Abstract
Purpose
Obesity is a well-recognized risk factor for impaired health-related quality of life (HRQOL). Nevertheless, few studies have investigated the mechanisms underlying the obesity-HRQOL associations. In this study, we explored potential mediators of the associations between obesity and HRQOL.
Methods
Body mass index (BMI), an indicator of obesity, and HRQOL data were available for the 34,565 individuals 20 years of age and older participating in the cross-sectional Korea National Health and Nutrition Examination Survey 2007–2012. HRQOL was measured by the EuroQol five-dimension descriptive system. Path analysis was performed to assess the contributions of obesity-related diseases and self-rated health (SRH) on the relationships between obesity and HRQOL.
Results
In men, obesity was negatively associated with HRQOL through diabetes mellitus, hypertension, and dyslipidemia and positively associated with HRQOL through SRH. These opposite indirect effects offset one another and produced a non-significant association between obesity and HRQOL in men. However, in women, obesity was directly associated with HRQOL and indirectly associated with HRQOL through diabetes mellitus and SRH. Since these associations were in the same negative direction, the negative obesity-HRQOL association was clearly observed in women.
Conclusions
Obesity was negatively associated with HRQOL through obesity-related diseases in both genders. However, in men, the positive association between obesity and SRH resulted in a non-significant association of obesity with HRQOL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obesity and overweight are among the major public health concerns worldwide; their prevalence has risen markedly in previous decades. A recent study reported that the worldwide proportion of adults with body mass index (BMI) ≥25 kg/m2 increased between 1980 and 2013: from 28.8 to 36.9% in men and from 29.8 to 38.0% in women [1]. Based on current trends, 2.16 billion adults are expected to have BMI ≥ 25 kg/m2 by 2030 [2]. In 2012, 24.5% of all new cancer cases were estimated to be related to high BMI defined as 25 kg/m2 or greater [3]. Maternal obesity during pregnancy is related to an increased risk of preterm birth, especially extremely preterm birth [4]. In addition, obesity is associated with increased risks of hyperinsulinemia, diabetes mellitus (DM), hypertension, dyslipidemia, systemic inflammation, and cardiovascular diseases [5].
Declines in health-related quality of life (HRQOL) have been found to predict an increased mortality in older adults and the elderly after adjusting for other risk factors [6, 7]. Numerous studies have identified the significance of certain biological and psychological factors in the etiology of impaired quality of life, and obesity is a well-established risk factor for decreased HRQOL [8–13], along with the other obesity-related metabolic diseases described [14]. Moreover, HRQOL is highly correlated with perceived health and symptoms of diseases; in fact, it is influenced more by the recognition of diseases than by the physically adverse effects of a disease [15]. Therefore, self-rated health (SRH) is likely to play an important role in impaired HRQOL.
Although many studies have reported an association between obesity and HRQOL, most have focused on the simple association between these two variables. Path analysis is a powerful statistical tool used to simultaneously assess interdependent associations among several variables; thus, it can be useful when addressing the mechanism associated with obesity-related HRQOL. In particular, because the effects of obesity on obesity-related diseases and SRH are expected to be involved in the mechanisms underlying the obesity-HRQOL association, it is necessary to investigate the roles of obesity-related diseases and SRH in the association between obesity and HRQOL. The objective of this study was to examine the relationship between obesity and HRQOL and determine the mediating effects of the pathologic conditions, including DM, hypertension, and dyslipidemia and SRH, on the relationship between obesity and HRQOL.
Methods
Our study analyzed data acquired from the Korean National Health and Nutrition Examination Survey (KNHANES) 2007–2012, a nationwide cross-sectional investigation performed in South Korea. This survey utilized stratified multi-stage clustered probability sampling to select a representative population of the non-institutionalized civilians of South Korea. KNHANES conducted face-to-face health interviews, health consciousness and behavior surveys, a nutrition survey, and standardized medical examinations to collect health- and nutrition-related data. KNHANES followed the Ethical Principles for Medical Research Involving Human Subjects defined by the Declaration of Helsinki, and collected a written informed consent form from every participant. Detailed descriptions of KNHANES are provided elsewhere [16, 17]. Since our study used anonymous public data, approval from the institutional review board was not required. A total of 37,604 adult participants aged 20 years or older completed KNHANES 2007–2012. Among them, participants with missing data on BMI (n = 2223) and HRQOL (n = 816) were excluded. Consequently, the study population included 34,565 individuals aged 20 years or older (14,624 men and 19,941 women), who were investigated for both BMI and HRQOL.
KNHNAES measured bodyweight to the nearest 0.1 kg on a calibrated balance-beam scale and height to the nearest 0.1 cm in an upright position using a stadiometer. We calculated BMI (kg/m2) by dividing bodyweight in kg by squared height in m. Obesity was defined as BMI ≥ 25 kg/m2, based on the Korean-specific definition [18]. Blood samples were collected from participants in the morning, after an 8-h overnight fast; these samples were analyzed using an auto-analyzer (Hitachi Automatic Analyzer model 7600; Hitachi, Tokyo, Japan). Blood pressure was measured three times in the sitting position with 5-min intervals, using a standard mercury sphygmomanometer (Baumanometer; WA Baum Co. Inc., Copiague, NY, USA). The first value was discarded, and the second and third values for the participants’ systolic and diastolic blood pressure were averaged and recorded. Metabolic diseases were defined based on the guidelines provided by the National Cholesterol Education Program Adult Treatment Panel III [19]. DM was defined as glucose ≥126 mg/dL or current treatment. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current treatment. Dyslipidemia was defined as high-density lipoprotein cholesterol <40 mg/dL, low-density lipoprotein cholesterol ≥160 mg/dL, total cholesterol ≥240 mg/dL, triglyceride ≥200 mg/dL, or current treatment.
To evaluate SRH, this study used the EuroQol visual analogue scale (EQ-VAS) [20] in which respondents were asked to evaluate their SRH on a vertical scale ranging from zero, the worst imaginable health, to 100, the best imaginable health.
To assess HRQOL, this study used the EuroQol five-dimension descriptive system (EQ-5D) [20], translated into Korean. Through the EQ-5D questionnaire, respondents evaluated their health status according to three levels of severity—no problem, some problems, and severe problems—for the following five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The EQ-5D score for each dimension was converted into a single index of healthFootnote 1 by applying a formula developed by the Korean Centers for Disease Control and Prevention [17]. The scores ranged from −0.171 to 1, with zero indicating death, negative scores meaning health states worse than death, and a score of one indicating ‘no problem’ with any of the above five dimensions. To facilitate the statistical analyses, this study multiplied the measured EQ-5D scores by 100; thus, the converted scores ranged from −17.1 to 100.
Because of the significant interaction between obesity and gender in the multivariable linear regression model, the data analyses were carried out separately for men and women. A basic statistical analysis was conducted with SAS 9.4 statistical software (SAS Institute, Cary, NC, USA). We used Student t test and χ 2 tests to compare the continuous and categorical variables, respectively, between non-obese and obese participants. The approximate relationship between BMI and HRQOL was evaluated by calculating the Spearman’s correlation coefficient. We performed a multivariable linear regression analysis to ascertain the association between obesity and HRQOL, after adjusting for the following variables: age, smoking status, alcohol consumption status, regular physical activity, education level, income, DM, hypertension, dyslipidemia, and SRH.
Mplus 7.3 statistical software (Muthén and Muthén 1998–2014) was used to conduct the path analysis. To examine the mechanistic pathway between obesity and HRQOL, we conducted a path analysis using structural equation modeling (SEM) after adjusting for age, smoking status, alcohol consumption status, regular physical activity, education level, and income. As lifestyle factors had highly skewed distributions with continuous measures (e.g., the total pack-years of smoking), this study used categorical lifestyle variables instead of continuous variables, as follows: smoking status (current smoker vs non- or ex-smoker), alcohol consumption status (at least once vs less than once a month), and regular physical activity (at least 20 min each session and three times a week vs less). This study opted for weighted least squares with mean- and variance-adjusted estimators, which is considered to be the appropriate approach for SEM with categorical endogenous variables because it allows for non-normality and is asymptotically efficient [21]. The hypothetical pathway was constructed based on the following well-documented causal relationships: between obesity and obesity-related diseases [22–24], between obesity and SRH [25], between SRH and HRQOL [26], between obesity and HRQOL [12, 13], and between obesity-related diseases and HRQOL or SRH [27]. In the pathway, the hypothesized mediators were DM, hypertension, dyslipidemia, and SRH. We examined several pathways from obesity to HRQOL, including the (1) direct effect, (2) indirect effect via DM only, (3) indirect effect via hypertension only, (4) indirect effect via dyslipidemia only, (5) indirect effect via SRH only, (6) indirect effect via DM and SRH, (7) indirect effect via hypertension and SRH, and (8) indirect effect via dyslipidemia and SRH. Since the mechanisms by which obesity influences each dimension of the HRQOL are likely to differ, this study additionally performed path analyses for each dimension of the HRQOL as the outcome. We tested the significance of the indirect effects using the bias-corrected bootstrap procedure as described by Preacher and Hayes with B = 5000 bootstrap samples [28, 29]. The indirect effect was considered if the confidence interval of the unstandardized coefficient (B), assessed using the bias-corrected bootstrapping, did not include zero. To accommodate the complex sampling structure of the KNHANES data, we performed path analyses with stratification, cluster, and sampling weights.
The χ 2 statistic is typically estimated to evaluate the model fit. However, the χ 2 test is very sensitive to the sample size and is potentially biased because a significant χ 2 test result may be obtained with a large sample size but not with a small sample size [30, 31]. Therefore, we did not use the χ 2 test as an indicator of overall fit. To test the goodness-of-fit of the model, several different fit indices less sensitive to sample size were used, including the root mean square error of approximation (RMSEA), comparative fit index (CFI), and Tucker–Lewis index (TLI). A model with the RMSEA ≤ 0.06, CFI ≥ 0.95, and TLI ≥ 0.95 was considered a good model [32]. A p < 0.05 was considered statistically significant.
Results
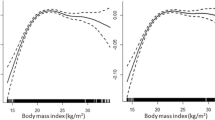
The mean age of the 34,565 participants was 50.2 years (SD = 16.4 years). The means of the BMI and EQ-5D values were 23.7 kg/m2 (SD = 3.4 kg/m2) and 92.9 (SD = 13.2), respectively. The overall prevalence of obesity was 32.0% (35.1% for men and 29.7% for women) (Table 1). The non-obese and obese groups differed significantly from one another in age, alcohol consumption, education level, income, DM, hypertension, dyslipidemia, SRH, and HRQOL, regardless of gender. The Spearman’s correlation analysis indicated that the BMI values correlated positively with HRQOL in men (r = 0.066; p < 0.001) and negatively with HRQOL in women (r = −0.164; p < 0.001) (Fig. 1). The results of the multivariable linear regression analysis showed that obese men did not differ significantly from non-obese men (B = 0.10; p = 0.601) in HRQOL but obese women scored significantly lower on HRQOL than did non-obese women (B = −1.45; p < 0.001) (data not shown).
Scatter plots with linear fit (solid line) for the correlation between body mass index (BMI) and health-related quality of life (HRQOL), measured by the EuroQol five-dimension descriptive system, in a men (n = 14,624) and b women (n = 19,941). Spearman coefficients were 0.066 (p < 0.001) and −0.164 (p < 0.001) in men and women, respectively
The path model for the HRQOL outcomes used data for 14,297 men (Fig. 2). The total effect of obesity on HRQOL was not significant (Table 2). The association between obesity and decreased HRQOL was significantly mediated by the following routes: via DM and SRH [B of the bias-corrected bootstrap = −0.074; standardized coefficient (β) of the original sample = −0.009; p < 0.05], via hypertension and SRH (B = −0.093; β = −0.011; p < 0.05), and via dyslipidemia and SRH (B = −0.080; β = −0.009; p < 0.05). SRH played a significant role in moderating the association between obesity and decreased HRQOL (B = 0.276; β = 0.033; p < 0.05). Regarding the goodness-of-fit measures for the path model for men, the RMSEA (0.056) and CFI (0.977) indicated a good model fit, but the TLI (0.563) did not. When analyzing each dimension, a significant total effect of obesity was found only for the mobility dimension of HRQOL (Table 3).
Hypothesized pathway in the association between obesity and health-related quality of life (HRQOL) measured by the EuroQol five-dimension descriptive system in men (n = 14,297). Covariates of age, smoking amount, alcohol consumption, metabolic equivalence task, income, and education were included in the model but not shown in the figure. Self-rated health was measured by the EuroQol visual analogue scale. Black and gray arrows indicate associations with p’s <0.05 and ≥0.05, respectively. B unstandardized regression coefficient, β standardized regression coefficient, OR odds ratio. *p < 0.01; **p < 0.001
A total of 19,459 women were analyzed for the path model for HRQOL outcomes (Fig. 3). The total effect of obesity on HRQOL was −0.593 (β = −0.049; p < 0.001; Table 2), and the direct effect was −0.493 (β = −0.041; p = 0.003). Among the hypothesized indirect pathways, only the route via DM and SRH were significant (B of the bias-corrected bootstrapping = −0.084; β of original sample = −0.007; p < 0.05). Some fit measures, such as the RMSEA (0.053) and CFI (0.980), for the women’s model indicated a good fit, but the TLI (0.619) did not. A significant total effect of obesity was found for all dimensions of HRQOL, with the exception of anxiety/depression (Table 3).
Hypothesized pathway in the association between obesity and health-related quality of life (HRQOL) measured by the EuroQol five-dimension descriptive system in women (n = 19,459). Covariates of age, smoking amount, alcohol consumption, metabolic equivalence task, income, and education were included in the model but not shown in the figure. Self-rated health was measured by the EuroQol visual analogue scale. Black and gray arrows indicate associations with p’s <0.05 and ≥0.05, respectively. B unstandardized regression coefficient, β standardized regression coefficient, OR odds ratio. *p < 0.01; **p < 0.001
Discussion
This study explored the potential causal pathways and mediating effects that may explain the association between obesity and HRQOL. Our findings indicated that DM commonly mediates the negative effect of obesity on HRQOL in both men and women. In men, the moderating effects of SRH canceled out the mediating effects of obesity-related diseases in the obesity-HRQOL association. A significant direct effect of obesity on HRQOL was observed only in women.
Several studies have reported that the relationship between obesity and HRQOL differs by gender, with negative association in women but positive or no significant association in men [14, 33–35]. Huang et al. reported that in a study in Taiwan, men with BMI ≥ 25 kg/m2 achieved better mental domain scores on subscales of the Short Form 36 health survey questionnaire (SF-36), such as vitality, social functioning, and mental health, than did other participants [34]. Women with BMI ≥ 25 kg/m2 had worse scores on all physical and mental domains than others in the study [34]. In a Korean study, compared to men of normal weight, overweight (23 ≤ BMI < 25 kg/m2) men older than 45 years of age had better work-related and psychosocial health scores on obesity-related quality of life measurement [35]. Other studies using KNHANES data reported gender differences in the association between obesity and HRQOL [14, 33].
Our path models showed that obesity was only directly associated with HRQOL in women; in men, it was indirectly associated with HRQOL through DM, hypertension, dyslipidemia, and SRH. Audureau et al. found significant indirect effects of obesity on HRQOL through obesity-related comorbidities in men, although the total effect of obesity was more apparent in women [36]. We speculated that obesity might affect HRQOL independently of obesity-related diseases, which may be the reason for the degree of difference in HRQOL between men and women. Psychological differences between genders, such as dissatisfaction with body shapes [37], weight self-stigmatization [9, 38], internalization of weight bias [8–11], and higher perceived health risk associated with obesity [39] could account for this disease-independent pathway.
Another interesting finding of our study was that obesity was positively associated with SRH in men. The mechanisms underlying the positive association between obesity and SRH in men alone have not yet been discovered, but several hypotheses can be proposed. First, obese men may be more mentally healthy than men of normal weight, which could lead to better SRH scores. In Zhu et al.’s study, obese adults with 25 ≤ BMI < 29 kg/m2 scored significantly better on the mental component of HRQOL as measured by the SF-36, than did adults of normal weight [40]. Another study reported that obese elderly men were less likely to have depressive symptoms than men of normal weight, but this association was not found in women [41]. Second, men tend not to see obesity as a problem. Gregory et al. reported that many obese adults, particularly men, do not recognize their body weight as a health risk [39]. Third, the historical backgrounds of the Korean population may strongly influence the conceptions of beauty and health. In general, Korea suffered from widespread poverty and starvation until late in the twentieth century [e.g., the gross domestic product (GDP) per capita was $156 in 1960] [42]; thus, obesity and being overweight may still be associated with well-being, affluence, and good nutrition.
To our knowledge, this is the first study to use path analysis to address the potential pathway from obesity to HRQOL through obesity-related diseases and SRH. Another strength of this study is the use of a nationwide representative sample to address our research questions. However, as with any cross-sectional design, causality cannot be ascertained; as such, reverse causation cannot be excluded. For example, bi-directional causality could exist between obesity and HRQOL [43]. Moreover, the TLI goodness-of-fit measure indicated a bad model fit. The measures of goodness-of-fit can be defined as follows: CFI = 1 − λ M/λ N, TLI = 1 − (λ M/df M)/(λ N/df N), and \({\text{RMSEA}}=\sqrt {{\lambda _{\text{M}}}/\left( {N - 1} \right)d{f_{\text{M}}}} ,\) where λ M and df M are the non-centrality parameter (NCP) and degree of freedom(df) for the specified model, respectively; λ N and df N note the NCP and df for the null model, respectively; and N indicates the sample size [44]. The NCP of the WLSMV was estimated by an adjusted χ 2 − df [45]. The TLI corrects for the df of the model which the CFI does not. Therefore, the low TLI indicates that some unnecessary paths (eg, adjustments or some confounders) were specified in our model: the fit was improved a bit further although our model had a lot fewer df compared to the null model. Another limitation of this study was that we did not include psychological variables in the path analysis models.
In conclusion, our results suggest gender differences in the mechanistic pathways from obesity to HRQOL and imply that obesity and obesity-related diseases should be treated and prevented based on gender. Longitudinal studies are needed to clarify the roles of metabolic abnormalities, SRH, and psychological factors in the obesity-HRQOL association.
Notes
The EQ-5D score = 1 − [0.05 + 0.096 × (1: some, 0: no or severe problem in mobility) + 0.418 × (1: severe, 0: no or some problem in mobility) + 0.046 × (1: some, 0: no or severe problem in self-care) + 0.136 × (1: severe, 0: no or some problem in self-care) + 0.051 × (1: some, 0: no or severe problem in usual activity) + 0.208 × (1: severe, 0: no or some problem in usual activity) + 0.037 × (1: some, 0: no or severe problem in pain/discomfort) + 0.151 × (1: severe, 0: no or some problem in pain/discomfort) + 0.043 × (1: some, 0: no or severe problem in anxiety/depression) + 0.158 × (1: severe, 0: no or some problem in anxiety/depression) + 0.05 × (1: severe problem in mobility or self-care or usual activity or pain/discomfort or anxiety/depression, 0: else)]. The EQ-5D score = 1 when no problem in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression.
References
Ng, M., Fleming, T., Robinson, M., Thomson, B., Graetz, N., Margono, C., et al. (2014). Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet, 384(9945), 766–781.
Kelly, T., Yang, W., Chen, C. S., Reynolds, K., & He, J. (2008). Global burden of obesity in 2005 and projections to 2030. International Journal of Obesity, 32(9), 1431–1437.
Arnold, M., Pandeya, N., Byrnes, G., Renehan, A. G., Stevens, G. A., Ezzati, M., et al. (2015). Global burden of cancer attributable to high body-mass index in 2012: A population-based study. The Lancet Oncology, 16(1), 36–46.
Cnattingius, S., Villamor, E., Johansson, S., Edstedt Bonamy, A. K., Persson, M., Wikstrom, A. K., & Granath, F. (2013). Maternal obesity and risk of preterm delivery. JAMA: The Journal of the American Medical Association, 309(22), 2362–2370.
Ahima, R. S., & Lazar, M. A. (2013). Physiology. The health risk of obesity—better metrics imperative. Science, 341(6148), 856–858.
Otero-Rodriguez, A., Leon-Munoz, L. M., Balboa-Castillo, T., Banegas, J. R., Rodriguez-Artalejo, F., & Guallar-Castillon, P. (2010). Change in health-related quality of life as a predictor of mortality in the older adults. Quality of Life Research, 19(1), 15–23.
Brown, D. S., Thompson, W. W., Zack, M. M., Arnold, S. E., & Barile, J. P. (2015). Associations between health-related quality of life and mortality in older adults. Prevention Science, 16(1), 21–30.
Latner, J. D., Durso, L. E., & Mond, J. M. (2013). Health and health-related quality of life among treatment-seeking overweight and obese adults: Associations with internalized weight bias. Journal of Eating Disorders, 1, 3.
Hilbert, A., Braehler, E., Haeuser, W., & Zenger, M. (2014). Weight bias internalization, core self-evaluation, and health in overweight and obese persons. Obesity, 22(1), 79–85.
Latner, J. D., Barile, J. P., Durso, L. E., & O’Brien, K. S. (2014). Weight and health-related quality of life: The moderating role of weight discrimination and internalized weight bias. Eating Behaviors, 15(4), 586–590.
Pearl, R. L., White, M. A., & Grilo, C. M. (2014). Weight bias internalization, depression, and self-reported health among overweight binge eating disorder patients., 22(5), E142–E148.
Lopez-Garcia, E., Guallar-Castillon, P., Garcia-Esquinas, E, & Rodriguez-Artalejo, F. (2016). Metabolically healthy obesity and health-related quality of life: A prospective cohort study. Clinical Nutrition (in press).
Fontaine, K. R., & Barofsky, I. (2001). Obesity and health-related quality of life. Obesity Reviews, 2(3), 173–182.
Park, S. S., Yoon, Y. S., & Oh, S. W. (2011). Health-related quality of life in metabolic syndrome: The Korea National Health and Nutrition Examination Survey 2005. Diabetes Research and Clinical Practice, 91(3), 381–388.
Smith, K. W., Avis, N. E., & Assmann, S. F. (1999). Distinguishing between quality of life and health status in quality of life research: A meta-analysis. Quality of Life Research, 8(5), 447–459.
Kweon, S., Kim, Y., Jang, M. J., Kim, Y., Kim, K., Choi, S., et al. (2014). Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). International Journal of Epidemiology, 43(1), 69–77.
Korea Center for Disease Control and Prevention. (2016). Korea National Health and Nutrition Examination Survey. 2016. Accessed September 23, 2016 from http://knhanes.cdc.go.kr.
Oh, S. W., Shin, S. A., Yun, Y. H., Yoo, T., & Huh, B. Y. (2004). Cut-off point of BMI and obesity-related comorbidities and mortality in middle-aged Koreans. Obesity Research, 12(12), 2031–2040.
Anonymous. (2002). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation, 106(25), 3143–3421.
EuroQol Group. (1990). EuroQol-a new facility for the measurement of health-related quality of life. Health Policy, 16(3), 199–208.
Finney, S. J., & DiStefano, C. (2006). Non-normal and categorical data in structural equation modeling. Charlotte, NC: Information Age.
Ishikawa-Takata, K., Ohta, T., Moritaki, K., Gotou, T., & Inoue, S. (2002). Obesity, weight change and risks for hypertension, diabetes and hypercholesterolemia in Japanese men. European Journal of Clinical Nutrition, 56(7), 601–607.
Oguri, M., Fujimaki, T., Horibe, H., Kato, K., Matsui, K., Takeuchi, I., & Yamada, Y. (2016). Obesity-related changes in clinical parameters and conditions in a longitudinal population-based epidemiological study. Obesity Research & Clinical Practice (in press).
Matsuda, M., & Shimomura, I. (2013). Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obesity Research & Clinical Practice, 7(5), e330–e341.
Svedberg, P., Bardage, C., Sandin, S., & Pedersen, N. L. (2006). A prospective study of health, life-style and psychosocial predictors of self-rated health. European Journal of Epidemiology, 21(10), 767–776.
Ocampo, J. M. (2010). Self-rated health: Importance of use in elderly adults. Colombia Médica, 41(3), 275–289.
Poljicanin, T., Ajdukovic, D., Sekerija, M., Pibernik-Okanovic, M., Metelko, Z., & Vuletic Mavrinac, G. (2010). Diabetes mellitus and hypertension have comparable adverse effects on health-related quality of life. BMC Public Health, 10, 12.
Preacher, K. J., & Hayes, A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891.
Hayes, A. F., & Preacher, K. J. (2010). Quantifying and testing indirect effects in simple mediation models when the constituent paths are nonlinear. Multivariate Behavioral Research, 45(4), 627–660.
MacCallum, R. C., Widaman, K. F., Preacher, K. J., & Hong, S. (2001). Sample size in factor analysis: The role of model error. Multivariate Behavioral Research, 36(4), 611–637.
Bentler, P. M., & Bonett, D. G. (1980). Significance tests and goodness of fit in the analysis of covariance structures. Psychological Bulletin, 88(3), 588.
Hu, L. T., & Bentler, P. M. (1999). Cutoff Criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6(1), 1–55.
Choo, J., Jeon, S., & Lee, J. (2014). Gender differences in health-related quality of life associated with abdominal obesity in a Korean population. BMJ Open, 4(1), e003954.
Huang, I. C., Frangakis, C., & Wu, A. W. (2006). The relationship of excess body weight and health-related quality of life: Evidence from a population study in Taiwan. International Journal of Obesity, 30(8), 1250–1259.
Song, H. R., Park, H. S., Yun, K. E., Cho, S. H., Choi, E. Y., Lee, S. Y., et al. (2010). Gender and age differences in the impact of overweight on obesity-related quality of life among Korean adults. Obesity Research & Clinical Practice, 4(1), e1–e82.
Audureau, E., Pouchot, J., & Coste, J. (2016). Gender-related differential effects of obesity on health-related quality of life via obesity-related comorbidities: A mediation analysis of a French Nationwide Survey. Circulation: Cardiovascular Quality and Outcomes, 9(3), 246–256.
Schwartz, M. B., & Brownell, K. D. (2004). Obesity and body image. Body Image, 1(1), 43–56.
Lillis, J., Levin, M. E., & Hayes, S. C. (2011). Exploring the relationship between body mass index and health-related quality of life: A pilot study of the impact of weight self-stigma and experiential avoidance. Journal of Health Psychology, 16(5), 722–727.
Gregory, C. O., Blanck, H. M., Gillespie, C., Maynard, L. M., & Serdula, M. K. (2008). Perceived health risk of excess body weight among overweight and obese men and women: Differences by sex. Preventive Medicine, 47(1), 46–52.
Zhu, Y., Wang, Q., Pang, G., Lin, L., Origasa, H., Wang, Y., et al. (2015). Association between body mass index and health-related quality of life: The “Obesity Paradox” in 21,218 adults of the Chinese general population. PLoS One, 10(6), e0130613.
Dong, Q., Liu, J. J., Zheng, R. Z., Dong, Y. H., Feng, X. M., Li, J., & Huang, F. (2013). Obesity and depressive symptoms in the elderly: A survey in the rural area of Chizhou, Anhui province. International Journal of Geriatric Psychiatry, 28(3), 227–232.
World Bank Group. (2016). GDP per capita. Accessed August 15, 2016 from http://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=KR.
Cameron, A. J., Magliano, D. J., Dunstan, D. W., Zimmet, P. Z., Hesketh, K., Peeters, A., & Shaw, J. E. (2012). A bi-directional relationship between obesity and health-related quality of life: Evidence from the longitudinal AusDiab study. International Journal of Obesity, 36(2), 295–303.
Kenny, D. A., & McCoach, D. B. (2003). Effect of the number of variables on measures of fit in structural equation modeling. Structural Equation Modeling, 10(3), 333–351.
Asparouhov, T., & Muthén, B. (2016). Computing the strictly positive Satorra-Bentler chi-square test in Mplus. Accessed August 15, 2016 from http://ww.statmodel2.com/examples/webnotes/SB5.pdf.
Funding
The study was not supported by any fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Park, S. Pathways linking obesity to health-related quality of life. Qual Life Res 26, 2209–2218 (2017). https://doi.org/10.1007/s11136-017-1565-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-017-1565-x