Abstract
Purpose
Summary scores derived from the Medical Outcomes Study HIV Health Survey (MOS-HIV) are used to assess treatment impacts among HIV-infected patients in Western settings, but have yet to be validated in rural, African settings. We examined the reliability, validity and responsiveness of scores among a prospective cohort of 947 HIV-1-infected adults initiating antiretroviral therapy between May 2003 and May 2004 in rural Uganda.
Methods
Physical (PHS) and mental health (MHS) summary scores were developed from baseline MOS-HIV sub-domains using exploratory factor analysis. Construct and discriminant validity were established by comparing mean summary scores across known groups of sociodemographic, clinical and health status characteristics. Effect sizes were calculated to assess responsiveness to therapy.
Results
Reliability of the PHS and MHS scores was 0.79 and 0.85, respectively. Mean baseline PHS and MHS scores varied significantly by CD4 cell count, HIV viral load, WHO stage of disease and Karnofsky performance status scores. By 12 months on antiretroviral therapy, PHS and MHS scores improved by 14.6 points (P < 0.001) and 13.9 points (P < 0.001), respectively.
Conclusions
PHS and MHS scores can be derived from the MOS-HIV and used to assess health status among cohorts of patients taking antiretroviral therapy in rural Uganda.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, antiretroviral therapy (ART) for people living with HIV and AIDS (PLHA) has become increasingly available throughout sub-Saharan Africa [1]. As a result, HIV is becoming a chronic, manageable illness requiring long-term therapy, and focus is shifting from palliative care to maintenance of health-related quality of life (QOL). Given this shift, it is important to be able to assess the influence of combination therapies on the QOL of patients taking ART. In Western settings, a number of health status measures have been developed to assess the impact of combination therapies on the QOL of PLHA taking ART [2]. The Medical Outcomes Study HIV Health Survey (MOS-HIV), which assesses 10 domains of functioning and well-being, is one of the most widely used instruments [3]. Two summary scores representing physical and mental health dimensions have been constructed and validated for use as endpoints in clinical trials in US populations [4]. However, the summary scores have yet to be validated for use in rural, African settings.
The use of summary measures as endpoints instead of individual domain scores is often preferred, as it simplifies the data analysis and interpretation of findings [4]. In addition, summary measures do not suffer from floor and ceiling effects, which are potential problems for the individual domain scores [5]. This is of particular importance in very sick or very healthy populations. Given the criteria for ART initiation in sub-Saharan Africa, generally CD4 cell count less than 200 or WHO stage 3 or 4 disease [6], those eligible for ART are often extremely sick or severely symptomatic. As such, it is important to assess whether summary measures can be derived in this context, and if so, whether they are reliable and valid measures of health status. Among a rural Ugandan cohort of PLHA initiating ART, we evaluated whether physical and mental health summary scores could be derived from the MOS-HIV, if the summary scores demonstrated reliability and validity, and whether the summary scores were responsive to ART over time.
Methods
Study population
We used data collected as part of the Home-based AIDS Care Project, a three-year randomized trial designed to compare different strategies for monitoring ART in rural Uganda. This cohort was described previously [7, 8] and is discussed briefly here. 710 HIV-infected women and 237 HIV-infected men were enrolled from Tororo and Busia Districts in Eastern Uganda between May 2003 and May 2004. To be eligible for the study, participants had to be ≥18 years old, have confirmed HIV-1 infection, be clients of the AIDS Service Organization, be a resident in the study catchment area, and have a CD4 cell count <250 cells/μl or severe HIV disease, defined as WHO stage 3 or 4 or recurrent herpes zoster. Isolated pulmonary tuberculosis was not an inclusion criterion.
After enrollment, participants’ households were visited weekly by lay workers that re-supplied ART and other drugs, conducted a pill count and assessed participants’ health using a standardized checklist. No clinic visits were scheduled following enrollment, but participants were encouraged to visit the hospital or clinic if ill. In addition, participants were taken to the clinic if they experienced specifically defined symptoms or were severely ill during the home visit. CD4 and viral load testing was conducted every 3 months with blood samples collected during home visits [9]. Participants were provided education regarding ART adherence and sexual risk behavior reduction at enrollment and were visited every 3 months by a certified HIV/AIDS counselor assigned at enrollment. Counselors collected social and behavioral data and provided ongoing counseling on topics including adherence, social support, disclosure, sexual behavior, HIV discordance and family planning.
Procedures
Surveys were administered in face-to-face interviews at ART initiation and every 3 months thereafter. On average, the survey took 30 min to complete. Only data from study participants with no prior ART experience who provided baseline data within 2 weeks of ART initiation were included in this analysis. Data were double entered using Epi-Info 2002 (CDC, Atlanta, GA, USA) and analyzed in Stata 8.2 (StataCorp, College Station, TX, USA). The study was approved the Uganda National Council of Science and Technology, and the Institutional Review Boards of the Centers for Disease Control and Prevention, the Uganda Virus Research Institute and Tulane University; all participants provided written consent at enrollment.
Measures
Health-related quality of life
Health-related quality of life was measured using a Luganda version of the Medical Outcomes Study HIV Health Survey (MOS-HIV), which was culturally adapted for use in rural southwestern Uganda [10]. We translated the survey into six additional languages spoken in our study area: Ateso, Lugisu, Lunyole, Japadhola, Kiswahili and Samia. We also modified the 35-item instrument for two reasons: (1) due to the large number of biologic, clinical and psychosocial variables being collected on a quarterly basis throughout the trial, we wanted to prioritize variables thought particularly relevant to the study context and (2) to ensure comprehension and consistency across the seven languages spoken in the study area.
Modifications included dropping some of the original items used to assess three sub-domains and splitting a single item into three separate questions for a fourth sub-domain. Only one of the five original questions was used to assess general health perceptions. The question asked respondents to rate their health in general on a 5-level response format ranging from ‘excellent’ to ‘poor’. To assess respondents’ role functioning, one of two original items was used. The item asked respondents whether their health keeps them from working at a job, digging, doing work around the house or attending school. Health distress was measured using two of the original four items; respondents were asked whether they were afraid because of their health and whether they felt weighed down by health problems. The single item used to measure health transition in the MOS-HIV was split into three separate questions to better assess this domain. Respondents were asked to rate three items in comparison with 30 days ago on a 5-level response format ranging from ‘much better’ to ‘much worse’: (1) physical health, (2) emotional condition and (3) ability to care for and provide for children. For this study, the single item comprising the overall QOL domain was omitted. No changes were made to the wording of the items included in the modified version of the Luganda MOS-HIV used in our study.
The resulting 29-item instrument assessed ten sub-domains of functioning and well-being, including physical functioning, role functioning, general health perceptions, pain, health transition, mental health, cognitive functioning, health distress, social functioning and vitality. All items referred to the past 30 days. Items for each sub-domain were summed and then transformed to a 0–100 scale, with higher scores reflecting better functioning and well-being. All sub-domains in the English [3] and Luganda [10] versions of the MOS-HIV have previously demonstrated excellent internal consistency, test–retest reliability, and construct and discriminant validity.
Health status and clinical measures
Two global measures of health status were assessed by clinicians at ART initiation, including WHO stage of HIV disease [11] and the Karnofsky performance status (KPS) score. KPS scores lower than 80 indicate moderate to severe impairment of functioning [12]. The item from the MOS-HIV health transition domain that asked respondents to rate their physical health compared to 30 days ago was used to classify study participants into one of three categories: those whose physical health had improved stayed the same or worsened. CD4 cell count and HIV-1 viral load were assessed at ART initiation and quarterly thereafter.
Sociodemographic characteristics
Demographic variables measured included gender, age, education, marital status and main source of income. Due to the small number of single respondents who had never previously married, we combined single, separated and divorced participants into one category, and married and cohabiting participants into another category. For income, the ‘dependent’ category refers to those who reported receiving money from others as their main source of income.
Statistical analyses
The underlying factor structure of the ten MOS-HIV sub-domains was assessed using principal components factor analysis with oblique rotation [4]. We conducted exploratory rather than confirmatory factor analysis due to the modifications made to the survey instrument and the belief that people living with HIV in rural Uganda might respond differently to the survey items than people in the West. The eigenvalues and scree plot were examined to determine the number of factors. The ‘score’ command in STATA generated the factor score coefficients that were used to calculate the physical (PHS) and mental health summary scores (MHS). Summary scores were standardized to scales with a mean of 50 and a standard deviation of 10 [4]. To assess internal consistency reliability, Cronbach’s alpha coefficients were calculated for MOS-HIV sub-domains containing two or more items and both summary scores. Coefficients ≥0.70 are considered to be sufficiently reliable for group comparisons [3].
To assess whether mean PHS and MHS scores varied in the expected directions by demographic characteristics, we examined differences using two-sample t tests for independent samples or one-way ANOVA. To test the hypotheses that the summary scores would demonstrate good construct validity and discriminate between stages of HIV disease, we tested for differences in mean scores by known clinical and other health-related variables using two-sample t tests for independent samples or one-way ANOVA. For one-way ANOVAs with significant F statistics, multiple comparisons were conducted using the Bonferroni test to discern differences between groups. Comparisons were based on baseline CD4 cell count strata (<50, 50–99, 100+), plasma HIV-1 viral load (<5 log10 copies/ml vs. ≥5 log10 copies/ml), WHO disease stage (stage 1, stage 2, stages 3 and 4), KPS (≥80 vs. <80) and patient response to the health transition item relating to physical health (improved, stable and worsened).
To assess the responsiveness of PHS and MHS scores to antiretroviral therapy over time, we tested for significant differences in mean scores between baseline and 12-month follow-up using paired t tests. We also calculated effect sizes (Mean2-Mean1/Baseline SD) [13]. A 95% confidence interval was calculated for each effect size using the formula proposed by Hedges and Olkin [14]. To compare the sensitivity of summary scores and clinical markers to ART, we also calculated effect sizes for CD4 cell count and HIV-1 viral load between baseline and 12-month follow-up. Effect sizes ≥0.8 were considered large [15] and indicated greater sensitivity to clinical change [16].
Results
Assessment of factor structure
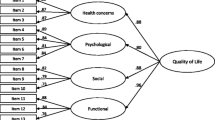
Principal components factor analysis of the MOS-HIV sub-domains at baseline yielded two factors that explained approximately 64% of the variance in the MOS-HIV sub-domains. Both oblique and orthogonal rotations were employed to aid interpretation of the factors. Based on the items that loaded onto each factor, it was clear that factor 1 represented mental health and factor 2 corresponded to physical health. The two factors were correlated (r = 0.62). Sub-domains loading on the physical health summary (PHS) included physical function, role function, general health perceptions, bodily pain and health transition. For the mental health summary (MHS), mental health, cognitive function and health distress sub-domains loaded most strongly. Similar to previous factor analyses of the MOS-HIV measures [4], social function and vitality loaded on both factors. However, the sub-domains loaded more strongly on the MHS and were, therefore, included in the construction of the MHS score. The commonalities observed indicate that the two factor solution explained a large portion of the variance in each scale, ranging from 0.425 (for health transition) to 0.793 (for mental health) (Table 1). PHS and MHS scores were generated after oblique rotation by transforming the scored factor loadings to scales with a mean of 50 and a standard deviation of 10 [4]. All subsequent data analyses were performed using the transformed PHS and MHS scores. The means and standard deviations of the MOS-HIV sub-domains and summary scores prior to ART initiation are shown in Table 2.
Reliability of MOS-HIV scales, PHS and MHS
All MOS-HIV scales with two or more items showed high internal consistency reliability, with Cronbach’s alpha values greater than or equal to 0.79. The estimated reliability of the PHS was 0.79, and the MHS was 0.85, indicating that the scales included on each factor were highly correlated (Table 2).
Demographic variables and PHS and MHS
At baseline, there were no differences in mean PHS and MHS scores by gender, age or marital status, but both scores were associated with education level; patients with no education had significantly lower mean PHS and MHS scores than those with a post-primary education (PHS: 36.4 vs. 40.8, P < 0.001; MHS: 38.8 vs. 41.8, P = 0.013). PHS scores for participants with no formal education were also significantly lower when compared to those with primary education (36.4 vs. 39.8, P < 0.001). Main source of income was significantly associated with both scores, with respondents reporting dependent income scoring lower than respondents in each of the other three income categories (P < 0.001) (Table 3).
Validity of PHS and MHS
To explore construct and discriminant validity, we tested for differences in mean summary scores by a number of known clinical characteristics, including biologic markers of disease progression and global measures of health status. Mean scores differed significantly by CD4 cell count for PHS and MHS (Table 3). Patients with CD4 cell counts <50 cells/μl scored significantly lower than patients with CD4 cell counts of 100 or greater for both scores (PHS: 35.1 vs. 40.6, P < 0.001; MHS: 37.3 vs. 41.0; P < 0.001). Similarly, patients with viral loads ≥5 log10 copies/ml had significantly lower mean PHS and MHS scores compared to patients with viral loads <5 log10 (PHS: 38.3 vs. 41.1, P < 0.001; MHS: 39.0 vs. 42.2, P < 0.001). There was a clear decrease in both scores with advanced stage of HIV disease; for the PHS, respondents in WHO stages 3 and 4 scored significantly lower than those in stage 2 (36.7 vs. 39.7; P = 0.002) and stage 1 (36.7 vs. 40.6; P < 0.001). For the MHS, mean scores for those in WHO stages 3 and 4 were significantly lower compared to those in stage 1 only (38.6 vs. 41.1; P = 0.010).
At baseline, participants with KPS scores lower than 80 had lower mean PHS and MHS scores compared to those whose KPS scores were higher (PHS: 30.3 vs. 39.7, P < 0.001; MHS: 31.9 vs. 40.3; P < 0.001). Mean summary scores also differed significantly by patient-reported health status. Respondents who stated that their health improved or stayed the same in the past 30 days had significantly higher PHS and MHS scores compared to those whose health had worsened (PHS: 43.2 vs. 32.8, P < 0.001; MHS: 41.3 vs. 36.3, P < 0.001). The average difference in mean PHS scores ranged from 9.9 to 10.4 points for respondents with worsening health compared to those with improved or stable health. For the MHS, patients with worsening health scored 5.0 to 7.4 points lower than respondents with stable or improved health (Table 3).
Responsiveness of PHS and MHS
Physical and mental health summary scores improved substantially between ART initiation and 12-month follow-up. After 12 months on ART, mean PHS and MHS scores improved by 14.6 points (P < 0.001) and 13.9 points (P < 0.001), respectively. Effect sizes were large and positive, indicating a substantial positive effect of ART on PHS (ES: 1.5; 95% CI: [1.43, 1.64]) and MHS (ES: 1.3; 95% CI: [1.15, 1.35]). Clinical markers also changed dramatically between ART initiation and 12-month follow-up, with increases in mean CD4 cell count (ES: 2.8; 95% CI: [2.66, 2.92]) and decreases in mean viral load (ES: −5.7; 95% CI: [−5.91, −5.49]) observed (Table 4).
Discussion
Our study demonstrated that physical and mental health summary scores derived from the Medical Outcomes Study HIV Health Survey were generalizable to a large cohort of HIV-infected adults initiating ART in rural Uganda. Both scores demonstrated excellent reliability and good construct and discriminant validity. Importantly, we found that physical and mental health summary scores were responsive to ART use over time, with sensitivity comparable to that of CD4 cell count and HIV viral load.
Similar to previous studies of the MOS-HIV scales, we found evidence of two distinct factors representing physical and mental health [4, 10]. This finding suggests that the MOS-HIV summary scores, originally developed for use in Western settings, are also applicable to the rural Ugandan context. In non-Western countries, researchers interested in functional status are often faced with the decision to adapt existing scales or develop new instruments. While locally developed instruments may enhance the relevance and appropriateness of functional status measures [17], they limit the ability to make comparisons with other study populations. This is a key consideration when assessing the impact of ART on the functioning and well-being of HIV-infected populations. The MOS-HIV is commonly used to assess health-related quality of life in HIV-infected populations and has been culturally adapted for use in numerous countries [3], including Uganda [10]. Previous research in Western settings has suggested that physical and mental health summary scores may be useful in the evaluation of medical treatments [4]. Our study suggests that these summary measures may also be useful for assessing the impact of ART on quality of life in non-Western settings.
In our study population, PHS and MHS scores varied significantly by educational and economic status. These findings are consistent with previous studies among PLHA taking ART in the US and Italy, which found significantly lower scores among those with fewer years of education [18] and those who were unemployed [19]. Contrary to previous studies conducted in the US, Europe and Uganda, we did not find significant differences in PHS and MHS scores by gender [20–22], older age [19, 20, 23] or marital status [24]. The lack of association may reflect the composition of our study population, which was primarily composed of subsistence farmers in rural Uganda.
The summary measures varied significantly by clinical characteristics. Both scores were lower for patients with CD4 cell counts <50 and for patients with high HIV viral loads (≥5 log10 copies/ml) at baseline. These results concur with previous research [4] and support the validity of the physical and mental health summary scores for capturing differences in clinical measures.
The PHS and MHS scores were able to distinguish between stages of HIV infection. Respondents with asymptomatic infection (WHO stage 1) scored significantly higher than those with symptomatic infection (WHO stages 3 and 4) for both scores. In addition, the summary scores discriminated by global ratings of health status, including the clinician-assigned KPS and a patient self-assessment of health status. Patients who reported worsening health in the past 30 days had significantly lower mean PHS and MHS scores than patients whose health remained stable or improved. These results support the discriminant validity of the physical and mental health summary scores. Consistent with previous research [4], the average point differences between patients with worsening health and those with stable or improved health ranged from 9.0 to 10.0 points for the PHS and 5.0 to 7.0 points for the MHS.
Previous studies have suggested that a 5.0-point difference in summary scores is indicative of a clinically important change in health status [3, 25]. In our study, the PHS improved by 14.6 points and the MHS improved by 13.9 points by 12 months of ART. These large improvements indicate that the observed differences were clinically significant. However, while both health status and clinical measures responded to ART, the biologic markers, in particular viral load, appear to be more sensitive to treatment, as evidenced by the larger effect sizes observed. Our findings concur with previous research that demonstrated greater sensitivity to change over time for viral load as compared to CD4 cell count [26]. We recommend that where possible, both types of measures should be collected. However, in resource-poor settings with little or no access to viral load and CD4 cell count data, patient-reported measures of functioning and well-being may serve as important proxy indicators for observing changes in the health status of ART clients over time. More longitudinal studies are needed to examine the sensitivity and specificity of the MOS-HIV summary scores for assessing individual patient outcomes. While the MOS-HIV summary scores have demonstrated responsiveness in several US studies, assessments of the culturally adapted versions have not been able to establish responsiveness due to limited sample sizes [3]. Our study, conducted in a large cohort of ART patients, indicated that the summary scores are responsive to treatment in Uganda.
A few limitations must be considered when interpreting our results. First, the modifications made to the MOS-HIV may limit the comparability of our findings with other studies; however, the resulting summary scores demonstrated good construct and discriminant validity. In addition, the modified scale was similar to the 30-item MOS-HIV Health Survey [27], which was used to develop the original summary scores. Second, we were not able to disaggregate the effect of ART on QOL from other aspects of the home-based program due to the lack of a control group. It is possible that giving ART alone, in the absence of a comprehensive program, may result in smaller improvements in QOL over time. However, QOL scores tracked closely with CD4 cell counts [8] and previous research in our study population demonstrated substantial life-prolonging aspects of ART [9], suggesting a positive influence of ART over time. Lastly, while our findings demonstrate that the MOS-HIV summary scores are valid for assessing group outcomes, we did not assess whether the scores accurately predict individual patient outcomes. Further research is needed to examine the sensitivity and specificity of applying individual summary scores with individual patients’ biologic and clinical conditions.
In conclusion, this study demonstrated that physical and mental health summary scores can be derived from the MOS-HIV and used to assess health status among a cohort of patients taking ART in rural Uganda. The summary scores were highly reliable and demonstrated good construct and discriminant validity in our study population. In addition, they were responsive to ART use over time. The PHS and MHS scores are potentially useful for assessing the impact of treatment in cohort studies in rural Africa.
Abbreviations
- ANOVA:
-
Analysis of variance
- ART:
-
Antiretroviral therapy
- CD4:
-
Cluster of differentiation 4
- CDC:
-
US centers for disease control and prevention
- HIV:
-
Human immunodeficiency virus
- KPS:
-
Karnofsky performance status
- MHS:
-
Mental health summary score
- MOS-HIV:
-
Medical outcomes study HIV health survey
- PHS:
-
Physical health summary score
- PLHA:
-
People living with HIV and AIDS
- QOL:
-
Quality of life
- WHO:
-
World health organization
References
UNAIDS, World Health Organization. (2005). Progress on global access to HIV antiretroviral therapy: An update on “3 by 5”. In Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS) (pp. 1–55). World Health Organization (WHO).
Wu, A. W. (2000). Quality of life assessment comes of age in the era of highly active antiretroviral therapy. AIDS, 14(10), 1449–1451.
Wu, A. W., Revicki, D. A., Jacobson, D., & Malitz, F. E. (1997). Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV). Quality of Life Research, 6(6), 481–493.
Revicki, D. A., Sorensen, S., & Wu, A. W. (1998). Reliability and validity of physical and mental health summary scores from the Medical Outcomes Study HIV Health Survey. Medical Care, 36(2), 126–137.
Holmes, W., Bix, B., & Shea, J. (1996). SF-20 score and item distributions in a human immunodeficiency virus-seropositive sample. Medical Care, 34(6), 562–569.
World Health Organization. (2002). Scaling up antiretroviral therapy in resource limited settings: Guidelines for a Public Health Approach. Geneva: World Health Organization.
Bunnell, R., Ekwaru, J. P., Solberg, P., Wamai, N., Bikaako-Kajura, W., Were, W., et al. (2006). Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS, 20(1), 85.
Stangl, A. L., Wamai, N., Mermin, J., Awor, A. C., & Bunnell, R. (2007). Trends and predictors of quality of life among HIV-infected adults taking highly active antiretroviral therapy in rural Uganda. AIDS Care, 19(5), 626–636.
Mermin, J., Were, W., Ekwaru, J. P., Moore, D., Downing, R., Behumbiize, P., et al. (2008). Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: A prospective cohort study. The Lancet, 371(9614), 752–759.
Mast, T. C., Kigozi, G., Wabwire-Mangen, F., Black, R., Sewankambo, N., Serwadda, D., et al. (2004). Measuring quality of life among HIV-infected women using a culturally adapted questionnaire in Rakai district, Uganda. AIDS Care, 16(1), 81–94.
WHO, & The WHO International Collaborating Group for the study of the WHO staging system. (1993). Proposed ‘World Health Organization staging system for HIV infection and disease’: preliminary testing by an international collaborative cross-sectional study. The WHO International Collaborating Group for the Study of the WHO Staging System. AIDS, 7(5), 711–718.
Mor, V., Laliberte, L., Morris, J. N., & Wiemann, M. (1984). The Karnofsky performance status scale: An examination of its reliability and validity in a research setting. Cancer, 53(9), 2002–2007.
Kazis, L. E., Anderson, J. J., & Meenan, R. F. (1989). Effect sizes for interpreting changes in health status. Medical Care, 27(3), S178.
Hedges, L. V., & Olkin, I. (1985). Statistical methods for meta-analysis. New York: Academic Press.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum Associates.
Katz, J. N., Larson, M. G., Phillips, C. B., Fossel, A. H., & Liang, M. H. (1992). Comparative measurement sensitivity of short and longer health status instruments. Medical Care, 30(10), 917–925.
Bolton, P., & Tang, A. M. (2002). An alternative approach to cross-cultural function assessment. Social Psychiatry and Psychiatric Epidemiology, 37(11), 537–543.
Murri, R., Fantoni, M., Del Borgo, C., Visona, R., Barracco, A., Zambelli, A., et al. (2003). Determinants of health-related quality of life in HIV-infected patients. AIDS Care, 15(4), 581–590.
Liu, C., Johnson, L., Ostrow, D., Silvestre, A., Visscher, B., & Jacobson, L. P. (2006). Predictors for lower quality of life in the HAART era among HIV-infected men. Journal of Acquired Immune Deficiency Syndromes, 42(4), 470.
Mannheimer, S. B., Matts, J., Telzak, E., Chesney, M., Child, C., Wu, A. W., et al. (2005). Quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care, 17(1), 10–22.
Cederfjall, C., Langius-Eklof, A., Lidman, K., & Wredling, R. (2001). Gender differences in perceived health-related quality of life among patients with HIV infection. AIDS Patient Care STDs, 15(1), 31–39.
Babikako, H. M., Neuhauser, D., Katamba, A., & Mupere, E. (2010). Feasibility, reliability and validity of health-related quality of life questionnaire among adult pulmonary tuberculosis patients in urban Uganda: Cross-sectional study. Health and Quality of Life Outcomes, 8, 93.
Bajunirwe, F., Tisch, D. J., King, C. H., Arts, E. J., Debanne, S. M., & Sethi, A. K. (2009). Quality of life and social support among patients receiving antiretroviral therapy in Western Uganda. AIDS Care, 21(3), 271–279.
Sowell, R. L., Seals, B. F., Moneyham, L., Demi, A., Cohen, L., & Brake, S. (1997). Quality of life in HIV-infected women in the south-eastern United States. AIDS Care, 9(5), 501–512.
Cohen, C., Revicki, D. A., Nabulsi, A., Sarocco, P. W., & Jiang, P. (1998). A randomized trial of the effect of ritonavir in maintaining quality of life in advanced HIV disease. AIDS, 12(12), 1495.
Tarwater, P. M., Gallant, J. E., Mellors, J. W., Gore, M. E., Phair, J. P., Detels, R., et al. (2004). Prognostic value of plasma HIV RNA among highly active antiretroviral therapy users. AIDS, 18(18), 2419.
Wu, A. W., Rubin, H. R., Mathews, W. C., Ware, J. E., Jr, Brysk, L. T., Hardy, W. D., et al. (1991). A health status questionnaire using 30 items from the Medical Outcomes Study: Preliminary validation in persons with early HIV infection. Medical Care, 29(8), 786.
Acknowledgments
This study was funded by the Centers for Disease Control and Prevention through the President’s Emergency Plan for AIDS Relief and the US Department of Health and Human Services. The authors wish to thank the participants and study team in Tororo, especially Kenneth Khana. We also wish to thank Stevens Bechange for data management and Drs. Dominique Meekers, Paul Hutchinson and Lisanne Brown for providing comments on earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Rights and permissions
About this article
Cite this article
Stangl, A.L., Bunnell, R., Wamai, N. et al. Measuring quality of life in rural Uganda: reliability and validity of summary scores from the Medical Outcomes Study HIV Health Survey (MOS-HIV). Qual Life Res 21, 1655–1663 (2012). https://doi.org/10.1007/s11136-011-0075-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-011-0075-5