Abstract
Alumina-supported cobalt metal catalysts are studied in the preparation of 1-phenylethanol by the selective hydrogenation of acetophenone. X-ray diffraction was used to investigate the phase of the catalysts. Effects of reaction temperatures, Co loading, and catalysts preparation method on the activity of the hydrogenation of acetophenone and the selectivity of 1-phenylethanol were studied. The results show that the preparation method and Co loading influence the dispersion of the catalyst. The hydrogenation of carbonyl groups has priority to hydrogenation of phenyl groups over alumina-supported Co catalysts. The selectivity of 1-phenylethanol decreases when the reaction temperature is increased under the same conversion conditions. With the Co loading increasing, the reaction activity increases while the selectivity of 1-phenylethanol decreases. The selectivity of 1-phenylethanol reaches 98% when the conversion is up to 98.2% at 373 K over the catalyst prepared by impregnation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
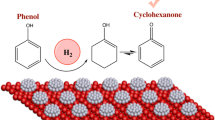
The selective hydrogenation of aromatic ketones to aromatic alcohols is an important reaction in the fine chemical industry [1–3]. So far, the hydrogenation of acetophenone has been successfully studied on Pd [4, 5], Pt [2, 6], Ru [7], Ni [8, 9], and Cu [10] catalytic systems. It is well known that the hydrogenation of acetophenone is a complex multi-step reaction due to competitive hydrogenation between phenyl and carbonyl groups in one molecule (Fig. 1) [6]. The products are strongly dependent on catalyst composition. The products are 1-phenylethanol and ethylbenzene over Pd-based catalyst [4, 5]. In contrast, the products are 1-phenylethanol, ethylbenzene, methylcyclohexylketone, cyclohexylethanol and ethylcyclohexane over Pt-based catalyst [2, 6]. Over Ru-based catalysts, the main products are 1-phenylethanol, methylcyclohexylketone, and cyclohexylethanol. The main products over Raney Ni catalyst include 1-phenylethanol, ethylbenzene, methylcyclohexylketone and cyclohexylethanol. It is important to find an appropriate active component to improve the activity and selectivity of 1-phenylethanol.
Cobalt is commonly considered to be a good catalyst for the hydrogenation of carbonyl groups and was widely used in hydrogenation of cinnamaldehyde [11, 12]. However, few studies have been carried out on the application of Co-based catalyst over selective hydrogenation of acetophenone.
The aim of this investigation is to demonstrate the catalytic performance of alumina-supported Co catalysts on the selectivity in acetophenone hydrogenation. X-ray diffraction was used to investigate the phase of the catalysts. The effect of reaction temperature, Co loading and the preparation method on the activity and selectivity of 1-phenylethanol were studied. High selectivity of 1-phenylethanol was obtained.
Experimental
Materials and methods
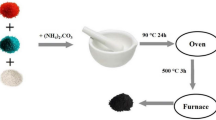
Coprecipitation: an aqueous solution of (NH4)2CO3 (A.R., Shanghai Sihewei Chemicals Co.) was added to an ethanol solution of Al(NO3)3 (A.R., Shanghai zhengxin reagent factory) and Co(NO3)2 (A.R., Shanghai zhengxin reagent factory) and the pH was adjusted to 7. The mixture was stirred for 30 min. The sample was filtered under vacuum, aged at room temperature for 24 h, dried at 373 K for 10 h, and then heated to 773 K at a rate of 10 K/min and calcined in air at 773 K for 6 h.
Impregnation: isovolumetric impregnation was adopted. The required amount of Co(NO3)2 was dissolved in water and added the required amount of Al2O3 support. The mixture was kept for 24 h at room temperature, dried at 313 K for 4 h and 373 K for 10 h, then heated to 673 K at a rate of 1 K/min and calcined at 673 K for 4 h.
The alumina-supported Co metal catalysts were produced by reducing the oxide precursors in hydrogen at 773 K for 4 h.
Catalyst characterization
XRD examination was performed using a X-ray diffract meter (Bruker axs/D8 Foucs) with Cu Kα radiation (λ = 0.15406 nm).
Hydrogenation reaction
Liquid-phase hydrogenations of acetophenone were carried out in a 500 mL stirred batch reactor. The catalyst (0.8 g), acetophenone (5 mL, Sinopharm Chemical Reagent. Co. Ltd.), and ethanol (160 mL, Sinopharm Chemical Reagent. Co. Ltd.) were loaded into the reactor, then the reactor was sealed. Reactions were performed under a total pressure of 2 MPa. The reaction products were identified with GC–MS (Agilent-6890GC-5973MS) and analyzed by GC (SHIMADZU GC-2010).
Results and discussion
Catalyst characterization
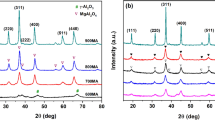
Figs. 2 and 3 show XRD patterns of the alumina-supported cobalt oxides precursors and metal catalyst. As shown in Fig. 2, mainly Co3O4 exists on the γ-Al2O3 support. It can be seen that with increasing Co loading, the intensity of the XRD diffraction pattern of Co3O4 is improved, which means the particle diameter of Co3O4 increased. It can also be seen that, at the same Co loading amount, the Co/Al2O3 oxide precursors prepared by impregnation showed higher XRD peak intensity of Co3O4 than the catalyst prepared by coprecipitation, which means that the sample prepared by coprecipitation showed higher dispersion and smaller particle diameter of Co3O4. Fig. 3 shows that Co3O4 converted to Co metal after reduction, which means the active components in the selective hydrogenation of acetophenone is Co0. But a few diffraction peaks of Co3O4 appeared on the catalyst when the Co loading is below 28.6%, and the XRD peak intensity of Co3O4 decreases with the Co loading increasing, which means the oxides with lower Co loading were less reducible.
Acetophenone hydrogenation on alumina-supported Co catalyst
Fig. 4 displays the product distribution (mol %) as a function of time during the selective hydrogenation of acetophenone over the alumina-supported Co catalyst. It is shown that PE and EB are the major products for hydrogenation of acetophenone over alumina-supported Co catalyst. This indicates that the hydrogenation of carbonyl groups has priority over the hydrogenation of phenyl groups on alumina-supported Co catalysts. The concentration of PE first increases and then decreases with reaction time, and the concentrations of EB slowly boosts with time. This means the formation of 1-phenylethanol and ethylbenzene is a consecutive reaction in which 1-phenylethanol is the intermediate for ethylbenzene [5]. When the conversion is close to 100% at 353 K, a small amount of 1-cyclohexylethanol (0.2%) was produced.
Effect of reaction temperature on acetophenone hydrogenation
A significant enhancement in the activity was observed with the reaction temperature increasing (Fig. 4). The conversion of acetophenone reaches 95.8% after 4 h at 353 K, while it reaches at 97.6% after 0.5 h at 393 K.
The selectivity of 1-phenylethanol dropped rapidly along with reaction temperature increasing under the same conversion rate conditions (Fig. 5). This is due to the dehydration reaction of 1-phenylethanol to styrene and styrene is consecutively transformed to ethyl benzene by hydrogenation at high reaction temperature [13]. The conversion rate of the 1-phenylethanol to ethyl benzene increased rapidly with temperature.
Effect of Co loading on acetophenone hydrogenation
The effects of Co loading on the selectivity hydrogenation of acetophenone were also studied. The results are shown in Table 1. With the increase of the Co loading on the alumina support, the activity increases quickly but the selectivity of 1-phenylethanol decreases. Over the 4.8% Co/Al2O3 catalyst, the conversion is 2% within 2 h and the selectivity of 1-phenylethanol is 100%. Over the 44.4% Co/Al2O3 catalyst, the conversion reaches 96.4% within 0.5 h and the selectivity of 1-phenylethanol is 31.1%. The Co loading shows significant influence on the catalytic hydrogenation activity, and the increase of activity is higher than the increase of Co loading.
Effect of preparation method on hydrogenation performance
The effect of preparation method on the hydrogenation performance was also studied, and the results are shown in Table 2. The catalyst prepared by impregnation shows higher selectivity of 1-phenylehtanol and lower activity. After 1 h, the catalyst prepared by impregnation forms 1-phenylethanol with high selectivity (99.1%) and shows low hydrogenation conversion (only 35.2%). The selectivity of 1-phenylethanol reached 98% when the conversion was up to 98.2% at 373 K. From the XRD pattern, it has been seen that the catalyst prepared by impregnation shows lower dispersion, which may be the reason of lower active of the catalyst prepared by impregnation.
Conclusions
The application of alumina-supported Co metal catalyst in the preparation of 1-phenylethanol by selective hydrogenation of acetophenone is reported. The results show that the sample prepared by coprecipitation has higher dispersion. PE and EB are the major products in reaction. The hydrogenation of carbonyl groups has priority over the hydrogenation of phenyl groups on alumina-supported Co catalysts. The selectivity of 1-phenylethanol decreased with the reaction temperature increasing. When the Co contents increases, the reaction activity increases while the selectivity of 1-phenylethanol decreases. The catalyst prepared by impregnation showed higher selectivity of 1-phenylehtanol and lower activity. The selectivity of 1-phenylethanol reached 98% when the conversion is up to 98.2% at 373 K.
References
Reddy BM, Rao KN, Reddy GK (2009) Catal Lett 131(1–2):328
Liu HP, Lu GZ, Guo Y et al (2009) Catal Commun 10(9):1324
Quintanilla A, Bakker JJW, Kreutzer MT et al (2008) J Catal 257(1):55
Chen CS, Chen HW (2004) Appl Catal A 260(2):207
Ji YL, Ma XB, Wu XJ et al (2007) Appl Catal A 332(2):247
Chen CS, Chen HW, Cheng WH (2003) Appl Catal A 248(1–2):117
Casagrande M, Storaro L, Talon A et al (2002) J Mol Catal A 188(1–2):133
Hamarthibault S, Masson J, Fouilloux P et al (1993) Appl Catal A 99(2):131
Masson J, Vidal S, Cividino P et al (1993) Appl Catal A 99(2):147
Bertero NM, Apesteguia CR, Marchi AJ (2008) Appl Catal A 349(1–2):100
Chen XF, Li HX, Dai WL et al (2003) Appl Catal A 253(2):359
Li HX, Chen XF, Wang MH et al (2002) Appl Catal A 225(1–2):117
Jiang L, Zhu YF, Xiang YZ et al (2007) Chin J Catal 28(03):281
Acknowledgments
The work was supported by Excellent Young Teachers funding schemes of Zhe-jiang Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X.B. Preparation of 1-phenylethanol by selective hydrogenation of acetophenone over alumina-supported Co catalysts. Reac Kinet Mech Cat 102, 417–424 (2011). https://doi.org/10.1007/s11144-010-0268-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-010-0268-x