, Mohseni Madjid (2006) J Hazard Mat B 128:130
Yu H, Zhang K, Rossi C (2007) J Photochem Photobiol A Chem 188:65
Ge H, Chen G, Yuan Q, Li H (2005) Cat Today 110:171
Schiesser WE (1991) The numerical methods of lines. Integration of partial differential equations. Academic Press, San Diego
http://www.epa.gov/athens/learn2model/part-two/onsite/estdiffusion.html
Bouzaza A, Vallet C, Laplanche A (2006) J Photochem Photobiol A Chem 177:212
Acknowledgments
The authors highly appreciate the financial support that the Ministry of Science, Education and Sport of Republic of Croatia has given for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marečić, M., Jović, F., Kosar, V. et al. Modelling of an annular photocatalytic reactor. Reac Kinet Mech Cat 103, 19–29 (2011). https://doi.org/10.1007/s11144-011-0299-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-011-0299-y