Abstract
Objective
To estimate the extent to which HIV-related variables, cognition, and other brain health factors interrelate with other HIV-associated symptoms to influence function, health perception, and QOL in older HIV+ men in Canada.
Design
Cross-sectional structural equation modelling (SEM) of data from the inaugural visit to the Positive Brain Health Now Cohort.
Setting
HIV clinics at 5 Canadian sites.
Subjects
707 men, age ≥ 35 years, HIV+ for at least one year, without clinically diagnosed dementia.
Main outcome measures
Five latent and 21 observed variables from the World Health Organization’s biopsychosocial model for functioning and disability and the Wilson–Cleary Model were analysed. SEM was used to link disease factors to symptoms, impairments, function, health perception, and QOL with a focus on cognition.
Results
QOL was explained directly by depression, social role, health perception, social support, and quality of the environment. Measured cognitive performance had direct effects on activity/function and indirect effects on participation, HP and QOL, acting through self-reported cognitive difficulties and meaningful activities.
Conclusion
The biopsychosocial model showed good fit, with RMSEA < 0.05. This is the first time the full model has been tested in HIV. All of the domains included in the model are theoretically amenable to intervention and many have evidence-based interventions that could be harnessed to improve QOL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The experience of living with HIV has changed substantially over the past two decades, as HIV infection has shifted from a disease with a dire prognosis to a manageable chronic condition [1,2,3]. Quality of life (QOL) is replacing survival as the central concern of patients and clinicians alike [1]. As people age with HIV, issues related to brain health, both mood and cognition, have emerged as preoccupations, with cognitive impairment a particular worry. Even with full viral suppression, cognitive challenges seem to be common, the underlying causes are debated, and prognosis is not yet clear [4,5,6,7,8].
Brain health is a multi-dimensional construct reflecting the brain’s role in cognition, mood, emotional stability, motivation, and energy. Cognition can be characterized in terms of performance on neuropsychological tests (NP), here termed cognitive ability; and patient-reported difficulty in performing cognitively demanding activities, here termed cognitive difficulties. Lower cognitive ability may or may not be associated with cognitive difficulties [9]. Mental health comorbidities, notably anxiety and depression, are also very common, and may co-occur with or exacerbate cognitive difficulties, low cognitive ability, or both [10]. The inter-relationships between these aspects of brain health can be challenging to disentangle in clinical and research contexts. Moreover, brain health variables are only some of the many factors that affect QOL, either directly or indirectly, through a potentially complex structure.
Of the brain health constructs, cognition is perhaps that which can be most impactful as there is good evidence that cognition can affect important real-life activities in people with HIV, including adherence to medication [11,12,13], performance of tasks important for daily life, or role and social function [7, 13,14,15,16], driving [17], and employment [18]. Perhaps the largest study to date in HIV (n = 267) tracing the links between cognitive ability, difficulties, and function in everyday life found associations between NP test performance, reported cognitive difficulties, and performance-based tests of everyday activities using simulated shopping and financial tasks [19]. NP test performance sufficiently poor to support a diagnosis of HIV-Associated Neurocognitive Disorder (HAND) interferes with life roles and social function, as well as global QOL [13]. Likewise, lower cognitive ability on NP testing has been shown to have a negative impact on health-related and global QOL [20,21,22].
However, the links between cognition and QOL become less clear as additional variables are considered. The literature has not definitively established whether NP test performance or reported cognitive difficulty is most relevant for predicting downstream outcomes like function and QOL [19]. Other work argues that the apparent relationship between cognition and real-life function is explained by other, correlated, variables such as age, education, mood, or immune function [15].
This literature has used multiple regression, a method that has inherent limitations for such questions. Fully elucidating the complex structure and relationships between and among factors that contribute to QOL in HIV, and situating cognition within that structure, requires a comprehensive theoretical model and a statistical approach that goes beyond correlation or even multivariate regression models [23]. Structural equation modelling (SEM) is well-suited to address this complexity: it combines factor analysis, correlation, path analysis, and regression with the use of latent variables and is designed for testing a priori hypothesized relationships among multiple correlated variables [24]. SEM has been applied in HIV, using Wilson and Cleary’s 1995 model (W–C Model) to link clinical variables and health domains of HRQL [25]. To illustrate, Vidrine et al. [26] used an SEM model to test the fit of the W–C model to data from 348 persons with HIV treated with combination antiretroviral therapy who were recruited in 2000 from urban centres in the United States. They demonstrated that disease status (nadir CD4 cell count) explained health-related QOL (HRQL: physical and mental health), through the presence (paths) of symptoms (pain), function (ability to maintain a household), and participation (ability to participate in meaningful life activities). Other constructs, education, occupation, smoking, alcohol, and illicit drug use also acted on these variables.
Several groups have used SEM to investigate contributors to aspects of QOL in women. Logie et al. [27] conducted an SEM linking stigma to health perception among 173 African and Caribbean Black women living with HIV in Ontario, Canada. This group used a theoretical model based on fundamental cause theory which conceptualizes the linkages between social contexts and health disparities. They also extended this model in a 2017 publication [28] to include QOL which was a latent construct formed by the 6 domains of the WHOQOL-HIV [29]. They found that stigma, racism, social support, and depression had direct paths to QOL, which is not surprising given that the latent construct of QOL was formed by some of these constructs (psychological, social relationships, spirituality/beliefs). Alsayed et al. [30] applied SEM in a sample of 178 HIV+ American women, and found that overall QOL was influenced by depressive symptoms, social support, physical function, and self-perceived general health. Their measure of overall QOL was a single item with a 5-point ordinal scale. A 2018 study of Chinese pregnant women living with HIV (n = 101) [31] found that depression and anxiety mediated perceived social support as a contributor of HRQL. Their measure of HRQL was the EQ-5D which includes an item for anxiety/depression. None of these SEM models for HRQL or QOL included constructs related to cognition.
Thus, while difficulty with cognitively demanding activities is an important concern for persons living with HIV, the contribution of cognitive impairment to cognitive difficulties, the relationships between cognitive difficulties and other brain health constructs, and the downstream effects of all these brain health domains on function and QOL has yet to be assessed within a comprehensive model. The aim of this analysis was to estimate the extent to which HIV-related variables, cognition, and other brain health factors interrelate with other HIV-associated symptoms to influence function, health perception, and QOL in older HIV + men in Canada.
Methods
A cross-sectional analysis was carried out on data from the inaugural assessment of participants in the Positive Brain Health Now (BHN) Cohort [32] with recruitment between 2014 and 2016 from five Canadian sites.
Population
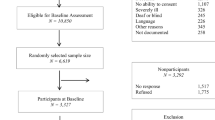
Details on the sample have been reported previously [32]. Participants were ≥ 35 years old, HIV+ for at least 1 year, and able to communicate adequately in either French or English. Excluded were people with Stage 3 or more on the Memorial Sloan–Kettering dementia severity scale, [33], a non-HIV-related neurological disorder likely to affect cognition, active CNS opportunistic infection, known psychotic disorder, substance dependence or abuse within the past 12 months or life expectancy of < 3 years as judged by the treating physician. Owing to the preponderance of men in this cohort (≈ 85%) and the differences between men and women in terms of personal and environmental characteristics, the analysis was restricted to men only.
Model
The model tested in this study combines two theoretical models that are commonly applied in the health field to understand how a complex set of outcomes may be linked. The World Health Organization’s 2001 International Classification of Functioning, Disability, and Health (ICF) [34] is a framework to explain links between body function/structure, activity/function, and participation, incorporating contextual factors (individual and environmental). The W–C Model incorporates these same elements, under different rubrics, and is a framework for linking clinical (biologic) variables, symptoms, functional status, through to health perceptions (HP) and overall QOL. The two models are complementary [25]. The combined ICF/W–C model shown in Fig. 1 comprises eight rubrics (categories of variables): Characteristics of the Individual, Characteristics of the Environment, and from left to right, Biological Variables, Impairment/Symptoms, Activity/Function, Participation, HP, and QOL.
Measurement
Figure 2 shows the variables or constructs situated under the eight rubrics of the ICF/W–C model. In addition to directly measured (manifest) variables, the specified model also includes five latent variables: anxiety, depression, sleep, QOL, and HP. Ten patient-reported outcome measures (PROs) contributed data for many of the constructs: RAND-36 [35], WHOQOL-HIV BREF [36], Hospital Anxiety and Depression Scale (HADS) [37], WHO5 Well-Being Index for mood [38], three items from Starkstein’s Apathy Scale for motivation [39, 40], selected items from Revised Sign and Symptom Check-List for HIV [41], Perceived Deficits Questionnaire (PDQ) [42] for cognitive difficulties; EuroQol EQ-5D-3L [43]; Patient Generated Index (PGI) for individualized QOL [44], and the Trier Inventory for Chronic Stress (TICS) [45]. Total scores from measures such as the PDQ were entered directly under their respective constructs. Other measures contain test items that encompass more than one rubric represented in the model. These measures were disaggregated and their items were assigned to the appropriate construct. For example, the WHOQOL includes items assessing the environment, social support, and beliefs: these were extracted for inclusion under the relevant rubrics in the domain, with only a single item included under the rubric QOL (“How would you rate your quality of life?”).
For ease of interpretation of the path coefficients, PROs were converted to a 0 to 100 scale with 100 indicating the most positively valenced value for the construct (e.g., better mood, higher function, less pain). This required reverse scoring of some.
Table 1 details the measures that contributed to each observed and latent variable:
Personal characteristics
This rubric includes age, education, and personal beliefs (Domain VI of the WHOQOL-HIV BREF) [36].
Environmental characteristics
This rubric includes social support, quality of the environment (Domain V of the WHOQOL-HIV BREF) [36], and external sources of stress as captured by the TICS [45].
Biologic variables
This rubric included a comorbidity index derived from the Charlson Index [46] and two HIV-related variables: duration of HIV infection and a diagnosis of AIDS. The latter was defined based on either: (i) a history of AIDS-defining illnesses (ADI) or; (ii) among those without (i), a nadir CD4 < 200 cells/μL.
Impairment/symptoms
Cognitive ability was measured with a computerized cognitive test battery, the Brief Cognitive Ability Measure (B-CAM). Properties of the B-CAM have been reported elsewhere [47]. Briefly, the B-CAM comprises the following tasks: Corsi block task (forward and backward) [48], mini Trail-Making Test B [49], Eriksen flanker task (incongruent reaction time) [50], phonemic fluency [51], and recall of a list of 8 words. It encompasses the cognitive domains known to be affected by HIV [52] and important for function and QOL: executive function, memory, attention, and processing speed. The assumption that performance on these cognitive tests reflects a single latent variable (cognitive ability) in HIV is supported by the fit of the data to the Rasch model [53]. Rasch analysis is applied to produce a total score that quantifies ability in these HIV-relevant domains and can also be used to track the evolution of cognitive impairment over time.
Other observed variables under the Impairment/Symptom rubric were motivation, pain, vitality (fatigue), and HIV-specific signs and symptoms [41]. Finally, this rubric also included three latent variables. Two latent variables representing anxiety and depression were formed from the relevant Mental Health Index items of the RAND-36 [35], the HADS [37], and the WHO5 Well-Being [38]. A latent variable for sleep included a single item for sleep satisfaction from the WHOQOL-HIV BREF [36] and selected items from the Pittsburgh Sleep Quality Index [54], used in another context [55] and fit to a unidimensional and hierarchical model [53], and was scored from 0 to 11.
Activity/function
The self-reported frequency of cognitive difficulties in everyday activities was measured with the 20-item Perceived Deficits Questionnaire (PDQ) [42]. Other constructs under the Activity/Function rubric were physical functioning as measured by the corresponding index of the RAND-36 and number of hours per week of engagement in recreation and leisure activities as reported by the participant.
Participation
Limitation in fulfilling usual roles was measured by the three sub-scales of the RAND-36: Role Physical, Role Social, and Role Emotional.
HIV-associated HP and QOL
HP was assessed by a latent variable comprising the score from 0 to 100 on the EQ-5D VAS [43], one item from WHOQOL-HIV BREF, the General Health Perception subscale of the RAND-36 [35], scored from 0 to 100, and the EQ-5D utility score. QOL was represented by a latent variable comprised of the single item (5-point ordinal scale) for QOL from the WHOQOL-HIV BREF [36] and the score from 0 to 100 on the Patient Generated Index for individualized QOL (nominate, rate, and prioritize areas affecting QOL) [44].
Statistical methods
The ICF/W–C model was fit to the data using SEM with the software Mplus [20]. Robust maximum likelihood (MLM) estimation [24] was used as not all variables were normally distributed. As the population was gathered from five sites, this non-independence was adjusted for by including dummy variables in the model with paths to all other variables. Categorical variables with five or more levels were modelled as continuous variables; education was modelled as years. Variables within rubrics were allowed to correlate, as were items between personal and environmental characteristics. Personal and environmental factors were allowed paths to all variables in other rubrics. The model was developed sequentially, from left to right along the W–C model. Paths were allowed from left to right across each subsequent rubric, as well as from all personal and environmental factors. Correlations were added among all constructs within a single rubric as well as among all personal and environmental factors, and between the error terms of items arising from the same questionnaire. After removing non-significant paths, a rubric further along the left-to-right sequence was included along with all paths from the rubric immediately to the left and all personal and environmental factors. After removing non-significant paths to the newly added rubric, modification indices were examined and any suggested paths that crossed rubrics were added. This iterative procedure was followed until a final model including all seven rubrics was developed. Table 2 includes details on the model progression.
Two methods were used to handle missing data. People with missing data on more than half of the variables considered were removed from the analysis. Otherwise, single imputation was performed using Statistical Analysis System (SAS version 9.4) statistical software MI procedure. A single imputation was viewed as the most reasonable option as data from all visits (up to five) were used in the imputation; full information maximum likelihood (FIML) would not have used any data outside of what was included in the SEM model. The imputed value for the first visit was used in the current SEM.
Between site variation was examined using the intraclass correlation coefficient (ICC). ICCs were calculated using the random effects model that corresponded to the distribution of each variable: normal, binary, or multinomial.
Goodness of fit of the model was assessed using: root mean square error of approximation (RMSEA), Comparative Fit Index (CFI), and standardized root mean squared residual (SRMR). The Tucker–Lewis Fit Index was not considered since paths to the site variables from each of the variables in the model were retained regardless of their significance status.
When depicting these relationships only direct paths are shown; measured (manifest) variables are shown in rectangles and latent variables are shown in ovals. Paths were modelled only between variables in different rubrics and are shown as single-headed arrows, dark lines if the path is significant at p < 0.05, and dotted lines if 0.05 < p<0.10. Correlations between variables under the same rubric were numerous and are presented separately. Total effects are estimated from any and all direct and indirect paths.
Results
Five sites participated in the BHN cohort and provided data for this analysis on a total of 707 men, with two sites in Montreal contributing 173 and 225 men each, Vancouver 117, Hamilton 45, and Toronto 147. This sample excluded men (n = 13) who were missing more than half of the variables considered for the analysis. Overall, 74% had no missing data and, among the 34 variables included in the model, the number of missing variables never exceeded seven.
Table 1 presents the unimputed data on characteristics of the participants on the model variables corresponding to each rubric of the model, along with intraclass correlation coefficients across sites. The average age of the men was 53 years (SD: 8.3); the majority were between 45 and 59 years, and the oldest was 81 years; all were taking antiretroviral treatment. Cognitive ability was measured with the B-CAM, scored out of 35 (mean: 20.0; SD: 4.6). For the variables scored out of 100, means ranged from 54 (vitality) to 82 (physical function). All but two intraclass correlations were below 5%; cognitive ability and comorbidity index were between 5% and 10%. Fit of the model was good (RMSEA: 0.042; SRMR: 0.028, with an acceptable CFI: 0.952) [56].
The five latent variables forming the measurement portion of this model were constructed theoretically, and modelled along with paths from four variables representing the five sites. Three of the five latent variables fell in the Symptom/Impairment rubric. Depression was formed by the HADS depression scale, the WHO-5, and the sum of three RAND-36 depression items, and explained 65%, 80%, and 74% of each, respectively. The anxiety latent consisted of the HADS anxiety scale and the sum of two RAND-36 anxiety items, explaining 71% and 77%, respectively. Sleep was formed by an item from the HIV WHOQOL and a measure scored based on a Rasch model created with other data, and explained 55% and 47% of these two items. The HP latent variable explained 62% of the EuroQol VAS, 41% of the EuroQol utility score, 67% of the GH subscale of the RAND-36, and 60% of an HIV WHOQOL item. The QOL latent variable explained 61% of the WHOQOL item but only 25% of the PGI measure used to form it. Site explained between 0.7% and 1.6% of the variance of the latent variables. The measurement model had an adequate fit, with an RMSEA of 0.060, an SRMR of 0.029, and a CFI of 0.960.
Table 3 shows how each variable explained the others in the model via direct paths. The table is ordered from left to right starting from Personal and Environmental factors and then according to progression through the rubrics of the ICF/WC models.
The parameters of each path are given: beta (β) is interpreted as a regression coefficient (for every one unit difference in the exogenous x variable in the path, the endogenous y variable in the path changes by β units); the standard error (se) is interpreted as the standard deviation of the estimated β; the p value is determined from β/se, the equivalent of a t test, and the ‘p’ column indicates with a * if the path is significant at p < 0.05 and a ^ when 0.05 < p<0.10. The StdXY column provides the standardized regression coefficient which is needed to compare across paths as each combination of path variables, x and y, have different measurement scales; standardization is with respect to the standard deviation (SD) of the variable. The path with the highest StdXY is the path where the explanatory relationship is the strongest. As an illustration, the first path shown is from age to comorbidity with a β of 0.038 (se: 0.006) and a StdXY of 0.233. The β indicates that people who differ by one year of age score, on average, 0.038 points higher on the comorbidity index. The StdXY indicates that those who differ by 1 SD on age, differ by 0.233 of the SD of comorbidity; this path is significant at p < 0.05. Along with four paths to the biological variables (one of which is not significant), age has paths to four of the symptom/impairment variables, to one of the activity/function variables (physical), and to social role under participation. The path between environmental sources of stress and anxiety has the highest value of StdXY (0.499).
Table 4 presents total paths, calculated from both direct and indirect paths, to HP and to QOL. Table 5 shows the total paths to the cognitive constructs. The strongest paths to HP were from quality of the environment, depression, pain, and physical function, with StdXY of 0.374, 0.335. 0.334, and 0.332, respectively. Depression and quality of the environment were also strongly associated with QOL, along with health perception (StdXY of 0.599, 0.0.550, and 0.489, respectively).
The focus of this paper was on the role of cognition in the context of other brain health and functional outcomes in QOL and, therefore, the number of paths in our analysis is large. Because of this, the full model is shown in Fig. 3 in a supplementary file in a series of five panels showing direct paths from variables related to (a) personal factors; (b) environmental factors; (c) HIV-related biology variables; (d) symptoms and impairments; and (e) activity/function, participation, and HP. For this paper, we present models related to brain health constructs, particularly cognitive constructs. Supplementary Fig. 3a shows only those direct paths to and from cognitive constructs and to QOL. Paths between two variables are shown with black (p < 0.05) or dotted (p: 0.05 < p<0.10) lines; paths to or from a group of variables are shown in large blue lines and the groups are shown by a blue box enclosure (Fig. 3).
a Direct paths to and from cognitive constructs leading to HP and QOL. b Direct paths to quality of life. Measured (manifest) variables are shown in rectangles and latent variables are shown in ovals. Paths shown in black or blue lines were significant at the p < 0.05 level; paths in grey lines associated with values 0.05 < p < 0.10. (Color figure online)
Cognitive ability
Variables influencing the B-CAM measure of cognitive ability were age, education, quality of the environment, and environmental sources of stress. Cognitive ability in turn linked to cognitive difficulties (PDQ) and to the extent of engagement in meaningful activities. Cognitive ability affected participation, HP and QOL only indirectly, i.e., through its impact on cognitive difficulties and meaningful activities.
Cognitive difficulties
Multiple variables contributed to self-reported cognitive difficulties, including cognitive ability, anxiety, pain, vitality, a count of HIV signs and symptoms, and external sources of stress from the Environment rubric, all via direct paths. Indirect contributions were made by age, education, personal beliefs, external sources of stress, environmental quality, and social support (see Table 5). Interestingly, depression was not directly linked to cognitive difficulties, although it had a direct impact on HP and QOL. Cognitive difficulties influenced all aspects of Participation: fulfilment of social, emotional, and physical roles. Through the path to social role, cognitive difficulties had an (indirect) impact on QOL. The total effects (see Table 5) of cognitive performance and self-reported cognitive difficulties for both HP and QOL were very small (StdXY 0.002 and 0.006, respectively, for HP; 0.003 and 0.015, respectively, for QOL).
Other pathways to HP and QOL
Within the Biology rubric, comorbidity and duration of HIV had indirect effects on HP. Within the Impairment/symptoms rubric, depression and motivation influenced HP directly, anxiety and HIV symptoms influenced HP indirectly, and pain and vitality had both direct and indirect influences. Physical function, physical role, beliefs, and quality of the environment also contributed directly to HP, with 84% of the HP latent variable explained by model variables through direct and indirect paths. HP in turn directly explained QOL, as did depression, social role, quality of the environment, and the presence of social support, although many other symptoms/impairments and activity/function variables influenced QOL by way of indirect paths. The model explained 91% of the variance in the QOL latent variable.
Table 6 presents the correlations between variables under the Impairment/Symptom rubric, which includes cognitive ability (B-CAM). The highest correlations (0.57 to 0.83) were observed among vitality, anxiety, and depression, as well as between the sleep latent, vitality, and the anxiety and depression latents (0.64 to 0.68). The correlations of cognitive ability with anxiety and depression were low (r ≈ 0.2). Under the Activities and Participation rubrics, variables representing physical, cognitive, and meaningful activities showed little correlation, with the highest correlation, 0.31, observed between physical and cognitive activities (difficulties). In contrast, variables under the Participation rubric were correlated at approximately 0.6.
Alternative models
A feature of SEM is that multiple models can produce the same fit. An investigation into the lack of impact of a diagnosis of AIDS on variables subsequent in the model resulted in an alternative model with the same fit but significant paths from having an AIDS-defining illness to role physical (β = − 8.43) and to HP (β = − 1.65). These additions did not result in any other paths dropping out of the model.
Discussion
The ICF/WC-based biopsychosocial model tested here using SEM showed good fit to the data and 90% of the variation in the QOL latent variable was explained by model variables. The results showed that one or more aspects of brain health, the focus of the study, affected QOL through a cascade of health experiences linking biology, symptoms, activities, and participation, to HP and ultimately to QOL, although the total explanatory effects varied across the specific brain health constructs. While there have been other studies using SEM to explain complex relationships between constructs relevant to people living with HIV, this is the first that included cognitive constructs.
The BHN cohort was designed to disentangle the impact of cognition and other brain health variables on QOL in the presence of other impairments and limitations that also affect QOL. Cognitive ability, assessed based on performance tests included in the B-CAM, was influenced by age and education as well as characteristics of the environment. HIV-related variables were not influential. We also found that cognitive ability correlated only weakly (< 0.3) with other variables and latents within the same rubric, including depression and anxiety. However, cognitive ability was strongly associated with cognitive difficulties (β: 0.801; se: 0.113; see Table 3). Self-reported cognitive difficulties were also influenced by HIV signs and symptoms, pain, vitality, anxiety and environmental stress, and indirectly by age, education, and beliefs. Through this pathway, cognitive ability had an indirect but widespread impact on all aspects of living with HIV, including decreased engagement in meaningful activities, impaired participation in life’s roles, and through these, to impact QOL. Thus, in this non-demented sample, self-reported cognitive difficulties are good indicators of negative downstream effects on people’s lives.
Although cognitive ability and self-reported cognitive difficulties were strongly related, the two variables showed distinctly different sets of associations with the variables located downstream (to the left) and upstream (to the right) in the model (see Supplementary Fig. 3a, b).
While it is often reported that mood states, such as anxiety and depression, explain cognitive difficulties [57, 58], the observations here suggest that cognitive ability, measured from test scores, contributes unique information to understanding the basis of cognitive difficulties, as correlations of B-CAM with measures of symptoms of anxiety and depression were low (see Table 4, 5).
Furthermore, our analysis revealed that depressive symptoms act directly on QOL, as well as on the intermediate constructs of role participation and HP. This means that depression and anxiety symptoms do not account for the lower QOL observed among HIV-positive men with lower cognitive ability and/or cognitive difficulties in everyday activities.
A strength of the SEM approach is the use of latent variables to represent constructs such as HP and QOL. This overcomes some of the limitations of the previous literature linking cognition to QOL that used social function or mental or physical health indices as proxies for QOL [22, 59, 60], or total scores from multi-item dimensional questionnaires in which items assess multiple constructs in addition to cognitive problems [20, 21].
The current results generally concord with those of other studies that have used different measures. Several groups have observed the correlation between measured and self-reported cognition [19, 22, 61,62,63]. Tozzi et al. concluded, in a study published in 2003 (n = 111) using a regression approach, that QOL is influenced by cognitive impairment and by the ability to engage in activities of everyday living [22]. This study differs from ours in that 33% were untreated with antiretrovirals and they had been referred for neuropsychological testing because of risk or suspicion of cognitive impairment and 33% were cognitively impaired. Our study was of an unselected population, although response rates indicated that the well were less likely to enter [64]. Our study identified only indirect effects (or paths) by which cognition affects QOL in HIV. Previously, Heaton et al. [19] observed that results on neuropsychological testing no longer predicted function (employment status) when self-report measures were included in a regression model. Our results suggest that impairment on cognitive testing is only associated with role participation and QOL through self-reported cognitive difficulties and its impact on meaningful activity. Simioni reached a similar conclusion by demonstrating that prevalence of functional impact secondary to cognitive impairment was higher among patients with cognitive complaints than among non-complainers, and predicted poorer QOL [13].
Although the focus of the study was on cognition, this construct was one of several falling under the umbrella term of brain health. The effects of cognitive constructs on HP and QOL were not as strong as the other brain health constructs of anxiety, depression, vitality, and motivation indicating that a global approach to brain health is needed rather than focusing on single symptoms. These findings have implications for designing interventions to mitigate the impact of brain health on QOL in men living with HIV. The current SEM analysis highlights that multi-modal interventions are likely to be more effective than those that target single symptoms or impairments. While there is interest in cognitive training and rehabilitation, interventions addressing the determinants of self-reported cognitive difficulties, such as anxiety and health beliefs about life’s meaning and stigma (see Table 1) could also be of value. Symptoms of depression had a direct effect on QOL, highlighting the importance of treating this as well as considering this depression in trials of interventions targeting QOL either directly or indirectly. Interventions would also need to provide ways for to manage external sources of stress and environmental challenges as these were impactful at all levels of the biopsychosocial model.
A unique feature of this study was to consider cognition as a quantity rather than a classification (i.e., impaired or not). To this end, we used the B-CAM [47, 65] which is a computerized battery of neuropsychological tests [48,49,50,51], with a continuous score derived from the location of the item cut-points on the Rasch model. This is a mathematically valid and relatively low-burden approach for large-scale assessment.
A limitation of SEM is that even when a directional model fits the data, directionality in a causal sense can only be confirmed by analysis of longitudinal data or interventions aimed at modifying one of the variables in the cascade that links biology to QOL.
Conclusion
This is the first time that such a complete biopsychosocial model has been tested in HIV [66]. This view of HIV outcomes is very helpful in directing more specific analyses on key variables. For example, the “beliefs” domain in personal factors includes an item related to stigma and a second SEM paper focusing on this important experience has recently been completed [67]. In addition, all of the domains included in the model are theoretically amenable to intervention and many have evidence-based interventions that could be harnessed to improve QOL.
Measured cognitive performance is considered to have widespread effects and to be an important contributor to QOL. But in this non-demented and functional sample, other constructs contributed greater explanatory strength for QOL. Self-reported cognitive difficulties were an important source of information in understanding the negative impact of lower cognitive performance on patients’ lives. The current results add to growing evidence concerning the clinical impact of HIV-associated cognitive impairment.
Abbreviations
- ADI:
-
AIDS-defining illnesses
- B-CAM©:
-
Brief Cognitive Ability Measure
- cART:
-
Combination antiretroviral therapy
- CFI:
-
Comparative Fit Index
- CNS:
-
Central nervous system
- EQ-5D-3L:
-
EuroQol 5 dimensions with 3 levels
- EQ-5D-VAS:
-
Visual analogue scale for health rating from EuroQol
- HADS:
-
Hospital Anxiety and Depression Scale
- HADS-A:
-
HADS anxiety items
- HADS-D:
-
HADS depression items
- HIV_S&S:
-
HIV-specific signs and symptoms
- HP:
-
Health perception
- HRQL:
-
Health-related quality of life
- IAKL:
-
Instrumental activities of daily living
- ICF:
-
International classification of functioning, disability and health
- MHI:
-
Mental Health Index
- MHI-A:
-
Anxiety items from MHI
- MHI-D:
-
Depression items from MHI
- MLM:
-
Maximum likelihood
- MOT:
-
Motivation
- NP:
-
Neuropsychological
- PDQ:
-
Perceived Deficits Questionnaire
- PF:
-
Physical function
- PGI:
-
Patient Generated Index
- PRO:
-
Patient-reported outcome
- QOL:
-
Quality of life
- RAND-36 GHP:
-
General health perception
- RMSEA:
-
Root mean square error of approximation
- SD:
-
Standard deviation
- SEM:
-
Structural equation model
- SRM:
-
Standardized root mean squared residual
- STDXY:
-
Standardized regression coefficient
- TICS:
-
Trier inventory for chronic stress
- TLI:
-
Tucker–Lewis Fit Index
- WHO:
-
World Health Organization
- W–C:
-
Wilson–cleary
- WHO5:
-
World Health Organization 5-item well-being index
References
Cooper, V., Clatworthy, J., Harding, R., & Whetham, J. (2017). Measuring quality of life among people living with HIV: A systematic review of reviews. Health and Quality of Life Outcomes,15, 220.
Nakagawa, F., Lodwick, R. K., Smith, C. J., Smith, R., Cambiano, V., Lundgren, J. D., et al. (2012). Projected life expectancy of people with HIV according to timing of diagnosis. AIDS,26, 335–343.
Drewes, J., Gusy, B., & Ruden, U. (2013). More than 20 years of research into the quality of life of people with HIV and AIDS: A descriptive review of study characteristics and methodological approaches of published empirical studies. Journal of the International Association of Providers of AIDS Care (JIAPAC),12, 18–22.
Ances, B. M., & Clifford, D. B. (2008). HIV-associated neurocognitive disorders and the impact of combination antiretroviral therapies. Current Neurology and Neuroscience Reports,8, 455–461.
Gongvatana, A., Harezlak, J., Buchthal, S., Daar, E., Schifitto, G., Campbell, T., et al. (2013). Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. J Neurovirol,19, 209–218.
Antinori, A., Arendt, G., Becker, J. T., Brew, B. J., Byrd, D. A., Cherner, M., et al. (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology,69, 1789–1799.
Ellis, R. J., Rosario, D., Clifford, D. B., McArthur, J. C., Simpson, D., Alexander, T., et al. (2010). Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: The CHARTER Study. Archives of Neurology,67, 552–558.
Robertson, K. R., Su, Z., Margolis, D. M., Krambrink, A., Havlir, D. V., Evans, S., et al. (2010). Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology,74, 1260–1266.
Heaton, R. K., & Pendleton, M. G. (1981). Use of neuropsychological tests to predict adult patients’ everyday functioning. Journal of Consulting and Clinical Psychology,49, 807–821.
John, M. D., Greene, M., Hessol, N. A., Zepf, R., Parrott, A. H., Foreman, C., et al. (2016). Geriatric assessments and association with VACS index among HIV-infected older adults in San Francisco. Journal of Acquired Immune Deficiency Syndromes,72, 534–541.
Hinkin, C. H., Castellon, S. A., Durvasula, R. S., Hardy, D. J., Lam, M. N., Mason, K. I., et al. (2002). Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology,59, 1944–1950.
Ettenhofer, M. L., Foley, J., Castellon, S. A., & Hinkin, C. H. (2010). Reciprocal prediction of medication adherence and neurocognition in HIV/AIDS. Neurology,74, 1217–1222.
Simioni, S., Cavassini, M., Annoni, J. M., Rimbault, A. A., Bourquin, I., Schiffer, V., et al. (2010). Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS,24, 1243–1250.
Albert, S. M., Weber, E., Todak, G., et al. (1999). An observed performance test of medication management ability in HIV: Relation to neuropsychological status and medication adherence outcomes. AIDS and Behavior,3, 121–128.
Schifitto, G., Kieburtz, K., McDermott, M. P., McArthur, J., Marder, K., Sacktor, N., et al. (2001). Clinical trials in HIV-associated cognitive impairment: Cognitive and functional outcomes. Neurology,56, 415–418.
Tozzi, V., Balestra, P., Murri, R., Galgani, S., Bellagamba, R., Narciso, P., et al. (2004). Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. International Journal of STD and AIDS,15, 254–259.
Marcotte, T. D., Heaton, R. K., Wolfson, T., Taylor, M. J., Alhassoon, O., Arfaa, K., et al. (1999). The impact of HIV-related neuropsychological dysfunction on driving behavior. The HNRC Group. Journal of the International Neuropsychological Society,5, 579–592.
Rabkin, J. G., McElhiney, M., Ferrando, S. J., Van, G. W., & Lin, S. H. (2004). Predictors of employment of men with HIV/AIDS: A longitudinal study. Psychosomatic Medicine,66, 72–78.
Heaton, R. K., Marcotte, T. D., Mindt, M. R., Sadek, J., Moore, D. J., Bentley, H., et al. (2004). The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society,10, 317–331.
Osowiecki, D. M., Cohen, R. A., Morrow, K. M., Paul, R. H., Carpenter, C. C., Flanigan, T., et al. (2000). Neurocognitive and psychological contributions to quality of life in HIV-1-infected women. AIDS,14, 1327–1332.
Parsons, T. D., Braaten, A. J., Hall, C. D., & Robertson, K. R. (2006). Better quality of life with neuropsychological improvement on HAART. Health and Quality of Life Outcomes,4, 11.
Tozzi, V., Balestra, P., Galgani, S., Murri, R., Bellagamba, R., Narciso, P., et al. (2003). Neurocognitive performance and quality of life in patients with HIV infection. AIDS Research and Human Retroviruses,19, 643–652.
Byrne, B. M. (2012). Structural equation modeling wiht Mplus: Basic concepts, applications, and programming. London: Routledge Taylor & Francis.
Kline, R. B. (1998). Principles and practice of structural equation modeling (1st ed.). New York: Guilford Press.
Wilson, I. B., & Cleary, P. D. (1995). Linking clinical variables with health-related quality of life: A conceptual model of patient outcomes. Journal of the American Medical Association,273, 59–65.
Vidrine, D. J., Amick, B. C., III, Gritz, E. R., & Arduino, R. C. (2005). Assessing a conceptual framework of health-related quality of life in a HIV/AIDS population. Quality of Life Research,14, 923–933.
Logie, C. H., Jenkinson, J. I., Earnshaw, V., Tharao, W., & Loutfy, M. R. (2016). A structural equation model of HIV-related stigma, racial discrimination, housing insecurity and wellbeing among African and Caribbean Black women living with HIV in Ontario, Canada. PLoS ONE,11, e0162826.
Logie, C. H., Ahmed, U., Tharao, W., & Loutfy, M. R. (2017). A structural equation model of factors contributing to quality of life among African and Caribbean women living with HIV in Ontario, Canada. AIDS Research and Human Retroviruses,33, 290–297.
O’Connell, K. A., & Skevington, S. M. (2012). An international quality of life instrument to assess wellbeing in adults who are HIV-positive: A short form of the WHOQOL-HIV (31 items). AIDS and Behavior,16, 452–460.
Alsayed, N. S., Sereika, S. M., Albrecht, S. A., Terry, M. A., & Erlen, J. A. (2017). Testing a model of health-related quality of life in women living with HIV infection. Quality of Life Research,26, 655–663.
Xiaowen, W., Guangping, G., Ling, Z., Jiarui, Z., Xiumin, L., Zhaoqin, L., et al. (2018). Depression and anxiety mediate perceived social support to predict health-related quality of life in pregnant women living with HIV. AIDS Care,30, 1147–1155.
Mayo, N. E., Brouillette, M. J., & Fellows, L. K. (2016). Understanding and optimizing brain health in HIV now: Protocol for a cohort multiple randomized controlled trial. BMC Neurology,16(1), 8.
Price, R. W., & Brew, B. J. (1988). The AIDS dementia complex. Journal of Infectious Diseases,158, 1079–1083.
World Health Organization. (2001). International classification of functioning, disability and health (2nd revision ed.). Geneva: World Health Organization.
Hays, R. D., & Morales, L. S. (2001). The RAND-36 measure of health-related quality of life. Annals of Medicine,33, 350–357.
O’Connell, K. A., & Skevington, S. M. (2012). An international quality of life instrument to assess wellbeing in adults who are HIV-positive: A short form of the WHOQOL-HIV (31 items). AIDS and Behavior,16, 452–460.
Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica,67, 361–370.
Topp, C. W., Ostergaard, S. D., Sondergaard, S., & Bech, P. (2015). The WHO-5 well-being index: A systematic review of the literature. Psychotherapy and Psychosomatics,84, 167–176.
Starkstein, S. E., Fedoroff, J. P., Price, T. R., Leiguarda, R., & Robinson, R. G. (1993). Apathy following cerebrovascular lesions. Stroke,24, 1625–1630.
Mayo, N. E., Lourenco, C., Finch, L. E., & Fellows, L. K. (2015). Apathy, who cares? Stroke,45, e259–e298.
Holzemer, W. L., Hudson, A., Kirksey, K. M., Hamilton, M. J., & Bakken, S. (2001). The revised sign and symptom check-list for HIV (SSC-HIVrev). Journal of the Association of Nurses in AIDS Care,12, 60–70.
Sullivan, M., Edgley, K., DeHousx, E. (1990). A survey of multiple sclerosis, part 1: Perceived cognitive problems and compensatory strategy use. Canadian Journal of Rehabilitation, 4, 99–105.
Kind, P. (1995). The EuroQol instrument: An index of health-related quality of life. In B. Spilker (Ed.), Quality of life and pharmacoeconomics in clinical trials (pp. 191–201). Philadelphia: Lippincott-Raven Publishers.
Ruta, D. A., Garratt, A. M., Leng, M., Russell, I. T., & MacDonald, L. M. (1994). A new approach to the measurement of quality of life. The Patient-Generated Index. Medical Care,32, 1109–1126.
Petrowski, K., Paul, S., Albani, C., & Brahler, E. (2012). Factor structure and psychometric properties of the trier inventory for chronic stress (TICS) in a representative German sample. BMC Medical Research Methodology,12, 42.
Charlson, M. E., Pompei, P., Ales, K. L., & MacKenzie, C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases,40, 373–383.
Brouillette, M. J., Fellows, L. K., Palladini, L., Finch, L., Thomas, R., & Mayo, N. E. (2015). Quantifying cognition at the bedside: A novel approach combining cognitive symptoms and signs in HIV. BMC Neurol,15, 224.
Robbins, T. W., James, M., Owen, A. M., Sahakian, B. J., Lawrence, A. D., McInnes, L., et al. (1998). A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. Journal of the International Neuropsychological Society,4, 474–490.
Reitan, R. M., & Wolfson, D. (1985). The Halstead-Reitan neuropsycholgical test battery: Therapy and clinical interpretation. Tuscon, AZ: Neuropsychology Press.
Modirrousta, M., & Fellows, L. K. (2008). Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. Journal of Neuroscience,28, 14000–14005.
Delis, D. C., Kaplan, E., & Kramer, J. (2001). Delis Kaplan executive function system. San Antonio, TX: Peason.
Heaton, R. K., Clifford, D. B., Franklin, D. R., Jr., Woods, S. P., Ake, C., Vaida, F., et al. (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology,75, 2087–2096.
Andrich, D. (2005). Rasch models for ordered response categories. Encyclopedia of Statistics in Behavioral Science,4, 1698–1707.
Buysse, D. J., Reynolds, C. F., III, Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research,28, 193–213.
Bouchard, V., Duquette, P., & Mayo, N. E. (2017). Path to illness intrusiveness: What symptoms impact the life of people living with multiple sclerosis? Archives of Physical Medicine and Rehabilitation,98, 1357–1365.
Schermelleh-Engel, K., Moosbrugger, H., & Muller, H. (2003). Evaluating the fit of structural equations models: Tests of significance and descriptive goodness-of-fit measures. Methods of Psychological Research Online,8, 23–74.
Yoo-Jeong, M., Anderson, A., Rahman, A. F., Baumann, M., McBroom, J., Waldrop-Valverde, D. (2018). Associations of mood on objective and subjective cognitive complaints in persons living with HIV/AIDS. Journal of HIV and AIDS. https://doi.org/10.16966/2380-5536.146.
Claypoole, K. H., Elliott, A. J., Uldall, K. K., Russo, J., Dugbartey, A. T., Bergam, K., et al. (1998). Cognitive functions and complaints in HIV-1 individuals treated for depression. Applied Neuropsychology,5, 74–84.
Moore, R. C., Fazeli, P. L., Jeste, D. V., Moore, D. J., Grant, I., & Woods, S. P. (2014). Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS and Behavior,18, 1186–1197.
Morgan, E. E., Iudicello, J. E., Weber, E., Duarte, N. A., Riggs, P. K., Delano-Wood, L., et al. (2012). Synergistic effects of HIV infection and older age on daily functioning. Journal of Acquired Immune Deficiency Syndromes,61, 341–348.
Revicki, D. A., Chan, K., & Gevirtz, F. (1998). Discriminant validity of the medical outcomes study cognitive function scale in HIV disease patients. Quality of Life Research,7, 551–559.
Carter, S. L., Rourke, S. B., Murji, S., Shore, D., & Rourke, B. P. (2003). Cognitive complaints, depression, medical symptoms, and their association with neuropsychological functioning in HIV infection: A structural equation model analysis. Neuropsychology,17, 410–419.
Rourke, S. B., Halman, M. H., & Bassel, C. (1999). Neurocognitive complaints in HIV-infection and their relationship to depressive symptoms and neuropsychological functioning. Journal of Clinical and Experimental Neuropsychology,21, 737–756.
Mayo, N. E., Brouillette, M. J., & Fellows, L. K. (2018). Estimates of prevalence of cognitive impairment from research studies can be affected by selection bias. Journal of Acquired Immune Deficiency Syndromes,78, e7–e8.
Brouillette, M. J., Mayo, N., Fellows, L. K., Lebedeva, E., Higgins, J., Overton, E. T., et al. (2015). A better screening tool for HIV-associated neurocognitive disorders: Is it what clinicians need? AIDS,29, 895–902.
Ojelabi, A. O., Graham, Y., Haighton, C., & Ling, J. (2017). A systematic review of the application of Wilson and Cleary health-related quality of life model in chronic diseases. Health and Quality of Life Outcomes,15, 241.
Lam, A., Mayo, N. E., Scott, S., Brouillette, M. J., & Fellows, L. K. (2019). HIV-related stigma affects cognition in older men living with HIV. Journal of Acquired Immune Deficiency Syndromes,80, 198–204.
Acknowledgements
We want to thank the participants of the Positive Brain Health Now cohort study as well as the community members of the research team and the organizations. They represent COCQ -Sida (Ken Monteith); Aids Committee of Toronto (Sarah Schultz); Positive Living BC (Hesham Ali).
Funding
This project was supported by grants from the Canadian Institutes of Health Research (LKF, MJB, NM, TCO-125272), the CIHR Canadian HIV Trials Network (CTN 273), and salary support from the Fonds de Recherche Santé du Québec (LKF) and the Research Institute of the McGill University Health Centre (MJB). None of these funding agencies played any role in the design, data collection, analysis, or interpretation of the study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The project was approved by the Research Ethics Board of each of the participating institutions.
Informed consent
All participants provided informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mayo, N.E., Brouillette, MJ., Scott, S.C. et al. Relationships between cognition, function, and quality of life among HIV+ Canadian men. Qual Life Res 29, 37–55 (2020). https://doi.org/10.1007/s11136-019-02291-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-019-02291-w