Abstract
The bioactivity of two kiwifruit’s cultivars growing under organic and conventional conditions were studied and compared. The bioactive compounds were extracted with water and ethanol using similar conditions which are applied in pharmaceutical applications and for daily fruit consumption such as tea drink. Antioxidant radical scavenging assays [ferric-reducing/antioxidant power (FRAP); cupric reducing antioxidant capacity (CUPRAC); 2, 2-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS)], fourier transform infrared (FT-IR) and ultraviolet spectroscopy, two (2D-FL) and three-dimensional (3D-FL) fluorometry were used for the detection of biologically active metabolites derived from kiwifruits (total phenols, flavonoids, chlorophylls, carotenoids and ascorbic acid). The correlation between the total phenol content (TPC) and other bioactive compounds, and their total antioxidant capacities (TAC) was calculated for studied kiwifruit’s extracts. The interaction between drugs and human serum albumin (HSA) plays an important role in the distribution and metabolism of drugs. The properties of kiwifruit’s phenol extracts showed their ability to quench HSA, forming the complexes similar to the ones between the proteins and pure flavonoids such as quercetin. The cultivar ‘Bidan’ exhibited significantly higher TAC than the classic ‘Hayward’. In conclusion, for the first time ‘Bidan’ organic kiwifruit was analyzed and compared with widely consumed ‘Hayward’, using its bioactive and fluorescence properties. The influence of physiologically active kiwifruit’s compounds on human health, through our investigations in vitro and scientifically proven information, was explained. Relatively high content of bioactive compounds, high antioxidant and fluorescence properties of kiwifruit justify its use as a source of valuable antioxidants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants in general and fruits particularly have several compounds with antioxidant properties, which include ascorbic acid, carotenoids and polyphenols. Increased consumption of fruits protects cardiovascular diseases [1, 2]. Kiwifruit is widely consumed [3, 4]. There are many kiwifruit cultivars and the most known is ‘Hayward’. ‘Bidan’ is less spread than ‘Hayward’ and both cultivars belong to the Actinidia deliciosa species and is known for good taste [5–7]. There are some reports showing the differences between plants growing under specific conditions [8–13]. The effect of cultivation systems and fruit post harvest management on the antioxidant properties of apple, apricot and other plants was investigated [9, 10, 13]. The differences in the bioactivity between kiwifruit cultivars grown in conventional and organic conditions were less studied [7, 14]. Extraction with solvents of different polarity showed a variety of phenolic substances and their yield [15–17]. Therefore, it was decided to compare the nutritional and pharmaceutical properties of organic and conventional kiwifruit cultivars, using three solvents (ethanol, water and a mixture of 50 % ethanol and 50 % water). In order to receive reliable results of total antioxidant capacities three generally accepted assays (ABTS, FRAP and CUPRAC) were used [18–20]. As far as we know no results of such investigations have been published.
Materials and Methods
Chemicals
Trolox; phenolic standards, HSA, Tris, tris(hydroxymethy1) aminomethane; ABTS; Folin–Ciocalteu reagent; lanthanum (III) chloride heptahydrate; FeCl3 × 6H2O; CuCl2 × 2H2O; and 2,9-dimethyl-1,10-phenanthroline (neocuproine) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 2, 4, 6-Tripyridyl-s-triazine (TPTZ) was from Fluka Chemie (Buchs, Switzerland).
Sample Preparation
Kiwifruit cultivars ‘Hayward’ and ‘Bidan’ were grown under conventional and organic conditions in the orchard Heanam county (longitude 126° 15″ and latitude 34° 18″), Jeonnam province, Korea, 2010. Mean temperature was about 13–14 °C, rainfall—1,300 mm, and soil was loam. In fertilization of organic kiwifruit mature was applied (3 ton per 10 a). The fruits were sprayed three times with bio-control agents (Seva stop, Poex, white killer, produced in Korea). Any pesticides or herbicides were not applied during the growing process. Water drop irrigation (30 ton per week per 10 a) was usually used. Water is related to the dry matter of the fruit, but water stress enhances the phenolic content in plant. For the investigation five replicates of five fruits each at their commercial maturity stage (the degree of soluble solids content (SSC) was in the range of 6.8–7.5 %) were used. The samples were treated with liquid nitrogen in order to prevent oxidation of phenolic compounds and then lyophilized as previously described [5–7]. The bioactive compounds and the TACs were determined in ethanol (EtOH), water (H2O) and 50%EtOH + 50%H2O extracts of conventional (HC) and organic (HO) grown ‘Hayward’ and ‘Bidan’ (BC) and (BO) cultivars: HC, EtOH; HO, EtOH; HC, H2O; HO, H2O; HC, EtOH/H2O; HO, EtOH/H2O; BC, EtOH; BO, EtOH; BC, H2O; BO, H2O; BC, EtOH/H2O; BO, EtOH/H2O.
Determination of Bioactive Compounds and Total Antioxidant Capacities (TACs)
The extracts were phenols extracted with ethanol, water and a mixture of 50 % ethanol and 50 % water (concentration 20 mg/mL) during 1 h in a cooled ultrasonic bath [8, 9]. TPCs were determined by Folin-Ciocalteu method with absorbance measurements at 750 nm with spectrophotometer (Hewlett-Packard, model 8452A, Rockville, MD, USA). Total flavonoid content was determined by an aluminum chloride colorimetric method with some modifications. The absorbance was measured immediately against the blank at 510 nm [5–7].
For determination of ascorbic acid by CUPRAC assay, the aqueous phase was immediately examined after extraction-removal of La (III)-flavonoid complexes into ethyl acetate [21]. Total chlorophylls, chlorophylls a and b, and total carotenoids were extracted with 100 % acetone and determined spectrophotometrically at the following absorbances (nm) of 661.6, 644.8, and 470, respectively [22].
Determination of TACs, μmol TE/g DW
The TACs were determined by three complementary assays: (1) ABTS: ABTS.+ radical cation was generated by the interaction of ABTS (7 mmol/l) and K2S2O8 (2.45 mmol/l) with absorbance measurement at 734 nm. (2) FRAP: FRAP reagent of 900 μl was mixed with 90 μl of distilled water and 30 μl of kiwifruit samples. The absorbance was measured at 595 nm. (3) CUPRAC: The absorbance at 450 nm was recorded [18, 19].
Fluorometric Measurements
Fluorescence spectra for all kiwifruit water and ethanol extracts at a concentration of 0.01 mg/ml were recorded on a model FP-6500, Jasco spectrofluorometer, serial N261332 (Japan, equipped with 1.0 cm quartz cells and a thermostat bath. The three-dimensional spectra (3D-FL) were collected with subsequent scanning emission spectra from 250 nm to 500 at 1.0 nm increments by varying the excitation wavelength from 200 to 350 at 10 nm increments [23]. Quercetin (Q) was used as a standard. All solutions for protein interaction were prepared in 0.05 mol/l Tris–HCl buffer (pH 7.4), containing 0.1 mol/l NaCl. The final concentration of HSA was 2.0 × 10−6 mol/l. The HSA was mixed with quercetin in the proportions of HSA: extract = 1:1.
FT-IR Spectra Studies
Total phenols in the investigated kiwifruit extracts were studied by FT-IR spectroscopy. A Nicolet iS 10 FT-IR Spectrometer (Thermo Scientific Instruments LLC, Madison, WI, USA), with the smart iTRTM ATR (attenuated total reflectance) accessory was used to record IR spectra [7].
Statistical Analyses
To verify the statistical significance, mean ± SD of five independent measurements were calculated. Differences between groups were tested by two ways ANOVA. In the assessment of the TACs, Spearman correlation coefficients (R) were used. Linear regressions were also calculated. P-values of <0.05 were considered significant.
Results and Discussion
Total phenols, flavonoids, TACs, chlorophylls, carotenoids, and ascorbic acid
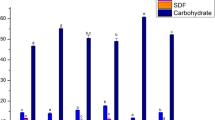
The results of the determination of the TPCs, flavonoids and TACs in the samples of conventional and organically grown kiwifruits by three solvent systems are shown in Table 1.
As can be seen, the TPCs and TACs by FRAP, CUPRAC and ABTS were significantly higher in EtOH/H2O extracts (P < 0.05) than in the other extracts. Total chlorophylls (a, b), total carotenoids and vitamin C (Table 2) did not show significant difference between the organically and conventionally grown kiwifruit cultivars. In ‘Bidan’ cultivar all the studied indices were significantly higher than in ‘Hayward’. The correlations between the phenolic compounds and the TACs were relatively high (R2 from 0.82 to 0.99).
Fluorometric data
The quenching properties of the kiwifruit samples in comparison with the pure flavonoid such as quercetin are shown in 3D -FL, which illustrated the elliptical shape of the cross maps. The results showed that the 3-D FL of cross section view of conventional kiwifruit phenolic extracts in comparison with organic were similar [7].
One of the main peaks for HSA was found at λex/em of 220/360 nm. The second main peak appeared for these samples at λex/em of 280/350 nm (Fig. 1a). The interaction of HSA and ‘Hayward’ (HC) and ‘Bidan’ (BC) kiwifruit extracts (Fig. 1b, c); HSA and Q (Fig. 1d), HSA, Q and BC (Fig. 1e) and HSA, Q and HC (Fig. 1f) showed slight change in the position of the main peak from 360 to 350 nm and the decrease in the fluorescence intensity (FI).
Some changes appeared when kiwifruit ethanolic extracts were added to HSA. Initially the main peak was at emission of 360 nm and FI of 984.11 (Fig. 2a and b, the upper lines are HSA). As a result of the reaction between the kiwifruit extracts and quercetin, the FI decreased (Fig. 2a and b, the decrease is in the middle and low lines in comparison with the upper one). The following decrease in the FI (%) occurred during the interaction of quercetin and ethanol phenolic extracts: HSA + Q = 65.5; HSA + BC = 14.2; HSA + BC + Q = 71.1; HSA + BO = 14.5; HSA + BO + Q = 80.2; HSA + HC = 11.7; HSA + HC + Q = 71.3; HSA + HO = 13.4; HSA + HO + Q = 61.3. Water phenolic extracts showed slightly different numbers: HSA + Q = 65.5; HSA + BC = 13.5; HSA + BC + Q = 70.9; HSA + BO = 12.8; HSA + BO + Q = 78.7; HSA + HC = 10.1; HSA + HC + Q = 70.3; HSA + HO = 9.6; HSA + HO + Q = 60.1. The interaction between ethanol and water kiwifruit phenolic extracts and HSA showed that the extracts have a strong ability to quench the intrinsic fluorescence of HSA by forming complexes. The results of binding affinity of HSA and extracted total phenols showed that the main quenching effect was with ‘Bidan’ organic + quercetin (Fig. 2b, with the lowest line of FI = 209.78). The main peak was at λem/ex = 350/280 nm. Our very recent results showed that the fluorescence was significantly quenched, because of the conformation of the BSA changes in the presence of pure flavonoids and kiwifruit extracts [23]. This interaction between the quercetin and HSA was investigated using tryptophan fluorescence quenching. It is in agreement with others that quercetin, as an aglycon, is more hydrophobic and demonstrates strong affinity toward HSA. Other cited results differ [24] from the reported by us, probably, because of the variety of antioxidant abilities of pure flavonoids and different fluorometric scanning ranges used in a similar study. There are no publications on the applications of 3D-FL, therefore our present conclusions, that 3D-FL can be used as an additional tool for the characterization of the total phenol extracts, correspond with our previous data [5–7]. The biological relevance of quercetin interaction in human organism is important from the point that this molecule of polyphenolic type extensively binds to HSA, the most abundant carrier protein in the blood, decreasing inflammation in atherosclerotic progression and regression. Our in vitro results of interaction of HSA and quercetin can be compared with other reports in vivo, showing the protective effects of quercetin on hepatic injury induced by different chemical reactions [24].
FT-IR spectra
The FTIR spectra of ethanol (Fig. 3, lines a, b, c, d) and water (Fig. 3, Insert) kiwifruit extracts (organic and conventional) were compared between them and also with standards in the range of common peaks (Table 3).
The best matching in the common range of the peaks was in kiwifruit ethanolic extracts: HC: BC = 97.15 %; BC: HO = 97.23 %; BO: HC = 96.05 %; BO: HO = 96.41 %; in water extracts the matching was lower: HC: BC = 93.36 %; BC: HO = 93.31 %; BO: HC = 94.35 %; BO: HO = 94.67 %. The matching (%) of the peaks in the region from 4000 to 650 cm−1 and from 2600 to 2400 cm−1 (Fig. 3, Table 3) showed the highest matching (99 %) between ‘Bidan’ conventional and ‘Hayward’ organic in two regions of phenols extracted with ethanol. The obtained peaks of total phenols in water and ethanol extracts were compared with different standards. The matching of tannic acid, catechin and quercetin in ethanol extracts was the highest between the phenolic compounds and kiwifruit extracts (Table 3). The highest matching was estimated in the region of 2600–2400 cm−1. Catechin and quercetin in the kiwifruit ethanolic extracts showed high matching of 86–89 %; and 84–86 %, respectively. Slightly different data were obtained in the water extracts, showing 90 % of matching with tannic acid in the range of 3404–3101 cm−1. These matching results for the first time showed that FT-IR spectra can be used for a rapid estimation of extracted bioactive compounds. The water extracts were relatively lower in the matching values than the ethanol, showing that the yield of extracted bioactive compounds also was lower in water than in ethanol extracts. Quercetin and catechin exhibited the highest matching in the investigated fruit extracts in comparison with flavonoids hesperidin and fisetin; caffeic, p-coumaric, gallic and tannic acids. In our previous study, the FT-IR spectra data showed that the main bands in the kiwifruit samples slightly shifted [7]. A shift in the difference between the standards and the investigated samples can be explained by the extraction procedures of the total phenols.
It is well known that consumption of fruits with high contents of bioactive compounds and high antioxidant capacity prevents and treats even the most dangerous diseases [1, 2], therefore it is recommended to use fruits in daily diet [20]. An interest was shown by scientific community concerning organically versus conventionally grown fruits and vegetables [9–12]. In the present investigation conventional and organically grown kiwifruit cultivars ‘Hayward’ and ‘Bidan’ were studies with the aim to find the best among them for human consumption. It must be underlined once again that these samples were at the same stage of ripening and were grown in the same geographic and climatic conditions. Therefore, no doubt, that the determined data were reliable. So, we found that ‘Hayward’ and ‘Bidan’ ethanolic extracts of the organic grown cultivars contain higher amounts of the studied bioactive compounds, but these results were not always significant.
Our results of phenolics (mg GAE/g DW) in ‘Hayward’ kiwifruit patterns were slightly higher than in other studies 2.19 [3], 2.94 [4], and 3.5 [14], and equal to 0.784 mg GAE/g FW [8]. High levels of total phenolics and their discrepancies, which were determined in this study (Table 1), if compared to previous reports [5, 7, 23], can be explained due to different extraction procedures of phenolics, different genetic attributes as well as ecological conditions, plant genotype, cultivation site and technological practices and year of harvest [8]. The same patterns were reported for other fruits [1, 6, 20]. Reports indicate that organic and conventional fruits may differ on a variety of sensory qualities [9, 11]. The results of our recent investigation [5] support the data of the above cited authors. The differences in the composition of organic and conventional vegetables depend on various kinds of organic mature and the growing seasons [5, 9]. Apples from organic production showed a higher content of total phenolics than apples from integrated cultivation [9]. The TACs, TPCs and total carotenoids showed better performance under organic system in comparison with the integrated [10]. Our data are in correspondence with others [16] that solvent chemical properties (hexane, ethyl acetate, acetone, methanol, and a methanol:water mixture), such as polarity, can differentially impact the efficiency with which different bioactive compounds are recovered from foods. This could lead to differences in estimated biological activity such as TAC. The different polarity of the solvents was shown by others [15], comparing ethyl acetate, methanol, n-hexane, n-butanol and water extracts from mungbean seeds and sprouts. The TAC of all samples was higher in methanol extract than in ethyl acetate, probably showing that hydrophilic (polar) antioxidants were in the majority in the tested extracts. So, Fisk et al. [25] reported that the TAC ranged from 1.6 to 2.3 AA equivalents/g FW with no significant difference between treatments. Similar results were reported in different fruits [26, 27]. As it could be seen the differences between organic and conventional kiwifruit were not significant. There may be differences in the secondary metabolites between two fruits grown by two methods immediately after harvest. The plant grown by organic method contains higher amount of secondary metabolites, therefore it is exposed to plant cell wall fragments and microorganism materials (or pathogens) with higher ability. Some of these materials are known as elicitors, which are defined as substances that induce plant defense responses in plants. These elicitors certainly induce secondary metabolites. However, it will be difficult to see differences in these metabolites unless the samples are analyzed immediately after harvest. There may be differences in cell wall thickness and lignin contents because most elicitors induce cell wall thickening and lignification along with induction of secondary metabolites. This discussion is in full agreement with others that limited the spectrum of species examined [10]. The intake of organic foods leads to some advantages [12, 13], such as the ingestion of high contents of phenolic compounds and vitamin C, and a low content of nitrates and pesticides. The amount of polyamines, substances stimulating cellular division, is increased. A higher content of these compounds could explain some of the effects of the diet on patients affected by certain syndromes.
Conclusions
The antioxidant capacity of the studied ethanol and water extracted samples is significantly different (P < 0.05): according to the used tests was significantly minimal (P < 0.05) in water, and the highest was in ethanol:water mixture. ‘Bidan’ cultivar showed higher bioactivity than ‘Hayward’. Higher bioactivity was in organic kiwifruits, but not always significant. Positive antioxidant and quenching properties of kiwifruit justify the use of this fruit as a source of valuable antioxidants.
Abbreviations
- 2D and 3D-FL:
-
Two and three-dimensional fluorescence spectra
- ABTS:
-
2,2-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid)
- CUPRAC:
-
Cupric reducing antioxidant capacity
- FRAP:
-
Ferric-reducing/antioxidant power
- FT-IR:
-
Fourier transform infrared spectroscopy
- HSA:
-
Human serum albumin
- TAC:
-
Total antioxidant capacities
- TPC:
-
Total phenol content
References
Paredes-López O, Cervantes-Ceja M, Vigna-Pérez M, Hernández-Pérez T (2010) Berries: improving human health and healthy aging, and promoting quality life—A review. Plant Foods Hum Nutr 65:299–308
Duttaroy AK, Jøorgensen A (2004) Effects of kiwi fruits consumption in human volunteers on platelet aggregation and plasma lipids in vitro. Platelets 15:287–292
Tavarini S, Degl’Innocenti E, Remorini D, Massai R, Guidi L (2008) Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem 107:282–288
Jeong CH, Lee WJ, Bae SH, Choi SG (2007) Chemical components and antioxidative activity of Korean gold kiwifruit. Han’guk Sikp’um Yongyang Kwahak Hoechi 36:859–865
Park YS, Leontowicz H, Leontowicz M, Namiesnik J, Suhaj M, Cvikrova M, Martincova O, Weisz M, Gorinstein S (2011) Comparison of the contents of bioactive compounds and the level of antioxidant activity in different kiwifruit cultivars. J Food Comp Anal 24:963–970
Sarbu C, Nascu-Briciu RD, Kot-Wasik A, Gorinstein S, Wasik A, Namiesnik J (2012) Classification and fingerprinting of kiwi and pomelo fruits by multivariate analysis of chromatographic and spectroscopic data. Food Chem 130:994–1002
Park YS, Kyung S-H, Kang S-G, Park Y-K, Namiesnik J, Leontowicz H, Leontowicz M, Ezra A, Trakhtenberg S, Gorinstein S (2012) Organic and conventional kiwifruit, myths versus reality: antioxidant, antiproliferative, and health effects. J Agric Food Chem 60:6984–6993
Mikulic-Petkovsek M, Schmitzer V, Slatnar A, Stampar F, Veberic R (2012) Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J Food Sci 77:C1064–C1070
Mikulic-Petkovsek M, Slatnar A, Stampar F, Veberic R (2010) The influence of organic/integrated production on the content of phenolic compounds in apple leaves and fruits in four different varieties over a 2-year period. J Sci Food Agric 90:2366–2378
Leccese A, Bureau S, Reich M, Renard MGCC, Audergon J-M, Mennone C, Bartolini S, Viti R (2010) Pomological and nutraceutical properties in apricot fruit: cultivation systems and cold storage fruit management. Plant Foods Hum Nutr 65:112–120
Bourn D, Prescott J (2002) A comparison of the nutritional value, sensory qualities, and food safety of organically and conventionally produced foods. Crit Rev Food Sci Nutr 42:1–34
Lima GPP, Vianello F (2011) Review on the main differences between organic and conventional plant-based foods. Int J Food Sci Tech 46:1–13
Camin F, Perini M, Bontempo L, Fabroni S, Faedi W, Magnani S, Baruzzi G, Rapisarda P (2011) Potential isotopic and chemical markers for characterising organic fruits. Food Chem 125:1072–1082
Amodio ML, Colelli G, Hasey JK, Kader AA (2007) A comparative study of composition and postharvest performance of organically and conventionally grown kiwifruits. J Sci Food Agric 87:1228–1312
Kim DK, Jeong SC, Gorinstein S, Chon S-U (2012) Total polyphenols, antioxidant and antiproliferative activities of different extracts in mungbean seeds and sprouts. Plant Foods Hum Nutr 67:71–75
Bae H, Jayaprakasha GK, Crosby K, Jifon JL, Patil BS (2012) Influence of extraction solvents on antioxidant activity and the content of bioactive compounds in non-pungent peppers. Plant Foods Hum Nutr 67:120–128
Mastrodi Salgado J, Baroni Ferreira TR, de Oliveira Biazotto F, dos Santos Dias CT (2012) Increased antioxidant content in juice enriched with dried extract of pomegranate (Punica granatum) peel. Plant Foods Hum Nutr 67:39–43
Ozgen M, Reese RN, Tulio AZ Jr, Scheerens JC, Miller AR (2006) Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J Agric Food Chem 54:1151–1157
Apak R, Guclu K, Ozyurek M, Karademir SE (2004) Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem 52:7970–7981
Proteggente AR, Pannala AS, Paganga G, Van Buren L, Wagner E, Wiseman S, Van De Put F, Dacombe C, Rice-Evans CA (2002) The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Rad Res 36:217–233
Ozyurek M, Guçlu K, Bektasoglu B, Apak R (2007) Spectrophotometric determination of ascorbic acid by the modified CUPRAC method with extractive separation of flavonoids-La(III) complexes. Anal Chim Acta 588:88–95
Boyer RF (1990) Isolation and spectrophotometric characterization of photosynthetic pigments. Biochem Educ 18:203–206
Gorinstein S, Haruenkit R, Poovarodom S, Park YS, Vearasilp S, Suhaj M, Ham KS, Heo BG, Cho JY, Jang HG (2009) The comparative characteristics of snake and kiwifruits. Food Chem Toxicol 47:1884–1891
Xiao JB, Chen TT, Cao H, Chen LS, Yang F (2011) Molecular property-affinity relationship of flavanoids and flavonoids for human serum albumin in vitro. Mol Nutr Food Res 55:310–317
Fisk CL, McDaniel MR, Strik BC, Zhao Y (2006) Physicochemical, sensory, and nutritive qualities of hardy kiwifruit (Actinidia arguta‘Ananasnaya’) as affected by harvest maturity and storage. J Food Sci 71:S204–S210
Egea I, Sanchez-Bel P, Romojaro F, Pretel MT (2010) Six edible wild fruits as potential antioxidant additives or nutritional supplements. Plant Foods Hum Nutr 65:121–129
Fernandez-Lopez JA, Almela L, Obon JM, Castellar R (2010) Determination of antioxidant constituents in cactus pear fruits. Plant Foods Hum Nutr 65:253–259
Acknowledgments
This research was partly supported by the Rural Development Administration (RDA), Korea.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article was written in memory of my dear brother Prof. Simon Trakhtenberg, who died in November 2011, who encouraged me and our research group during all his life. Shela Gorinstein
Rights and permissions
About this article
Cite this article
Park, Y.S., Im, M.H., Ham, KS. et al. Nutritional and Pharmaceutical Properties of Bioactive Compounds in Organic and Conventional Growing Kiwifruit. Plant Foods Hum Nutr 68, 57–64 (2013). https://doi.org/10.1007/s11130-013-0339-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-013-0339-z