Abstract
The aim of this work was to evaluate (i) the phenol and flavonoid recovery and bioaccessibility indexes and (ii) the antioxidant activity of both types of non-defatted and defatted chia seeds during the in vitro gastrointestinal digestion. The ground samples were subjected to in vitro simulated gastrointestinal digestion, and the resultant fractions were extracted and subjected to spectrophotometric assays. The results pointed to increasing concentrations of polyphenolic compounds during digestion, although only a low-medium percentage of phenols and a low percentage of flavonoids were available for absorption in the intestinal tract. In addition, the high level of fats seemed to have a negative effect on the bioaccessibility of flavonoids. Further studies should be undertaken to better understand the stabilization of the bioactive compounds of chia and to improve their bioaccessibility. Meanwhile, the present study represents a solid base for studying the bioavailability of bioactive compounds of chia seeds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chia (Salvia hispanica L.) is a herbaceous plant, native to southern Mexico and northern Guatemala, that has been consumed since ancient times by Mesoamerican populations [1]. Seeds (the part of the plant that is consumed) have been widely studied because of their high content of proteins and oils (mainly polyunsaturated fatty acids in the form of omega-3 and omega-6 fatty acids) [2]. Additionally, they contain a high proportion of bioactive compounds, such as antioxidant dietary fiber and polyphenolic compounds [3]. Because of this, and for their techno-functional properties, the seeds could be used as potential ingredients for developing new functional foods. Nevertheless, as mentioned by Gullón et al. [4], the beneficial effects and effectiveness of products with a high content of bioactive compounds depends on (i) fibre intake, (ii) fibre composition, (iii) the bioactive compounds associated with the fibre, especially polyphenolic compounds, which are directly related to the antioxidant properties [5] and (iv) the changes produced during gastrointestinal digestion. Any compound can be considered potentially effective for human health but only if remains bioaccessible after all the phases involved in gastrointestinal digestion, which is why availability for absorption after gastrointestinal digestion must be assessed [6]. Among in vitro , ex vivo and in vivo models, simulated in vitro gastrointestinal digestion represents a simple, fast and valid alternative to evaluate bioaccessibility [4]. The scientific literature contains a high number of contributions dealing with the bioactive compound content and antioxidant capacity of chia seeds. However, no information is available on the changes that chia polyphenolic compounds undergo during gastrointestinal digestion, or on their bioaccessibility. Thus, the aim of this work was to evaluate (i) the recovery and bioaccessibility indexes of phenols and flavonoids and (ii) their respective antioxidant capacity during in vitro gastrointestinal digestion of non-defatted and defatted chia seeds.
Material and Methods
Samples and Chemicals
The study was performed on two different chia samples: non-defatted and defatted chia seeds obtained from Instituto de Agroquímica y Tecnología de Alimentos (IATA); Spanish National Research Council (CSIC). The samples were ground with a mill to obtain flour.
Simulated In Vitro Gastrointestinal Digestion
The in vitro gastrointestinal digestion of samples was performed following the method described by Gullón et al. [7], subjecting the samples to oral, gastric and intestinal phases. Individual digestions were carried out for each phase. At the end of each phase of gastrointestinal digestion, the digestion mixtures were centrifuged for 12 min at 8000 g and 4 °C, yielding the chyme soluble fraction (CSF) and the pellet fraction (PF). Both fractions were lyophilized and stored at −20 °C until further use.
Extraction Method
Undigested and digested samples (0.05–0.3 g) were mixed with 5–10 mL of methanol-water (80–20, v/v) and sonicated for 15 min, after which, the samples were centrifuged for 10 min, 8000 g at 4 °C. The supernatants were collected and the pellets were mixed with 5–10 mL of acetone:water (70:30, v/v) and the same steps were repeated. The supernatants were combined and evaporated to dryness. Five milliliters of methanol were added to the residue, and the mixture was well shaken in a Vortex for 2 min. The methanolic extract was filtered with a 0.45 μm filter and stored a − 20 °C until further use.
Recovery Index and Bioaccessibility Index
To evaluate the effect of the matrix composition on the digestion of the phenolic group (phenolic acids and flavonoids) two different indexes were studied, following the indications of Ortega et al. [8]: the recovery percentage and bioaccessibility percentage. The recovery percentage allows the amount of the phenolic group present in the complete digest (CSF and PF) to be measured after mouth, gastric and intestinal digestion of the test food according to: Recovery index (%) = (PCDF/PCTF) × 100, where PCDF is the total phenol content (mg) in the digested (CSF + PF) and PCTF is the total phenol content (mg) quantified in test matrix.
For each phenol group, bioaccessibility is defined as the percentage of polyphenolic compounds solubilised in the absorbed fraction (IN) after the intestinal dialysis phase. Thus, this index defines the proportion of the polyphenolic compounds that could become available for absorption into the systematic circulation: Bioaccessibility index (%) = (IN/IN + OUT) × 100. where, IN is the absorbed fraction and OUT is the non-absorbed fraction.
Total Phenolic (TPC) and Total Flavonoid (TFC) Contents
The TPC of the samples was estimated using the Folin-Ciocalteu method, as proposed by Singleton and Rossi [9]. Gallic acid (GA) was used as reference standard and the results were expressed as mg GA equivalents/g of fresh weight matrix (FW). TFC was established by means of the method described by Blasa et al. [10]. The reference standard was rutin and the results were expressed as mg rutin equivalents (RE)/g of FW matrix.
Determination of Polyphenolic Compounds
The polyphenolic profiles of all the samples obtained for each phase of in vitro gastrointestinal digestion were determined by high performance liquid chromatography (HPLC) following the methodology described by Lucas-Gonzalez et al. [11]. The polyphenolic compounds were identified by comparing UV absorption spectra and retention times of each compound with those of pure standards injected in the same conditions.
Antioxidant Capacity
The antioxidant capacity was assessed by means of four in vitro spectrophotometric assays.
The DPPH assay was performed using the stable radical 2,2-diphenyl-1-picrylhydrazyl, following the method proposed by Brand-Williams et al. [12]. Trolox was used as reference standard and the results were expressed as mg Trolox equivalents/g of FW matrix. The ferric reducing antioxidant power (FRAP) was assessed by means of the potassium ferricyanide-ferric chloride method described by Oyaizu [13], using Trolox as reference standard. The results were expressed as mg Trolox equivalents/g of FW matrix. The TEAC-ABTS assay was assessed using the method proposed by Gullón et al. [7]. Trolox was again used as reference standard and the results were expressed as mg Trolox equivalents/g of FW matrix. Ferrous ions chelating activity (FIC) was determined establishing the inhibition of Fe2+-ferrozine complex formation after adding Fe2+ to the test material according to the method described by Carter [14]. EDTA was used as reference standard and results were expressed as mg EDTA/g of FW matrix.
Statistical Analysis
The results are expressed as the mean ± SD of two parallel trials (n = 4) and compared using the statistical program JMP 13.1.0 (SAS Institute Inc., Cary, USA). The differences between the mean values obtained for the antioxidant capacity and those obtained for the different phases of in vitro gastrointestinal digestion were analyzed by one-way analysis of variance (ANOVA). Tukey’s post hoc test was applied for comparison of the means, and differences were considered significant at p < 0.05. The total phenolic and flavonoid contents were correlated with the antioxidant capacity results through Pearson’s correlation test: the positive/negative strength of correlation was considered as follows: low for +/−0.1 < r < +/−0.3, moderate for +/−0.3 < r < +/−0.7, and strong for r > +/− 07; for values of r < +/− 0.1 the variables were considered not to be correlated.
Results and Discussion
Recovery Index and Bioaccessibility Index
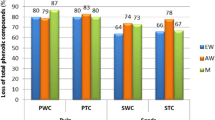
The percentage of recovery allows the amount of phenolic and flavonoid compounds recovered after oral, gastric and intestinal digestion to be compared with the amount undigested test food. Figures 1 and 2 show the recovery index for the total phenolic content and total flavonoid content obtained after the oral, gastric and intestinal phases, respectively. As regards the recovery index for total phenolic acids, no statistical differences (p > 0.05) were found between non-defatted and defatted chia seeds after each phase of the gastrointestinal digestion process. The recovery index was lower (p < 0.05) for both samples after the oral phase than in the test matrix. However, after the gastric phase there were no significant differences (p > 0.05) from the test matrix. The highest recovery index values (p < 0.05) were found after the last phase of gastrointestinal digestion. It seems, then, that the oral phase negatively affects phenol recovery, while the same compounds were released from the matrix after the gastric phase and, particularly, after the intestinal phase. This release can be attributed to the acid and alkaline hydrolysis that occurs during the gastric and intestinal phases and agrees with the results obtained by Tagliazucchi et al. [15], who detected the gradual release of polyphenolic compounds during gastrointestinal digestion. The differences observed might also be related to the reactivity of the Folin-Ciolcateu reagent, since the main constituent can react with other non-phenolic compounds (e.g., vitamins, aminoacids and proteins) so that any assessment may be under- or overestimated [16]. A different situation was detected for the total flavonoid content: as shown in Figure 2, there were no statistical differences (p > 0.05) between non-defatted and defatted chia seeds, except in the recovery index obtained after the intestinal phase, which was lower (p < 0.05) for the defatted chia than the non-defatted sample. After the oral phase, the recovery index was significantly lower (p < 0.05) than in test matrix reference. Despite the increase observed there in the same index after the gastric phase (p < 0.05), it was still lower (p > 0.05) than that observed in the test matrix. After the intestinal phase the recovery index increased for non-defatted chia reaching a similar (p > 0.05) value to the test matrix, but no such increase was detected for defatted chia, where the recovery index remained similar (p > 0.05) to that observed after the gastric phase. Thus, the TFC was affected by the gastrointestinal digestion process, defatted chia having the worst response. Such behavior would be related to the chemical properties of these compounds, and it is known that flavonoids are not very stable and interact with food matrix compounds [9]. This decreasing trend observed for TFC agrees with the scientific literature: for example Lucas-Gonzalez et al. [11], found a low degree of TFC recovery for maqui berry extracts after the intestinal stage. In addition, the different response of defatted and non-defatted samples to gastrointestinal digestion would be related to the fat content; indeed, the gastrointestinal digestion process has previously been found to have a greater effect on samples with a lower fat content. For example, Ortega et al. [17] stated that a higher fat content in cocoa liquor had a protective effect against digestion, and related this phenomenon with better micellarization that favors the stability of polyphenols during digestion.
Figure 3 shows the bioaccessibility index obtained for TPC and TFC. The bioaccessibility index for phenolic acids was higher (p < 0.05) than for the flavonoids in the case of non-defatted chia, while similar values (p > 0.05) were obtained for defatted chia. Among samples there were statistical differences (p < 0.05) between the bioaccessibility index of flavonoids but not for phenolic acids (p > 0.05). Also, in this case, the higher fat content of non-defatted chia seeds affected the gastrointestinal digestion response of flavonoids. Generally, the greater the lipophilicity of a compound, the greater retention in the absorbed fraction (IN) and the lower the water phase solubilization in the unabsorbed fraction (OUT), reflecting lower potential bioaccessibility [17]. In the present study, the presence of fat in the food matrix seemed to promote the retention of flavonoids, mostly lipophilic polar molecules [18], in the IN fraction, leading to lower bioaccessibility. The obtained results agreed with those of Lucas-Gonzalez et al. [11], who found a bioaccessibility index for maqui berry extracts of 14% in the case of flavonoids and 78% for phenolic acid compounds, although other studies registered different trends: Ortega et al. [8], for example, found lower bioaccessibility values for phenolics (36%) than for flavonoids (64%) in pomegranate peel flour. Bioaccessibility was already found to be deeply influenced by the food matrix systems, their physicochemical properties and nutrients [7].
Table 1 shows the polyphenolic profile obtained before and after in vitro gastrointestinal digestion of non-defatted and defatted chia seeds. In undigested chia and defatted chia seeds 11 polyphenolic compounds were identified. Note that defatted chia seeds showed higher values (p < 0.05) for all the polyphenolic compounds detected than non-defatted chia seeds. Rosmarinic acid was the major compound detected and quantified, with values of 653.98 and 669.88 μg/g in non-defatted and defatted chia seeds, respectively, followed by daizdin and quercetin. These values were lower than those reported by Martínez-Cruz and Paredes-López [19] and Rahman et al. [20] for non-defatted and defatted chia seeds. After the intestinal phase (Table 1), seven polyphenolic compounds were detected in both chia and defatted chia seed samples. Again, for all the polyphenolic compounds detected, defatted chia seeds showed higher values (p < 0.05) than chia seeds. As with the undigested samples, rosmarinic acid was the main compound found, with values of 245.88 and 259.87 μg/g in non-defatted and defatted chia seeds, respectively.
As can be seen from the results obtained for the digested samples after in vitro gastrointestinal digestion, there was a drastic reduction in the levels of polyphenolic compounds. The results obtained are similar to those reported by Ortega et al. [8], who showed that after the intestinal digestion step, important losses of phenolic compounds are expected. As mentioned above, all the compounds detected were reduced in concentration (p < 0.05), with percentages of reduction between 62.40 and 82.89% compared with the initial concentration. While waiting for in vivo confirmation of the bioactivity and functionality, the bioaccessibility results for the bioactive compounds strongly suggest that chia and defatted chia seeds could be considered interesting functional food.
Antioxidant Capacity of Digested Samples
Table 2 shows the results obtained in antioxidant capacity assays for both non-defatted and defatted chia seeds after the different phases of gastrointestinal digestion, as well as, the percentage of variation (%Var). Comparison with the results obtained for the test matrix showed the best antioxidant capacity (p < 0.05) in the DPPH, ABTS and FRAP assays obtained for defatted chia. However, in the case of metal chelating activity (FIC assay), no statistical differences (p > 0.05) were found between non-defatted and defatted chia seeds. The results obtained in this work for these values were lower than those mentioned in the corresponding scientific literature. Sargi et al. [21] reported DPPH, ABTS and FRAP values of 640.74, 430.50 and 715.82 mg TE/g, respectively, for triturated homogenized chia seeds, while Marineli et al. [22] obtained higher antioxidant capacity values for DPPH and FRAP, with values of 116.04 and 112.81 mg TE/g, respectively. When the scavenging potential of chia polyphenolic compounds after the different phases of in vitro digestion was assessed, the DPPH values (Table 2) of non-defatted and defatted chia seeds after oral digestion decreased significantly (p < 0.05) with respect to initial values. In this assay, the defatted chia seeds had a higher (p < 0.05) antioxidant effect than non-defatted chia seeds. In the gastric phase, the DPPH values were higher (p < 0.05) than those obtained in the oral phase which means that the pH conditions and enzymes that act in the gastric step affect the non-defatted chia seeds. Again, in this phase defatted chia seeds had a higher (p < 0.05) antioxidant effect than non-defatted chia seed. After the intestinal phase, both non-defatted and defatted chia seeds had significantly (p < 0.05) higher DPPH values (increases of 11.90 and 28.67%, respectively) than the test matrix. In this phase, defatted chia seeds had higher (p < 0.05) DPPH values than non-defatted chia seeds, reflecting the behaviour recorded in the scientific literature. Chen et al. [23] stated that among 33 fruits, 29 samples increased their antioxidant values determined with the DPPH assay after gastric and intestinal digestion.
As regards the ABTS assay (Table 2), again the oral phase step decreased (p < 0.05) the ABTS values of both chia and defatted chia seeds with respect to undigested samples. As occurred in the DPPH assay, the defatted chia seeds showed a higher (p < 0.05) antioxidant capacity than non-defatted chia seeds. After the gastric phase, the antioxidant capacity of non-defatted chia seeds decreased (p < 0.05) with respect to test matrix 36.78% whilst this antioxidant capacity in defatted chia seeds increased 7%. The ABTS values of defatted chia seeds were higher (p < 0.05) than those of chia seeds. After the intestinal phase, the ABTS values increased (p < 0.05) significantly by 140 and 314% for both chia and defatted chia seeds, respectively. This behavior after the intestinal phase was hypothetically related to the release from the matrix of unextractable bioactive compounds and/or their chemical transformations into compounds with a greater antioxidant capacity [8]. With regards to the ferric reducing activity power (FRAP), the results obtained (Table 2) show that at the end of the oral phase defatted chia seeds showed higher values (p < 0.05) than non-defatted chia. This phase significantly decreased (p < 0.05) the reducing power in both chia samples compared with the test matrix. In the gastric phase, defatted chia seeds again showed higher values (p < 0.05) than non-defatted chia. No statistical differences (p > 0.05) were found in this phase between the FRAP values of both non-defatted or defatted chia seeds and the test matrix. In the last phase of digestion, The FRAP values increased (p < 0.05) over those of the undigested samples by 89.58 and 62.36% for non-defatted and defatted chia seeds, respectively, with no statistical differences (p > 0.05) between the samples analysed. The results obtained agree with those of Chandrasekara and Shahidi [24], who found that the FRAP values of dehulled and cooked grains of five millet varieties increased after gastrointestinal digestion, with a percentage of variation greater than 2700%. With regard to the ferrous ion chelating (FIC) activity (Table 2), no statistical differences were found (p > 0.05) between the chia samples at the end of oral phase or with respect to the test matrix (p > 0.05). In the gastric phase, defatted chia seeds had a higher (p < 0.05) antioxidant effect than chia seeds, the values of both being significantly lower (p < 0.05) than the initial values. The results obtained suggested that pH value as well as enzymatic activity increase the release and stability of compounds with chelating activity. The last phase of digestion significantly increased (p < 0.05) the %var. of both chia and defatted chia seeds (290 and 142%, respectively) over initial values. In this case, chia seeds had higher (p < 0.05) FIC values than defatted chia. These results agree with Gullón et al. [4], who found that the chelating activity of pomegranate peel flour increased significantly after the intestinal stage.
The results were correlated with those obtained from TPC and TFC; the Pearson correlation coefficients are shown in Table 3. Among the assays, there was a strong positive correlation (r > 0.7) between antioxidant capacity and the TPC results, a moderate correlation (0.7 < r > 0.3) between TFC and DPPH-FRAP-FIC and lower correlation (r < 0.3) between TFC and ABTS. After the oral phase, the correlation between TPC and AOA assays and TFC and FIC results became negative, while a strong positive correlation (r > 0.7) between antioxidant capacity and TFC was recorded. After the gastric phase the antioxidant capacity results were all strongly positively correlated (r > 0.7) with both TPC and TFC, while at the end of the gastrointestinal digestion the situation changed again: DPPH-ABTS-FRAP and TPC, and FIC and TFC had strong positive correlations (r > 0.7), while between TPC and FIC, and DPPH-ABTS-FRAP and TFC a negative correlation was obtained. The scientific literature contains several works that correlate the antioxidant capacity with the total phenolic and flavonoid contents, with varying results: several studies reported positive correlations [4, 25], while others mention very low or no correlation [11, 23]. The results we present here suggest that during the gastrointestinal digestion the antioxidant capacity detected could be attributed to the flavonoid content after the oral phase, to both the phenolic acids and flavonoid contents after the gastric phase and to the phenolic acids after the intestinal phase. These contrasting correlation results suggest that the antioxidant capacity may also be attributed to other antioxidant substances released or generated during the gastrointestinal digestion process.
Conclusions
The results showed that the concentration of polyphenolic compounds increase during digestion, although only a low-medium percentage of phenols and a low percentage of flavonoids are available for absorption in the intestinal tract. In addition, the presence of a high level of fats seems to have a negative effect on the bioaccessibility of flavonoids. It is important to note the divergence of the correlation results obtained between the total phenolic, total flavonoid content and antioxidant capacity. Despite the low bioaccessibility values obtained for both the phenolics and flavonoids, their potential antioxidant capacity could be a valid reason for the addition of non-defatted and defatted chia seeds as food ingredients. Further studies need to be carried out to understand the stability of chia bioactive compounds and to improve their bioaccessibility.
Abbreviations
- CSF:
-
Chyme-soluble fraction
- PF:
-
Pellet fraction
- IN:
-
Intestinal absorbed fraction
- OUT:
-
Intestinal unabsorbed fraction
- TFC:
-
Total flavonoid content
- TPC:
-
Total phenolic content
References
Capitani MI, Spotorno V, Nolasco SM, Tomás MC (2012) Physicochemical and functional characterization of by-products from chia (Salvia hispanica L.) seeds from Argentina. Food Sci Technol 45:94–102. https://doi.org/10.1016/j.lwt.2011.07.012
Ixtaina VY, Martínez ML, Spotorno V et al (2011) Characterization of chia seed oils obtained by pressing and solvent extraction. J Food Compos Anal 24:166–174. https://doi.org/10.1016/j.jfca.2010.08.006
Valdivia-Lopez MA, Tecante A (2015) Chia (Salvia hispanica): a review of native Mexican seed and its nutritional and functional properties. Adv Food Nutr Res 75:53–75. https://doi.org/10.1016/bs.afnr.2015.06.002
Gullón B, Pintado ME, Barber X, Fernández-López J et al (2015) Bioaccessibility, changes in the antioxidant potential and colonic fermentation of date pits and apple bagasse flours obtained from co-products during simulated in vitro gastrointestinal digestion. Food Res Int 78:169–176. https://doi.org/10.1016/ j.foodres.2015.10.021
Silva FGD, O’Callagahan Y, O’Brien NM, Netto FM (2013) Antioxidant capacity of flaxseed products: the effect of in vitro digestion. Plant Foods Hum Nutr 68:24–30. https://doi.org/10.1007/s11130-012-0329-6
Rein MJ, Renouf M, Cruz-Hernandez C, Actis-Goretta L, Thakkar SK, da Silva Pinto M (2013) Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br J Clin Pharmacol 75(3):588–602. https://doi.org/10.1111/j.1365-2125.2012.04425.x
Gullón B, Pintado ME, Fernández-López J et al (2015) In vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: changes in the antioxidant potential and bioactive compounds stability. J Funct Foods 19:617–628. https://doi.org/10.1016/j.jff.2015.09.056
Ortega N, Macià A, Romero MP, Reguant J, Motilva MJ (2011) Matrix composition effect on the digestibility of carob flour phenols by an in vitro digestion model. Food Chem 124:65–71. https://doi.org/10.1016/j.foodchem.2010.05.105
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16(3):144–158. https://doi.org/10.4172/2327-5146.1000289.
Blasa M, Candiracci M, Accorsi A, Piacentini MP, Albertini MC, Piatti E (2006) Raw Millefiori honey is packed full of antioxidants. Food Chem 97(2):217–222. https://doi.org/10.1016/j.foodchem.2005.03.039
Lucas-Gonzalez R, Navarro-Coves S, Pérez-Álvarez JA, Fernández-López J et al (2016) Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion. Ind Crop Prod 94:774–782. https://doi.org/10.1016/ j.indcrop.2016.09.057
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Oyaizu M (1986) Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jap J Nutr 44:307–315. https://doi.org/10.5264/eiyogakuzashi.44.307
Carter P (1971) Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal Biochem 40(2):450–458. https://doi.org/10.1016/0003-2697(71)90405-2
Tagliazucchi D, Verzelloni E, Bertolini D, Conte A (2010) In vitro bioaccessibility and antioxidant activity of grape polyphenols. Food Chem 120:599–606. https://doi.org/10.1016/j.foodchem.2009.10.030
Everette JD, Bryant QM, Green AM, Abbey YA, Wangila GW, Walker RB (2010) Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J Agric Food Chem 58:8139–8144. https://doi.org/10.1021/jf1005935
Ortega N, Reguant J, Romero MP, Macia A, Motilva MJ (2009) Effect of fat content on the digestibility and bioaccessibility of cocoa polyphenol by an in vitro digestion model. J Agric Food Chem 57(13):5743–5749. https://doi.org/10.1021/jf900591q
Materska M (2010) Evaluation of the lipophilicity and stability of phenolic compounds in herbal extracts. Acta Sci Pol Technol 9(1):61–69
Martínez-Cruz O, Paredes-López O (2014) Phytochemical profile and nutraceutical potential of chia seeds (Salvia hispanica L.) by ultrahigh performance liquid chromatography. J Chromatogr A 136:43–48. https://doi.org/10.1016/j.chroma.2014.04.007
Rahman MdJ, Costa de Camargo A, Shahidi F (2017) Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J Funct Foods 35:622–634. https://doi.org/10.1016/j.jff.2017.06.044
Sargi SC, Silva BC, Munise H et al (2013) Antioxidant capacity and chemical composition in seeds rich in omega-3: chia, flax, and perilla. Food Sci Technol 33(3):541–548. https://doi.org/10.1590/S0101-20612013005000057
Marineli RS, Moraes ÉA, Lenquiste SA, Godoy AT et al (2014) Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.). LWT-Food Sci Technol 59:1304–1310. https://doi.org/10.1016/j.lwt.2014.04.014
Chen GL, Chen SG, Zhao YY, Luo CX, Li J, Gao YQ (2014) Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind Crop Prod 57:150–157. https://doi.org/10.1016/j.indcrop.2014.03.018
Chandrasekara A, Shahidi F (2012) Bioaccessibility and antioxidant potential of millet grain phenolics as affected by simulated in vitro digestion and microbial fermentation. J Funct Foods 4(1):226–237. https://doi.org/10.1016/j.jff.2011.11.001
Baker I, Chohan M, Opara EI (2013) Impact of cooking and digestion, in vitro, on the antioxidant capacity and anti-inflammatory activity of cinnamon, clove and nutmeg. Plant Foods Hum Nutr 68:364–369. https://doi.org/10.1007/s11130-013-0379-4
Acknowledgements
This research was supported by the grant of Ministerio de Economía, de Industria y Competitividad for the project: GL2016-75687-C2-2-R (AEI/FEDER, UE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pellegrini, M., Lucas-Gonzalez, R., Sayas-Barberá, E. et al. Bioaccessibility of Phenolic Compounds and Antioxidant Capacity of Chia (Salvia hispanica L.) Seeds. Plant Foods Hum Nutr 73, 47–53 (2018). https://doi.org/10.1007/s11130-017-0649-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-017-0649-7