Abstract
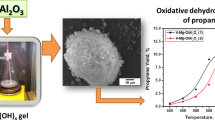
Nanocrystalline aerogel VOx/MgO catalysts for the oxidative dehydrogenation of propane with high surface area and uniform vanadium distribution were synthesized by co-gelation followed by supercritical drying. The catalysts were shown to have superior performance compared to nanocrystalline VOx/MgO catalysts prepared by impregnation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxidative dehydrogenation (ODH) of propane is an attractive way to produce propylene. The latter is an important industrial precursor for polymerization processes as well as for the synthesis of propylene oxide, cumene, acetic acid, etc. [1]. Considerable attention has been paid to the development of catalysts with high selectivity to propylene in the ODH. A V/MgO system was found to be one of the best in terms of activity and performance stability [2–4].
Of particular interest are the VOx/MgO catalysts prepared by impregnation of nanocrystalline MgO aerogel supports with a small crystallite size and high surface area, which are known to have superior adsorption and catalytic properties [5–9]. Such catalysts were shown to be more active in the oxidative dehydrogenation of butane than the VOx/MgO catalysts prepared in a conventional manner [10–12]. Unfortunately, the impregnation procedure used in these studies [10–12] for the vanadium deposition led to a considerable decrease in the catalyst surface area. In this study, we prepared VOx/MgO aerogel catalysts by a co-gelation procedure that excludes the impregnation stage, and compared their textural and catalytic properties with those of the catalysts prepared by impregnation.
Experimental
Mixed nanocrystalline V–Mg hydroxides were obtained by an aerogel technique similar to that known for the synthesis of AP–Mg(OH)2 aerogels [5, 6]. This technique starts with dissolving a magnesium ribbon in dry methanol to form a Mg(OCH3)2 solution. After addition of the gel-stabilizer (toluene), magnesium methoxide was slowly hydrolyzed with distilled water at vigorous stirring. Then, liquid VO(OC3H7)3 was added into the formed gel with a syringe. The resulting orange-colored gel was placed into an autoclave and dried under supercritical conditions for removal of solvent at 265 °C and 90 bar to give a mixed V–Mg hydroxide aerogel. Nanocrystalline VOx/MgO catalysts were obtained by the dehydration of the corresponding aerogel V–Mg hydroxides via their heating in a temperature-programmed regime to 550 °C in a tubular furnace with an intense air flow. The properties of the synthesized catalysts were compared with those of a sample prepared by the impregnation of the nanocrystalline aerogel MgO support synthesized by a similar procedure with an ethanol solution of NH4VO3 and also calcined at 550 °C.
The reactivity of the samples in the ODH of propane and the coke deposition kinetics were studied using a gravimetric pulse mass analyzer TEOM 1500 PMA (Thermo Scientific). This instrument allows one to control the sample weight and reaction temperature in a flow reactor with high precision [13]. The weight measurement precision was 5 μg with the maximum measurement frequency as high as 10 points per second. The reaction temperature was varied within the range of 400–600 °C, the reaction gas mixture was 91 vol.% (He) + 4.5 vol.% (O2) + 4.5 vol.% (C3H8). In some experiments, 4.5 vol.% of O2 was replaced with 9 vol.% of N2O. The reaction products were analyzed by GC.
Results and discussion
Textural characteristics
According to Table 1, the prepared mixed aerogel V–Mg hydroxides are characterized by very high surface areas exceeding 1000 m2/g, which are comparable to that of the vanadium-free Mg(OH)2 aerogel prepared by a similar method. The addition of different amounts of vanadium seems to have no significant effect on SBET of the resulting combined hydroxides. However, the subsequent transformation of the aerogel V–Mg hydroxides into corresponding VOx/MgO catalysts causes a considerable decrease in their surface area to 320–360 m2/g in a wide range of the vanadium concentrations (Table 1).
Still, this surface area is much higher than those of the catalysts with the similar vanadium loadings but prepared by impregnation of aerogel MgO supports with various vanadium precursors [10–12]. In this study, we compared the catalytic properties of our aerogel VOx/MgO catalysts with those of a catalyst prepared by the impregnation of the aerogel AP–MgO support. After supercritical drying, the Mg(OH)2 aerogel was dehydrated in vacuum at slowly increasing temperature to form AP–MgO with the surface area as high as 635 m2/g. Unfortunately, after the impregnation of this support with an ethanol solution of NH4VO3 and the following calcination at 550 °C, the surface area of this catalyst was only 160 m2/g. Still, this value exceeds those reported in the literature for similar materials [10–12].
TEM data
The morphology of the prepared aerogel VOx/MgO samples was studied by high-resolution TEM. According to the TEM data (Fig. 1), the 8%VOx/MgO aerogel catalyst consists mainly of spherical particles 200–300 nm in diameter. Lamellar particles about 100 nm in size are also present. The large agglomerates include the disordered wafer nanoscale (5–6 nm) crystallites of the face-centered cubic and orthorhombic symmetries. As revealed by the EDX analysis, the concentration of vanadium throughout the sample is almost uniform and is close to 4.3 at.%, which corresponds well to the number calculated from the total vanadium concentration. Thus, the developed aerogel method makes it possible to obtain a true nanocrystalline VOx/MgO system with the uniform distribution of the active component (VOx) throughout the support (MgO). In contrast, the traditional preparation method based on impregnating AP–MgO with the V(acac)3 solution gives a non-uniform composition of different magnesium vanadates (ortho- and pyrovanadates) distributed over the surface of aerogel MgO [12].
ODH of propane in the presence of O2
The bar diagram in Fig. 2 demonstrates a sufficient difference in the catalytic activity of the 8%VOx/MgO catalysts prepared either by impregnation of nanocrystalline MgO or by the aerogel method. The sample prepared by the aerogel technique was significantly more active at all the temperatures studied. This difference was especially large at moderate temperatures. For example, the propylene yield observed at 500 °C for the aerogel catalyst was almost three times higher than for the impregnated one. It is important that the sample used for comparison was prepared using the nanocrystalline aerogel MgO support and had relatively high surface area. According to the results of earlier studies, such catalysts are more active than those prepared by conventional methods [10–12].
Therefore, the developed aerogel technique provides the synthesis of VOx/MgO catalysts that are substantially more active in the ODH of propane than the catalysts prepared by conventional methods. This effect can be explained by the different distribution of vanadium species in the aerogel and supported VOx/MgO samples and by higher surface area of the former.
ODH of propane in the presence of N2O
Nitrous oxide is another oxidizing agent that can be used for oxidative dehydrogenation. The selectivity benefit of using nitrous oxide instead of oxygen in the ODH of propane is illustrated in Fig. 3. The use of N2O as an oxidizing agent provided the high selectivities towards propylene (85–100%) within the temperature range of 400-500 °C, whereas the use of O2 led to the drop in the selectivity at temperatures above 400 °C, probably due to the enhanced contribution of combustion processes (Fig. 3a). Besides, the selectivity in case of using N2O was higher at all the conversions obtained (Fig. 3b).
Unfortunately, we found that replacement of oxygen by nitrous oxide initiates the undesirable coke formation that leads to catalyst deactivation. The coke deposition was investigated by TEOM in the ODH of propane with N2O. Table 2 shows the amounts of coke deposited during the ODH of propane at 500 °C over aerogel VOx/MgO catalysts under different reaction conditions. It is obvious that the coke is not formed when O2 (4.5 vol.%) is used as the oxidizer. This is evidenced by the constant sample mass during the reaction and the yellowish color of the sample afterwards.
In the experiments with N2O, its concentration was fixed at 9 vol.% in order to keep the C3H8:oxidizer ratio the same. The coke formation rate increased with the vanadia content in the catalyst (Table 2), and for the sample 15%VOx/MgO, the amount of coke deposited reached almost 0.5 wt% after 40 min of the reaction. Besides, after the reaction, the color of the sample turned dark gray or even black, thus confirming the presence of coke on the surface.
The kinetics of the coke deposition was investigated to evaluate the order of the reaction with respect to propane at different temperatures. For this purpose, the coke deposition rates were measured at various concentrations of propane in the experiments carried out in TEOM over the aerogel 8%VOX/MgO catalyst with the feed containing 9 vol.% N2O. The apparent order (n) for the coke formation appeared to be 1.4 at 400 °C and increased to 2.4 at higher temperatures (450 and 500 °C) (Fig. 4). The obtained high values of the reaction order indicate that the negative effect of coking can be minimized by carrying out the ODH at low propane concentrations.
Thus, aerogel VOx/MgO samples prepared by co-gelation with subsequent supercritical drying and calcination in dry flowing air at 550 °C proved to have surface areas as high as 360 m2/g and homogeneous vanadium distribution. Their catalytic activity in the ODH of propane was substantially higher than that of the catalyst prepared by impregnation of the aerogel MgO support. These results suggest that the preparation method developed may be promising for the synthesis of catalysts for the oxidative dehydrogenation.
References
Eisele P, Killpack R (1993) Propene. Ullmanns encyclopedia of industrial chemistry. VCH, Weinheim, 211 pp
Cavani F, Ballarini N, Cericola A (2007) Catal Today 127:113
Klisinska A, Samson K, Gressel I, Grzybowska B (2006) Appl Catal A: General 309:10
Balderas-Tapia L, Hernandez-Perez I, Schacht P, Cordova IR, Aguilar-Rios GG (2005) Catal Today 107–108:371
Utamapanya S, Klabunde KJ, Schlup JR (1991) Chem Mater 3:175
Klabunde KJ, Stark JV, Koper OB, Mohs C, Park DG, Decker S, Jiang Y, Lagadic I, Zhang D (1996) J Phys Chem 100:12142
Mishakov IV, Zaikovskii VI, Heroux DS, Bedilo AF, Chesnokov VV, Volodin AM, Martyanov IN, Filimonova SV, Parmon VN, Klabunde KJ (2005) J Phys Chem B 109:6982
Mishakov IV, Bedilo AF, Richards RM, Chesnokov VV, Volodin AM, Zaikovskii VI, Buyanov RA, Klabunde KJ (2002) J Catal 206:40
Mishakov IV, Heroux DS, Chesnokov VV, Koscheev SG, Mel’gunov MS, Bedilo AF, Buyanov RA, Klabunde KJ (2005) J Catal 229:344
Pak C, Bell AT, Tilley TD (2002) J Catal 206:49
Chesnokov VV, Bedilo AF, Heroux DS, Mishakov IV, Klabunde KJ (2003) J Catal 218:438
Vidal-Michel R, Hohn KL (2004) J Catal 221:127
Perez-Ramirez J, Gallardo-Llamas A, Daniel C, Mirodatos C (2004) Chem Eng Sci 59:5535
Acknowledgement
Financial support of this work by the Russian Foundation for Basic Research (Grants 06-03-32540 and 06-03-32512) and CRDF (project RUE1-2893-NO-07) is acknowledged with gratitude.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishakov, I.V., Ilyina, E.V., Bedilo, A.F. et al. Nanocrystalline aerogel VOx/MgO as a catalyst for oxidative dehydrogenation of propane. React Kinet Catal Lett 97, 355–361 (2009). https://doi.org/10.1007/s11144-009-0041-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-009-0041-1