Abstract
Purpose
Sleep disturbance is common in late life. While social interaction is a basic human concern, few studies have explored the linkage between interpersonal relationships and sleep disturbance. The present study examines the reciprocal associations between weak social networks outside the household and sleep disturbance in elderly, as well as the underlying mechanisms.
Methods
We utilized data from a nationally representative longitudinal survey of community-dwelling elderly in Singapore (n = 1417; ≥ 60 years). Participants were assessed three times over 6 years (2009, 2011, 2015). Measures included strength of social networks outside the household, restless sleep (sleep disturbance), and the mediating variables of depressed mood, chronic diseases, and cognitive impairment. A cross-lagged mediation analysis was conducted.
Results
Bootstrapping results showed that weaker social networks were related to more restless sleep via more depressed mood. Also, restless sleep was negatively associated with social networks through depressed mood. The other mediators examined were not significant.
Conclusions
Weak social networks and restless sleep reciprocally influence each other through depressed mood. Recognition of this interplay can inform efforts in improving elderly’s sleep quality, social networks, and psychological well-being.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep disturbance (used interchangeably with restless sleep, insomnia, poor sleep quality, sleep complaint, sleep fragmentation, and sleep problem) [1,2,3] is an important aspect of quality of life [4]. It is prevalent in late life, with over one-third of elderly in the United States (U.S.) reporting sleep complaints [5]. Given the unfavorable consequences of sleep disturbance including poor health outcomes and increased healthcare utilization, it is essential to understand its determinants among elderly. The literature has primarily focused on the role of health factors, including depressive symptoms and physical illness [3]. Surprisingly, despite the fact that social interaction is a fundamental human concern [6], data on the linkage between interpersonal relationships and sleep disturbance is lacking, particularly from longitudinal research [1, 4, 7]. A notable exception is a 4-year study which showed that poorer marital quality predicted more sleep disturbance in elderly [8]. It also reported that more sleep disturbance predicted poorer marital quality in the future, thereby revealing a mutually reinforcing cycle between weak marital relations and poor sleep.
The present study addresses two gaps in the literature of the linkage between interpersonal relationships and sleep disturbance among elderly. First, social networks with friends and relatives outside the household have been understudied. We posit the importance of analyzing strength of social networks outside the household especially for the elderly as they tend to live with very few household members or even live alone after the demise of their spouses and/or when their children have moved out [9]. Cross-sectional data have suggested that stronger relations with friends and relatives may lead to less sleep disturbance [10, 11]. There is also evidence suggesting the reverse of this association as a longitudinal study has revealed that poor sleep quality limits social activities (e.g., visiting friends and relatives) [12]. In view of these argument and findings, we examined the potential reciprocal associations between weak social networks outside the household and sleep disturbance in elderly.
Second, there is a lack of empirical scrutiny of the underlying mechanisms of the linkage between interpersonal relationships and sleep disturbance [1, 7]. One suggested mechanism is poor health [13], but the mediating effect of poor health remains to be tested. Here, we investigated the mediating role of three health conditions—depressed mood, chronic diseases, and cognitive impairment—to explain the reciprocal associations between weak social networks outside the household and sleep disturbance. Studying these intertwined health factors together enables the assessment of their relative importance [14]. Furthermore, deeper knowledge of how sleep disturbance is related to social networks is crucial as it can inform interventions for the enhancement of elderly’s quality of life [1].
The literature on depressive symptoms suggests that depressed mood is a potential mediator for the reciprocal associations between weak social networks and sleep disturbance. Weaker social networks are linked with greater depressive symptoms among the elderly [9]. Depressive symptoms share some common physiopathology with sleep disturbance, such as altered activity of the hypothalamic–pituitary–adrenal (HPA) axis [15]. Gerontological studies have documented that depressive symptoms contribute to sleep disturbance [3]. There is also evidence showing that sleep disturbance elevates depressive symptoms [15]. Further, depressive symptoms may result in social withdrawal and are detrimental to the maintenance of social networks [14]. Collectively, the reciprocal associations between weak social networks and sleep disturbance may be mediated by depressed mood.
The presence of chronic diseases is another potential mediator of the reciprocal associations between weak social networks and sleep disturbance. Lack of social ties may result in comorbidity [16], which is a risk factor for sleep disturbance [3, 17]. Regarding the reverse direction, given its linkage with metabolism, thermoregulation, immunofunction, and respiratory and cardiovascular processes, poor sleep quality increases the risk of comorbidity [17]. Comorbidity may in turn hinder socializing outside the household in elderly [14].
Finally, some studies have shown that weak social networks increase the risk of cognitive impairment [18], and that elderly who are cognitively impaired experience more sleep disturbance than their cognitively normal counterparts [19]. In addition, sleep disturbance may contribute to cognitive impairment through neurodegeneration and neurotransmitter changes [20], and poor cognitive function may inhibit social activities and result in weaker social networks [14]. These perspectives and findings together suggest that cognitive impairment may also be a mediator.
All in all, weak social networks outside the household may be reciprocally associated with sleep disturbance in elderly through depressed mood (Hypothesis 1), chronic diseases (Hypothesis 2), and cognitive impairment (Hypothesis 3). Here, we examined these hypotheses using longitudinal data collected in three waves over 6 years from a sample of community-dwelling elderly in Singapore.
Methods
Participants
The data used in the present investigation are from the Panel on Health and Ageing of Singaporean Elderly (PHASE), which is a nationally representative longitudinal survey of community-dwelling elderly (citizens and permanent residents) in Singapore. Data collection/analysis was approved by the Institutional Review Board at National University of Singapore. In 2009 (Time 1; T1), a single-stage stratified (by age, gender, and ethnicity, based on the 2007 population distribution) random sampling method was adopted to recruit participants aged 60 years or above. A total of 8400 elderly was drawn from the national database of dwellings. Individuals aged ≥ 75 years, and non-Chinese (Malays and Indians) were oversampled by a factor of two to ensure sufficient numbers in these groups for future analyses. 1195 addresses were found to be invalid. Of the rest, 4990 elderly (69.3% response rate) were interviewed face-to-face with a structured survey at their residence after informed consent. 357 of these participants had died before the T2 data collection in 2011. 1530 individuals refused to participate in the follow-up interviews at T2 or were not contactable. At T2, 3103 follow-up interviews were conducted. Of these T2 participants, 423 had died before the T3 data collection in 2015. 1108 individuals refused to participate in the follow-up interviews at T3 or were uncontactable. At T3, 1572 follow-up interviews were conducted. Incentives for the T1, T2, and T3 survey interviews were SG$15, 30, and 30, respectively.
Figure 1 illustrates the sample selection for the current study. At all three time points, proxy interviews (mostly spouse or adult child) were conducted when the elderly were unable to respond themselves due to health reasons. At T2 and T3, a cognitive test [Short Portable Mental Status Questionnaire (SPMSQ)] [21] was administered as a screening tool. Proxy interviews were also conducted for the elderly who were identified as having severe cognitive impairment (8–10 errors on SPMSQ). Elderly assessed through proxy interviews were excluded here because key variables of interest were not assessed through the proxies.
The analytical sample involved the 1417 elderly who were able to provide self-reported data and were not severely cognitively impaired in all three waves. This sample size was sufficient for our proposed cross-lagged mediation analysis [22]. Table 1 presents the characteristics of the analytical sample at baseline. Attrition analysis revealed that the values of the focal variables and covariates assessed at T1 were similar (η2s ≤ 0.05) between the analytical sample and the individuals who participated at T1 only, indicating that attrition was not a serious concern [23]. The analytical sample at baseline (53.6% aged 60–69, 36.0% aged 70–79, 10.4% aged 80 or above; 42.2% males, 74.0% Chinese) resembled the 2007 population (54.3% aged 60–69, 32.9% aged 70–79, 12.8% aged 80 or above; 45.1% males, 75.0% Chinese).
Measures
Five focal variables were assessed in all three waves: social networks, restless sleep, depressed mood, chronic diseases, and cognitive impairment.
Social networks with friends and relatives outside the household
Strength of social networks outside the household was assessed with the modified Lubben’s social network scale [24]. Local research has reported findings that support its validity [9]. It comprises 12 items and captures network size, frequency of contact, and closeness with relatives and friends outside the household (e.g., “How many friends do you see or hear from at least once a month?” [0 = 0 friends, 5 = 9 friends or more], “How often do you see or hear from relatives with whom you have the most contact?” [0 = never, 5 = always]). A higher score indicates stronger social networks outside the household (score range 0–60; Cronbach’s alpha = 0.92, 0.87, 0.88 for T1, T2, and T3, respectively).
Restless sleep
Using a commonly accepted methodology [1, 2, 25,26,27], we operationalized sleep disturbance in terms of restless sleep. The “my sleep was restless” item was extracted from the 11-item Center for Epidemiological Studies Depression (CES-D) Scale [28], which has been found to be a valid measure in the local context [9]. Similar to some other surveys [2], in PHASE, participants’ responses were recorded on a 3-point scale (0 = none/rarely, 1 = sometimes, 2 = often).
Depressed mood
We measured depressed mood using the 5 mood items (e.g., “I felt sad”) of the CES-D Scale (3-point: 0 = none/rarely, 1 = sometimes, 2 = often) [28]. The “my sleep was restless” item and other somatic items of the scale were excluded [2, 26, 27]. The interpersonal items were also excluded because social networks was a focal variable. These practices are commonly exercised to avoid item overlapping and effect size inflation in the analysis [16]. A higher total score (score range 0–10; Cronbach’s alpha = 0.78, 0.74, 0.78) indicates a higher level of depressed mood.
Chronic diseases
Participants reported their number of chronic diseases, with a maximum of 10 diseases: heart attack, other heart condition, cancer, cerebrovascular disease, high blood pressure, diabetes, respiratory illness, digestive illness, renal/kidney or urinary tract ailment, and liver/gallbladder ailment.
Cognitive impairment
Level of cognitive impairment was assessed with the 10-item SPMSQ [21], which tests orientation to time and place, memory, current event information, and executive function (score range 0–10; higher score indicating greater cognitive impairment). A previous local study has illustrated its convergent validity [29]. The reliability of SPMSQ was relatively low (Cronbach’s alpha = 0.71, 0.64, 0.60). Despite these values, we retained this validated instrument as is and did not remove any item to deliberately increase its reliability [30].
Covariates
We included seven sociodemographic and two health behavior covariates [14]: age, gender, ethnicity, education level, housing type (an indicator of socioeconomic status in Singapore), marital status, household size (number of people the participant lived with), current smoking status, and physical exercise (average score of 2 items [walking for exercise purposes, playing a game of sport]; 1 = not at all, 5 = every day).
Analysis
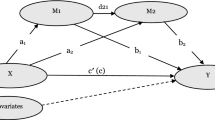
Using Mplus version 7.4, we carried out a cross-lagged mediation analysis [31] with the mean- and variance-adjusted weighted least squares (WLSMV) estimation (Fig. 2), which adopts a pairwise present approach to handle missing data. In our statistical model, we regressed the mediating variables (depressed mood, chronic diseases, and cognitive impairment)time t on social networkstime t−1, and regressed restless sleep time t on the mediating variablestime t−1 and social networkstime t−1. Also, we regressed the mediating variablestime t on restless sleeptime t−1, and regressed social networkstime t on the mediating variablestime t−1 and restless sleeptime t−1. Covariates (e.g., age), concurrent covariances (e.g., T1 social networks with T1 depressed mood), and autoregressive effects (e.g., effect of T1 depressed mood on T2 depressed mood) were taken into account.
Cross-lagged mediation analysis. Black lines refer to the impact of social networks on the mediating variables (depressed mood, chronic diseases, and cognitive impairment) and restless sleep, and that of the mediating variables on restless sleep. Gray lines refer to the impact of restless sleep on the mediating variables and social networks, and that of the mediating variables on social networks. Permitted by our dataset, nine covariates assessed at T1 were controlled for in the prediction of T2 variables. For the prediction of T3 variables, household size and exercise assessed at T2, as well as other covariates assessed at T1 (age, gender, ethnicity, education level, housing type, marital status, smoking) were controlled for. For simplicity, covariates, concurrent covariances, and autoregressive effects are not shown
The model fit indices of CFI, RMSEA, and WRMR were considered. While a CFI value of < 0.90 indicates a poor model fit, for RMSEA and WRMR, values larger than 0.10 and 1.00 suggest model rejection [32]. We did not consider χ2 statistic (significant value suggests that the model does not fit the data well) and TLI (value of < 0.90 suggests a poor model fit) as χ2 is likely to be statistically significant when the sample size is large, and TLI tends to be biased and appear low when the model is complex [33].
Indirect effect is the product term of the regression coefficient of the T1 predictor–T2 mediator relationship and that of the T2 mediator–T3 outcome relationship. When these two coefficients were significant, we computed the indirect effect and evaluated its significance level with reference to the 95% bias-corrected bootstrap confidence intervals (CI95). A CI95 that excludes zero indicates a significant indirect effect. Bootstrapping procedures (2000 samples) were employed because the assumption of a normal distribution of indirect effects is often violated [34].
Results
About one-third (28, 30, and 38% at T1, T2, and T3, respectively) of our sample of Singaporean elderly experienced restless sleep (reporting “sometimes” or “often” on “my sleep was restless”). Cross-lagged mediation analysis reported the following model fit findings: χ2 (63) = 207.65, p < .001, CFI = 0.98, TLI = 0.88, RMSEA = 0.04, WRMR = 0.49. Given our large sample size and complex model, attention should be given to CFI, RMSEA, and WRMR, which suggest that our model fitted the data well. Results showed that 21, 17, 73, 41, and 20% of variance of T2 social networks, T2 depressed mood, T2 chronic diseases, T2 cognitive impairment, and T2 restless sleep were accounted for. In addition, 23, 15, 38, 31, and 22% of variance of T3 social networks, T3 depressed mood, T3 chronic diseases, T3 cognitive impairment, and T3 restless sleep were explained.
There was a significant impact of T2 restless sleep on T3 social networks (standardized coefficient [β] = − 0.08, p = .026). However, the effect of T1 restless sleep on T2 social networks, and those of T1 (T2) social networks on T2 (T3) restless sleep were non-significant. Overall, there was little evidence revealing that social networks and restless sleep were directly related (Figs. 3, 4).
Impact of social networks on the mediating variables (depressed mood, chronic diseases, and cognitive impairment) and restless sleep, and that of the mediating variables on restless sleep. Standardized coefficients are reported. Solid lines indicate statistically significant paths (p < .05), while dotted lines denote non-significant paths. For simplicity, covariates, concurrent covariances, and autoregressive effects are not shown
Impact of restless sleep on the mediating variables (depressed mood, chronic diseases, and cognitive impairment) and social networks, and that of the mediating variables on social networks. Standardized coefficients are reported. Solid lines indicate statistically significant paths (p < .05), while dotted lines denote non-significant paths. For simplicity, covariates, concurrent covariances, and autoregressive effects are not shown
Weaker social networks at T1 led to higher levels of depressed mood at T2 (β = − 0.08, p = .002). A similar pattern was observed for the temporal relationship between T2 social networks and T3 depressed mood (β = − 0.17, p < .001). Moreover, T1 depressed mood contributed to T2 restless sleep (β = 0.11, p = .001), and T2 depressed mood contributed to T3 restless sleep (β = 0.08, p = .019). Bootstrapping procedures revealed that the indirect effect of T1 social networks on T3 restless sleep via T2 depressed mood was statistically significant (indirect effect = − 0.006, CI95 = [− 0.017, − 0.001]).
Restless sleep at T1 entailed higher levels of depressed mood at T2 (β = 0.13, p < .001). There was a similar temporal relationship between T2 restless sleep and T3 depressed mood (β = 0.15, p < .001). Further, there was an impairing effect of T1 depressed mood on T2 social networks (β = − 0.07, p = .012), and that of T2 depressed mood on T3 social networks (β = − 0.08, p = .003). Bootstrapping procedures showed that T1 restless sleep had a significant indirect effect on T3 social networks through T2 depressed mood (indirect effect = − 0.011, CI95 = [− 0.024, − 0.003]). In sum, our data supported Hypothesis 1: the reciprocal associations between weak social networks and restless sleep were mediated by depressed mood.
The paths linking chronic diseases and cognitive impairment with social networks and restless sleep were non-significant, with the association between T1 chronic diseases and T2 restless sleep as the only exception (β = 0.09, p = .001). Overall, chronic diseases and cognitive impairment were not significant mediators. Hypotheses 2 and 3 were not supported.
Discussion
Sleep disturbance is prevalent in late life. Its prevalence in our sample of elderly in Singapore was 28–38%, which was comparable to that in the U.S [5]. Nevertheless, there has been a lack of research on the linkage between sleep disturbance and social networks outside the household, an important aspect of the elderly’s social quality of life. Specifically, although several mediating processes might be present as suggested by some prior studies, their unique and relative contributions have not been subject to empirical investigation. The present longitudinal study addressed these longstanding research gaps, and revealed that restless sleep is reciprocally associated with weak social networks, and that these associations are mediated by depressed mood.
These mediated associations can be explained by the documented mutual influences between sleep disturbance and depressive symptoms, and those between weak social networks and depressive symptoms. Aging is associated with an attenuated circadian rhythm which disrupts sleep and mood regulation [35]. Furthermore, various age-related changes in the HPA axis increase nocturnal awakenings and sleep fragmentation in the elderly [36] and are linked with depressive symptoms [37]. Psychologically, depressed individuals may ruminate on their negative thoughts [38], and such arousal impairs sleep quality [39, 40]. Simultaneously, sleep disturbance, which could be regarded as a stressor [41], may elevate depressive symptoms through arousing maladaptive beliefs and hopelessness [42].
Existing literature has also documented the mutual influences between weak social networks and depressive symptoms. Weaker social networks are associated with greater depressive symptoms in the elderly [9]. Conversely, depressive symptoms drive social withdrawal [14]: depressed individuals may find it difficult to offer support and return favors to social contacts. The (anticipated) failure to meet the social norm of reciprocity may reduce efforts in maintaining social networks.
Collectively, weak social networks and restless sleep reciprocally influence each other through depressed mood, as illustrated in the present longitudinal study. To strengthen the causal claims, future studies may attempt to manipulate social networks in a laboratory setting [43] and examine its effect on mood and sleep. Studies in which researchers modulate sleep quality [44] to investigate its impact on mental health and social networks are also useful. Moreover, to consolidate our understanding of the mediating effect of depressed mood, future studies should address the role of the relevant processes (e.g., HPA axis activity, rumination) in the social networks—sleep disturbance linkage.
Contrary to some previous research [16, 18], our data revealed that social networks were unrelated to chronic diseases and cognitive impairment. These differences across studies could be due to different operationalization and study time frame, which should be addressed in future meta-analyses. Our data also showed that only depressed mood was associated with restless sleep. This observation should not be interpreted as an artefact that the items for measuring these two constructs were from the same scale (i.e., CES-D Scale). The validity of the CES-D sleep item as a measure of restless sleep/sleep disturbance has been documented [25]. In fact, researchers have been using items from the CES-D Scale to examine the relationship between sleep disturbance and depressive symptoms [2, 26, 27]. Generally speaking, as described above, the mutual influences between depressive symptoms and sleep disturbance have been widely documented. Importantly, concurring our data, some past research has revealed that depressive symptoms are more strongly related to sleep disturbance than other health facets [45, 46]. Nevertheless, future studies should adopt more refined, multi-item measures (e.g., Pittsburgh Sleep Quality Index) [47] to quantify sleep disturbance. Sleep should also be evaluated objectively with actigraphy and polysomnography in the future. Replication studies using other measures for depressive symptoms will also be helpful.
We also acknowledge that we did not assess the quality of interpersonal relationships with household members, whereas previous research reported reciprocal associations between poor marital quality and sleep disturbance among elderly [8]. Future studies may consider the quality of different types of interpersonal relationships and examine how they are linked with sleep disturbance through pathways representing different aspects of impaired health. At the same time, the strength of the present study lies in its large sample size. Moreover, it involved a longitudinal design with three waves of data collection over a 6-year period, allowing us to utilize cross-lagged mediation analysis.
Furthermore, it is worth pointing out that depression is a psychiatric condition. Depressive symptoms are manifestations of depression, but they are not specific to depression. While our data have revealed a temporal impact of restless sleep on depressed mood, which is a depressive symptom, we cannot conclude that restless sleep causes depression. Also, while our data have revealed a temporal effect of depressed mood on restless sleep, they do not indicate that pre-clinical signs of depression, such as perceived worthlessness, cause restless sleep. Similarly, the findings that weak social networks were temporally associated with depressed mood do not indicate that weak social networks lead to a clinical diagnosis of depression.
In any event, the observed interplay among social networks, restless sleep, and depressed mood have three implications on health interventions. First, cultivation of social networks may benefit mood and sleep among elderly. One should note, however, that it is not necessarily useful to initiate treatment to reinforce social networks before tackling the problem of lack of motivation. Second, interventions aiming at improving elderly’s sleep quality may contribute to their psychological and social well-being. Third, treatments that address mood should also be in place to improve elderly’s sleep as well as their social networks. Collectively, these efforts may prevent the formation and break the links of the vicious cycle between weak social networks and sleep disturbance in late life.
References
Bassett, E., & Moore, S. (2014). Neighbourhood disadvantage, network capital and restless sleep: Is the association moderated by gender in urban-dwelling adults? Social Science & Medicine, 108, 185–193.
Leggett, A., Burgard, S., & Zivin, K. (2016). The impact of sleep disturbance on the association between stressful life events and depressive symptoms. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 71(1), 118–128.
Smagula, S. F., Stone, K. L., Fabio, A., & Cauley, J. A. (2016). Risk factors for sleep disturbances in older adults: Evidence from prospective studies. Sleep Medicine Reviews, 25, 21–30.
Zhang, H.-S., Li, Y., Mo, H., Qiu, D.-X., Zhao, J., Luo, J.-L., et al. (2017). A community-based cross-sectional study of sleep quality in middle-aged and older adults. Quality of Life Research, 26(4), 923–933.
National Sleep Foundation. (2003). The 2003 sleep in America poll. Retrieved May 4, 2017, from https://sleepfoundation.org/sleep-polls-data/sleep-in-america-poll/2003-sleep-and-aging.
Baumeister, R. F., & Leary, M. R. (1995). The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin, 117(3), 497–529.
Chen, J.-H., Waite, L. J., & Lauderdale, D. S. (2015). Marriage, relationship quality, and sleep among U.S. older adults. Journal of Health and Social Behavior, 56(3), 356–377.
Yang, H. C., Suh, S., Kim, H., Cho, E. R., Lee, S. K., & Shin, C. (2013). Testing bidirectional relationships between marital quality and sleep disturbances: A 4-year follow-up study in a Korean cohort. Journal of Psychosomatic Research, 74(5), 401–406.
Chan, A., Malhotra, C., Malhotra, R., & Ostbye, T. (2011). Living arrangements, social networks and depressive symptoms among older men and women in Singapore. International Journal of Geriatric Psychiatry, 26(6), 630–639.
Yao, K. W., Yu, S., Cheng, S. P., & Chen, I. J. (2008). Relationships between personal, depression and social network factors and sleep quality in community-dwelling older adults. Journal of Nursing Research, 16(2), 131–139.
Ailshire, J. A., & Burgard, S. A. (2012). Family relationships and troubled sleep among U.S. Adults: Examining the influences of contact frequency and relationship quality. Journal of Health and Social Behavior, 53(2), 248–262.
Baker, S., McBeth, J., Chew-Graham, C. A., & Wilkie, R. (2017). Musculoskeletal pain and co-morbid insomnia in adults: A population study of the prevalence and impact on restricted social participation. BMC Family Practice, 18(1), 17.
Troxel, W. M., Robles, T. F., Hall, M., & Buysse, D. J. (2007). Marital quality and the marital bed: Examining the covariation between relationship quality and sleep. Sleep Medicine Reviews, 11(5), 389–404.
Li, T., & Zhang, Y. (2015). Social network types and the health of older adults: Exploring reciprocal associations. Social Science & Medicine, 130, 59–68.
Bao, Y.-P., Han, Y., Ma, J., Wang, R.-J., Shi, L., Wang, T.-Y., et al. (2017). Cooccurrence and bidirectional prediction of sleep disturbances and depression in older adults: Meta-analysis and systematic review. Neuroscience & Biobehavioral Reviews, 75, 257–273.
Coyle, C. E., & Dugan, E. (2012). Social isolation, loneliness and health among older adults. Journal of Aging and Health, 24(8), 1346–1363.
Lemola, S., & Richter, D. (2013). The course of subjective sleep quality in middle and old adulthood and its relation to physical health. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 68(5), 721–729.
Holtzman, R. E., Rebok, G. W., Saczynski, J. S., Kouzis, A. C., Wilcox Doyle, K., & Eaton, W. W. (2004). Social network characteristics and cognition in middle-aged and older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 59(6), P278-P284.
Hita-Yanez, E., Atienza, M., & Cantero, J. L. (2013). Polysomnographic and subjective sleep markers of mild cognitive impairment. Sleep, 36(9), 1327–1334.
Yaffe, K., Falvey, C. M., & Hoang, T. (2014). Connections between sleep and cognition in older adults. Lancet Neurology, 13(10), 1017–1028.
Pfeiffer, E. (1975). A Short Portable Mental Status Questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatrics Society, 23(10), 433–441.
MacCallum, R. C., Browne, M. W., & Sugawara, H. M. (1996). Power analysis and determination of sample size for covariance structure modeling. Psychological Methods, 1(2), 130–149.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2 ed.). Hillsdale, NJ: Lawrence Erlbaum Associates.
Lubben, J., & Gironda, M. (2004). Measuring social networks and assessing their benefits. In C. Phillipson, G. Allan, & D. Morgan (Eds.), Social networks and social exclusion: Sociological and policy perspectives (pp. 20–34). Burlington, VT: Ashgate.
Burgard, S. A., & Ailshire, J. A. (2009). Putting work to bed: Stressful experiences on the job and sleep quality. Journal of Health and Social Behavior, 50(4), 476–492.
Devins, G. M., Edworthy, S. M., Paul, L. C., Mandin, H., Seland, T. P., Klein, G., et al. (1993). Restless sleep, illness intrusiveness, and depressive symptoms in three chronic illness conditions: Rheumatoid arthritis, end-stage renal disease, and multiple sclerosis. Journal of Psychosomatic Research, 37(2), 163–170.
Kutner, N. G., Bliwise, D. L., Brogan, D., & Zhang, R. (2001). Race and restless sleep complaint in older chronic dialysis patients and nondialysis community controls. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 56(3), P170-P175.
Kohout, F. J., Berkman, L. F., Evans, D. A., & Cornoni-Huntley, J. (1993). Two shorter forms of the CES-D depression symptoms index. Journal of Aging and Health, 5(2), 179–193.
Malhotra, C., Chan, A., Matchar, D., Seow, D., Chuo, A., & Do, Y. K. (2013). Diagnostic performance of short portable mental status questionnaire for screening dementia among patients attending cognitive assessment clinics in Singapore. Annals of the Academy of Medicine, Singapore, 42(7), 315–319.
Lin, X., & Leung, K. (2010). Differing effects of coping strategies on mental health during prolonged unemployment: A longitudinal analysis. Human Relations, 63(5), 637–665.
Newsom, J. T. (2015). Longitudinal structural equation modeling: A comprehensive introduction. New York: Routledge.
Wang, J., & Wang, X. (2012). Structural equation modeling: Applications using Mplus. Chichester: Wiley.
Kline, R. B. (2016). Principles and practice of structural equation modeling (4 ed.). New York: The Guilford Press.
Geiser, C. (2013). Data analysis with Mplus. New York: The Guilford Press.
Kondratova, A. A., & Kondratov, R. V. (2012). The circadian clock and pathology of the ageing brain. Nature Reviews Neuroscience, 13(5), 325–335.
Buckley, T. M., & Schatzberg, A. F. (2005). Aging and the role of the HPA axis and rhythm in sleep and memory-consolidation. American Journal of Geriatric Psychiatry, 13(5), 344–352.
Belvederi Murri, M., Pariante, C., Mondelli, V., Masotti, M., Atti, A. R., Mellacqua, Z., et al. (2014). HPA axis and aging in depression: Systematic review and meta-analysis. Psychoneuroendocrinology, 41, 46–62.
Zetsche, U., D’Avanzato, C., & Joormann, J. (2012). Depression and rumination: Relation to components of inhibition. Cognition and Emotion, 26(4), 758–767.
Thomsen, D. K., Mehlsen, M. Y., Hokland, M., Viidik, A., Olesen, F., Avlund, K., et al. (2004). Negative thoughts and health: Associations among rumination, immunity, and health care utilization in a young and elderly sample. Psychosomatic Medicine, 66(3), 363–371.
Thomsen, D. K., Yung Mehlsen, M., Christensen, S., & Zachariae, R. (2003). Rumination—relationship with negative mood and sleep quality. Personality and Individual Differences, 34(7), 1293–1301.
Wong, J. Y.-H., Fong, D. Y.-T., & Chan, K. K.-W. (2015). Anxiety and insomnia as modifiable risk factors for somatic symptoms in Chinese: A general population-based study. Quality of Life Research, 24(10), 2493–2498.
Sadler, P., McLaren, S., & Jenkins, M. (2013). A psychological pathway from insomnia to depression among older adults. International Psychogeriatrics, 25(8), 1375–1383.
Riva, P., & Eck, J. (Eds.). (2016). Social exclusion: Psychological approaches to understanding and reducing its impact. Cham: Springer.
Yang, P. Y., Ho, K. H., Chen, H. C., & Chien, M. Y. (2012). Exercise training improves sleep quality in middle-aged and older adults with sleep problems: A systematic review. Journal of Physiotherapy, 58(3), 157–163.
Quan, S. F., Katz, R., Olson, J., Bonekat, W., Enright, P. L., Young, T., et al. (2005). Factors associated with incidence and persistence of symptoms of disturbed sleep in an elderly cohort: The Cardiovascular Health Study. American Journal of the Medical Sciences, 329(4), 163–172.
Zimmerman, M. E., Bigal, M. E., Katz, M. J., Derby, C. A., & Lipton, R. B. (2013). Are sleep onset/maintenance difficulties associated with medical or psychiatric comorbidities in nondemented community-dwelling older adults? Journal of Clinical Sleep Medicine, 9(4), 363–369.
Buysse, D. J., Reynolds, C. F. III, Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213.
Acknowledgements
This research was supported by Singapore Ministry of Social and Family Development; Singapore Ministry of Health’s National Medical Research Council under its Singapore Translational Research Investigator Award as part of the project “Establishing a Practical and Theoretical Foundation for Comprehensive and Integrated Community, Policy and Academic Efforts to Improve Dementia Care in Singapore” (NMRC-STAR-0005-2009), and its Clinician Scientist - Individual Research Grant—New Investigator Grant (NMRC-CNIG-1124-2014); Duke-NUS Geriatric Research Fund. We thank Irene Teo and Rebecca Lau for their comments on the draft of this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Data collection/analysis was approved by the Institutional Review Board at National University of Singapore. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Cheng, G.HL., Malhotra, R., Chan, A. et al. Weak social networks and restless sleep interrelate through depressed mood among elderly. Qual Life Res 27, 2517–2524 (2018). https://doi.org/10.1007/s11136-018-1895-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-018-1895-3