Abstract
trans-Resveratrol (trans-R) has been reported to be a potential cancer chemopreventive agent. Although its cytotoxic activity against different cancer cell lines has been tested, its effect on human acute leukemia cell lines has scarcely been investigated, and only a few in vitro studies were performed using human breast epithelial cell lines. Due to its potential value for human health, demand for trans-R has rapidly increased, and new biotechnological strategies to obtain it from natural edible sources have been developed. Thus, grapevine cell cultures represent a reliable system of trans-R production since they biosynthesize trans-R constitutively or in response to elicitation. In addition, there are no studies deepen on the inhibitory effect of trans-R, produced by elicited grapevine cell cultures, on growth of human tumor cell lines. In this work, the effect of trans-R extracted from the culture medium, after elicitation of grapevine cell cultures, was tested on two human acute lymphocytic and monocytic leukemia cell lines, and one human breast cancer cell line. The effect of trans-R on cell proliferation was not only dose- and time-dependent but also cell type-dependent, as seen from the different degrees of susceptibility of cancer cell lines tested. As regards the effect of trans-R on cell cycle distribution, low trans-R concentrations increased cells in the S phase whereas a high trans-R concentration increased G0/G1 phase in all cell lines. Perturbation of the cell cycle at low trans-R concentrations did not correlate with the induction of cell death, whereas a high trans-R concentration, cell proliferation decreased as a result of increasing apoptosis in the three cell lines. In leukemia cells, trans-R up-regulated the expression of caspase-3 while trans-R-induced apoptosis in breast cells occur through a caspase-3-independent mechanism mediated by a down-regulation of Bcl-2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resveratrol is a simple molecule of stilbene nature that has been extensively investigated since the first scientific paper described its possible preventive effect against cancer in mice [1]. This 3,5,4′-trihydroxystilbene, whose active trans-form (trans-R) is found in low amounts in some edible fruits and many plant families, has become the focus of many studies and publications, especially due to its possible beneficial effect on human health [2].
As a result of its antioxidant [3] and anti-inflammatory [4] properties, it has been suggested that trans-R may play a role in the prevention of cardiovascular diseases and might also act as chemopreventive against certain types of cancer [2]. Moreover, trans-R appears to protect against obesity and diabetes [2], and neurodegenerative and ageing diseases [5]. Regardless of these potential benefits for human health, less than 10 human clinical trials have been described to characterize its protective role in these diseases, although more than one hundred experimental studies have been performed with animals, and numerous in vitro studies have been developed [2]. One of the most striking biological activities of trans-R that has been investigated is its chemopreventive effect against cancer. This property was first noted from an extract of Cassia quinquangulata that inhibit the three stages of carcinogenesis [1]. Since then, trans-R has been considered as a synonym of a naturally occurring molecule possessing chemopreventive activity, and research into this compound has gradually gathered momentum. Despite the limited number of human studies, there is sufficient available scientific data to suggest that trans-R is a disease-preventive compound in humans [2]. As regards its cancer chemopreventive activity based on animal studies, Vang et al. [2] concluded that there is sufficient evidence to support a chemopreventive effect of trans-R on the development of skin cancer in mice, and the results on the prevention of colon cancer were also considered promising. However, the effects of trans-R on other cancer types need to be investigated in more detail before recommending clinical trials. As regards its cytotoxic activity against cancer cell lines, although more than 900 in vitro studies have been carried out, its effect on human acute leukemia and breast cell lines have been scarcely investigated (only about 10 publications up to 2008 [6]) and no more than thirty using human breast cell lines [6, 7].
The inhibition of abnormal cell proliferation through the modulation of cell cycle progression is one of the most important strategies for chemoprevention as well as chemotherapy. trans-R inhibits the proliferation of cancer cell lines trough signalling molecules involved in regulating the cell cycle progression [8]. Moreover, multiple lines of evidence suggest that trans-R induces apoptosis by activating pro-apoptotic signalling molecules as well as by inhibiting anti-apoptotic molecules of intracellular signal transduction pathways [9]. Due to its high potential value for human health and the relatively low amounts obtainable from natural edible sources, new biotechnological strategies have been used to increase the levels of trans-R. In this way, grapevine cell cultures represent a reliable system of trans-R production because grapevine plants biosynthesize trans-R constitutively or in response to stress, which can be exploited by elicitation. In fact, the most significant success in increasing the trans-R content from Vitis cell cultures has been achieved using cyclodextrins (CDs) [10]. CDs are used in the food, pharmaceutical and chemical industries to solubilize non-polar compounds (like trans-R) in aqueous media. Under CD elicitation, grapevine cell cultures produce high levels of trans-R, mainly due to the fact that CDs chemically resemble the pectic oligosaccharides released from cell walls during fungal attack, and grapevine cells respond to their presence by synthesizing trans-R. In fact, Vitis cell cultures treated with CDs are able to produce more than 3,000 mg trans-R/l, which is secreted and accumulated in the culture medium, facilitating its extraction [10]. Therefore, CDs act not only as inducers of trans-R biosynthesis but also as promoters that remove trans-R from the medium, reducing both feedback inhibition and trans-R degradation, and allowing its accumulation in high concentrations [10]. In this work, the cytotoxic effect of this trans-R source extracted from the culture medium after elicitation of grapevine cell cultures with CDs was tested on two human acute lymphocytic and monocytic leukemia cell lines, and one human breast cancer cell line.
Materials and Methods
Plant Material
V. vinifera cv Monastrell calli were established in 1990 as described by Calderón et al. [11]. Grapevine cell cultures derived from them have been routinely maintained by periodical subcultures as described by Belchí-Navarro et al. [10].
Elicitor Treatments and trans-R Analysis
Elicitation experiments were performed in triplicate using 12–14 day old Monastrell cell cultures as described by Belchí-Navarro et al. [10]. After elicitation, aliquots of the extracellular medium were analyzed in a HPLC (Agilent, Germany) equipped with ESI-MS (Agilent, Germany) as described by Lijavetzky et al. [12] using Data Analysis v 2.1.
Human Cancer Cell Lines and Culture Conditions
Human acute leukemia cell lines, JURKAT E.6 and THP-1, were cultured as described by Fernández-Pérez et al. [13]. The human breast epithelial cell line MCF-7, an estrogen receptor-positive cell line derived from an in situ carcinoma, was grown in EMEM medium supplemented with 10 % fetal bovine serum, 2 mM glutamine, 100 g/ml streptomycin, 100 U/ml penicillin, 1 mM sodium pyruvate and 0.1 mM amino acid solution (Sigma-Aldrich, Spain). JURKAT E.6 and THP-1 cells were seeded at 100,000 cells/ml and MCF-7 at 50,000 cells/cm2 in tissue culture flasks. All cells were maintained at 37 °C in a 5 % CO2 atmosphere with 95 % humidity, and were cultured every five days. Cell growth of three cell lines was characterized to ensure exponential growth throughout the experimental period, and a linear relationship between absorbance and cell number.
Measurements of Cell Growth by MTT or XTT Assays
All cells were seeded in 98-well microtiter plates at the different cell densities described above for 24 h. Then, cells were treated with different trans-R concentrations (10, 30 and 90 μM, using methanol as solvent at a final concentration of 0.13 %) for 24, 48, 72 and 96 h. At the end of each treatment, JURKAT E.6 and THP-1 cell proliferation was detected using XTT and phenazine methosulfate (Sigma Aldrich, Spain) as described by Fernández-Pérez et al. [13]. In the case of MCF-7 cell line, cell proliferation was measured with 1 mg/ml MTT solution (Sigma-Aldrich, Spain) for 4 h as described by Joe et al. [14]. Control cells were incubated under identical conditions without adding trans-R. In this case, in each microplate assay 10 % (v/v) DMSO was included as positive internal control while the culture medium and 1 % (v/v) methanol were included as negative and solvent controls, respectively. The effect of trans-R on the cell lines was quantified as the percentage of viability in relation with non-treated cell viability (control) (% cell viability = (absorbance test/absorbance control) x 100).
Cell Cycle Distribution Analysis by Flow Cytometry
The effect of trans-R on cell cycle distribution was evaluated by flow cytometry as described by Lee et al. [15] with minor modifications. For this, JURKAT E.6 and THP-1 cell lines were seeded at 5 × 105 cells/well and MCF-7 cell line at 3 × 105 cells/well in 12-well microtiter plates. All cell lines were treated with different trans-R concentrations (0, 10, 30 and 90 μM) and analyzed after 72 h incubation. After treatments, 200 μl of 1 × 106 cells/ml were fixed by addition of 2 ml of cold ethanol-phosphate buffered saline (PBS) (70–30 %) and then, samples were incubated for 30 min at 4 °C. Prior to staining, cells were centrifuged (150 g at 24 °C for 10 min) and resuspended in 800 μl of PBS. Cells were treated with 100 μl of RNase (1 mg/ml) and 100 μl propidium iodide (PI, 400 μg/ml) (Sigma-Aldrich, Spain) for 30 min at 37 °C in the dark. The fluorescence of stained cells was analyzed in a Fluorescence Activated Cell Sorting flow cytometry (Becton Dickinson, USA) and relative gated cells in each cell cycle phase were determined. Data acquisition and analysis were performed using CellQuest software for 30,000 cellular events per sample.
Apoptosis Analysis by Flow Cytometry
A double staining flow cytometric assay combining fluorescein diacetate (FDA) (Sigma-Aldrich, Spain) and PI was used for the detection of apoptotic cells. For this, cells were seeded in 12-well microtiter plates at the same cell density per well described above. All cell lines were treated with 10, 30 and 90 μM of trans-R, and apoptosis was measured after 72 h incubation. For this, cells were incubated with 20 μl of FDA solution (50 μg/ml) and 100 μl of PI (400 μg/ml) for 30 min at room temperature. Then, cells were analyzed in a Coulter Epics XL™ flow cytometer (Beckman Coulter, USA). Analyses were performed on 30,000 cells, which were acquired at 300 cells/s.
Western Blot of SDS-PAGE
Cells were harvested after 72 h of trans-R treatment, washed and lysed with cold lysis buffer PBS pH 7.4 with 1 % Nonidet P-40, 0.5 % desoxicolate, 0.1 % SDS and serine protease inhibitors (1 mM phenylmethylsulfonyl fluoride and 1 mM benzamidine hydrochloride) (Sigma-Aldrich, Spain). Protein concentration in the resulting lysate was determined according to the Bradford Bio-Rad protein assay using BSA as standard [16]. Proteins (20–30 μg) were separated by electrophoresis using 12 % polyacrylamide gels, and then transferred to PVDF membranes using a Mini Trans-Blot apparatus (BioRad, Spain) as described by Jiménez-Atiénzar et al. [17]. Then, PVDF membranes were incubated overnight at 4 °C in TBS-T (10 mM Tris-ClH, pH 7.4, 150 mM NaCl, 0.1 % Tween 20 containing 1 % non-fat milk) and the appropriated primary antibodies diluted as specified by the manufacturer. Membranes were washed and incubated with the corresponding HRP-conjugated secondary antibody (Sigma-Aldrich, Spain) at 1:2000 in TBS-T for 1 h, under constant stirring, at 25 °C. Finally, protein bands were detected on the membranes using the reaction medium for peroxidase activity staining as described by Jiménez-Atiénzar et al. [17]. In all the experiments, a control was carried out after the transfer by incubating a PVDF membrane with this staining solution.
Statistical Analysis
Experiments were repeated at least three times, and all data are given as the mean ± SD. Data were analyzed by ANOVA to confirm data variability and the multiple comparisons of means. All statistical analyses were performed with SPSS v.13.0. P values ≤0.05 were considered statistically significant.
Results and Discussion
Analysis by HPLC-ESI-MS
The extract obtained from elicited grapevine cell cultures was analyzed by HPLC-ESI-MS and showed only one peak, corresponding to trans-R with a high degree at 98 % purity, (results not shown), therefore, any potential cytotoxic activity of this extract could only be due to the presence of trans-R.
Effect of trans-R on Cell Proliferation
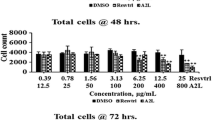
Proliferation arrest is one of the most important effects of trans-R on cancer cells from different origins [18]. To determine the inhibitory effect of trans-R on JURKAT E.6, THP-1 and MCF-7 cell lines, cells were treated with different trans-R concentrations for 96 h. The doses of trans-R (μM range) used in the experiments were comparable with the concentrations of trans-R that accumulate in liver and kidney following oral administration to mice [19]. The median inhibitory concentration (IC50) values after 72 h incubation were 29.3 ± 3.5, 21.7 ± 3.7 and 60.5 ± 2.9 μM for JURKAT E.6, THP-1, and MCF-7 cell lines, respectively. As shown by their IC50 values, both JURKAT E.6 and THP-1 cell lines were more sensitive to trans-R than MCF-7 cell line. This might be due to the higher proliferation rate of JURKAT E.6 and THP-1 cells compared with the MCF-7 cell line (data not shown). Leukemia cells may absorb and metabolize trans-R faster [20], and therefore manifest a stronger cytotoxic effect than that shown by MCF-7 cells. Some in vitro studies have tested the effect of trans-R obtained from different sources on HL60, a human promyelocytic leukemia cell line, and found higher IC50 values (around 70 μM, depending on the trans-R source used: P. cuspidatum extracts or chemically synthesized, [14, 21]) than those obtained in our experiments. As regards the trans-R effect on MCF-7 cells, IC50 values of around 100 μM have been found using both commercial (Sigma, [7, 22]) and natural trans-R sources (P. cuspidatum, [14]). In addition, trans-R showed a dose and time-dependent inhibitory effect on cell viability in all cancer cell lines (Fig. 1). In fact, Pozo-Guisado et al. [23] and Lee et al. [15] showed that treating human breast cancer cell lines with increasing trans-R concentrations resulted in a marked reduction in the number of viable cells in a time- and dose-dependent manner. However, although growth inhibition was dependent on trans-R concentration in all cell lines, it was more pronounced in JURKAT E.6 and THP-1 than in MCF-7 (Fig. 1a, b). In fact, MCF-7 cells were less sensitive to all concentrations of trans-R at all the incubation times tested (Fig. 1c). In addition, when trans-R concentrations ranging from 30 to 100 μM were used, cell growth decreased in all cell lines (p < 0.05) from 48 h of incubation onwards. Likewise, a low trans-R concentration (10 μM) increased cell viability in MCF-7 cells at 72 and 96 h of incubation compared with the control. This stimulatory effect at low trans-R concentrations was also observed in MCF-7 cells using trans-R concentrations of 10–20 μM [24]. The response of MCF-7 cells to treatment with low and high concentrations of trans-R was biphasic (Fig. 1c), as previously was reported [15, 22] for MCF-7 and HL-60 cell lines, respectively. However, this biphasic effect was cell type-dependent since it was not observed in JURKAT E.6 or THP-1 cell lines (Fig. 1a, b). Therefore, the effect of trans-R is not only dose- and time-dependent but also cell type-dependent, as seen from the different degrees of susceptibility of the three cell lines tested in this study.
Effect of trans-R on Cell Cycle Distribution
Having seen that trans-R decreased cell viability, the effect of this molecule on cell cycle distribution was analyzed (Fig. 2). For this, cells from JURKAT E.6, THP-1 and MCF-7 were exposed for 72 h to different trans-R concentrations (10, 30 and 90 μM) before staining with PI, and the DNA concentration in single cells was determined by flow cytometry. At a low trans-R concentration (10 μM), an accumulation of cells in S phase was observed in JURKAT E.6 (from 31.9 to 41.9 %) and THP-1 (from 20.6 to 33.9 %). In contrast, the populations of cells in the G0/G1 phase were reduced by this treatment (Fig. 2a, b). Similarly, the concentration of 30 μM caused an increase in the number of cells in the S phase and a corresponding decrease of cells in the G0/G1 phase. However, at a high trans-R concentration (90 μM), an accumulation of THP-1 and JURKAT E.6 cells in the G0/G1 phase (from 8 to 13.7 and from 20.4 to 54.8 %, respectively) and a decrease of cell fraction in the S phase was observed compared with the treatment of 30 μM of trans-R (Fig. 2a, b). When MCF-7 cells were incubated with a low trans-R concentration (10 μM), no effect on cell cycle distribution was observed (Fig. 2c). In contrast, 30 μM trans-R resulted in a significant accumulation of cells in the S phase (from 14.9 to 46.8 %), concomitant with a diminishing number of cells in the G2/M phase compared with the control (p < 0.05). However, the histogram of the DNA content in MCF-7 cells treated with 90 μM trans-R showed a significant increase in G0/G1 phase cells (from 53.5 to 62.9 %), while the population of cells in the S phase was significantly lower than that observed using 30 μM trans-R (p < 0.05). Taken as a whole, these results on cell cycle distribution indicate that concentrations of trans-R between 10 and 30 μM increase the number of cells in the S phase, particularly in JURKAT E.6 and THP-1, whereas a high concentration increases the cell population in the G0/G1 phase in all cell lines. This biphasic effect of trans-R on cell cycle distribution has been also described by Pozo-Guisado et al. [25] and Singh et al. [7] working with MCF-7 cells. These authors reported that at a low trans-R concentration (50 μM), cell accumulation in the S phase could be due to the low rates of cell death in the face of a significant decrease in cell proliferation. Similarly, Komina et al. [8] and Wesierska-Gadek et al. [18] also observed this behaviour in human HL-60 leukemia and MCF-7 cells, respectively. Moreover, Lee et al. [15] observed a significant increase of cells in the G0/G1 phase in HL-60 leukemia cells treated with 100 μM trans-R, while the population of cells in S- and G2/M-phase decreased. Different explanations have been given for cell cycle blockage in the S phase after treatment with trans-R. The effect could be related to the direct involvement of trans-R in DNA replication through the inhibition of ribonucleotide reductase and DNA polymerase activities [26], which are essential for DNA synthesis. In this sense, the decrease in ribonucleotide reductase activity could contribute to anti-proliferative activity of trans-R by partially inhibiting cell cycle progression through the S phase. Many other studies have correlated trans-R effects with key protein regulation in the cell cycle, where it would decrease the protein activities that control DNA transcription (e.g., NF-κB) [27] or activate proteins that regulate cell cycle progression (e.g., p53 and p21WAF1/CIP1) [23]. Indeed, Pozo-Guisado et al. [23] suggested that the treatment of MCF-7 cells with trans-R, induced cell accumulation and blockage in the S phase as a result of p21WAF1/CIP1 (cyclin-dependent kinase inhibitor) induction in the presence of increased levels of p53 protein.
Effect of trans-R on cell cycle distribution in JURKAT E.6 (a), THP-1 (b) and MCF-7 (c) cell lines. Cells were treated with 0, 10, 30 and 90 μM trans-R for 72 h. The different phases of cell cycle were represented as G0/G1 (black), S (white) and G2/M (gray). Treatments significantly different from control were considered at p < 0.05. Data are presented as mean ± SD
trans-R Induces Apoptotic Cell Death
Perturbation of the cell cycle is a prerequisite for the induction of apoptosis. For this reason, the possible apoptotic effect of trans-R on JURKAT E.6, THP-1 and MCF-7 cell lines was analyzed by flow cytometry using FDA-PI double staining (Fig. 3). It was seen that concentrations ranging from 0 to 30 μM of trans-R did not affect the percentage of apoptotic cells in any of the three cell lines. In contrast, a high trans-R concentration (90 μM) increased the percentage of apoptotic cells in all cases (p < 0.05). These results suggest that treatment with low trans-R concentrations decreases cell proliferation by blocking the cell cycle rather than by inducing cell death. On the contrary, at high trans-R concentrations, cell growth diminishes as a result of increasing cell death in all cell lines. Similar results were described by Pozo-Guisado et al. [23] and Singh et al. [7] using MCF-7 cells. In fact, these authors observed that 50 μM trans-R did not alter chromatin distribution in the nuclei of MCF-7 cells, whereas a concentration of 100 μM induced chromatin condensation and nuclear fragmentation, which suggests the presence of apoptotic cells. Similarly, Lee et al. [15] and Joe et al. [14] demonstrated that high concentrations of trans-R (100 and 300 μM, respectively) provoked apoptosis in HL-60 leukemic cells. As it can be observed in Fig. 3, leukemic cells showed different degrees of sensitivity at high trans-R concentrations since the percentage of apoptotic JURKAT E.6 cells was greater than that found in THP-1 cells, suggesting a differential response according to the leukemic cell type in question.
To deepen our knowledge of the apoptotic effect of high trans-R concentrations, we studied how this compound affects the expression levels of Bcl-2 and caspase-3, proteins which are involved in the regulation of cell apoptosis in all three cancer cell lines. Bcl-2 belongs to a family of proteins that prevent irreversible cellular damage. Bcl-2 is an integral membrane protein mainly located in the outer membrane of mitochondria and its over-expression prevents cells from undergoing apoptosis in response to a variety of stimuli [28]. Caspase-3 protein is a member of the cysteine-aspartic acid protease family. The sequential activation of caspases plays a central role in the execution-phase of cell apoptosis, and this protease is considered to be the most important executor caspase and can be activated by any of the initiator caspases [29]. The three cancer cell lines were treated with 10, 30 and 90 μM of trans-R, and the expression levels of anti-apoptotic Bcl-2 and caspase-3 proteins were determined by Western blotting after 72 h of incubation (Fig. 4). As it can be observed, trans-R up-regulated the expression of caspase-3 in JURKAT E.6 and THP-1 but Bcl-2 expression was not significantly up-regulated compared with control cells. Moreover, trans-R induced apoptosis through caspase-3 activation in JURKAT E.6 and THP-1 cell lines in a dose-dependent manner. Similar results were found by Cakir et al. [21] in leukemia cells treated with trans-R, where apoptosis was induced through caspase-9 activation and subsequent caspase-3 activation. In contrast, the expression of caspase-3 in MCF-7 cells was found to be negligible at all the trans-R concentrations tested (data not shown), suggesting the absence of functional caspase-3 [7]. In fact, Janicke [30] showed that MCF-7 cells were caspase-3 deficient. Moreover, trans-R inhibited the expression of anti-apoptotic gene product Bcl-2 in MCF-7 cells (Fig. 4). Indeed, the levels of Bcl-2 were down-regulated in a dose-dependent manner, an effect that was especially highlighted when a high trans-R (90 μM) concentration was used. Similar results were found [22] when a human breast cancer cell line was treated with 105 μM trans-R. They also observed that the levels of an anti-apoptotic Bcl-x protein were down-regulated. In addition, Pozo-Guisado et al. [31] suggested the induction of caspase-3/-8-independent apoptosis through the down-regulation of Bcl-2 in trans-R-treated MCF-7 cells. In agreement with this author, our data strongly suggest that trans-R-induced apoptosis in MCF-7 cells could take place through a caspase-3-independent mechanism, which is mediated by the down-regulation of Bcl-2.
In conclusion, the effect of natural trans-R on cell proliferation is not only dose- and time-dependent but also cell type-dependent, as seen by the different degrees of susceptibility of the leukemia and breast cancer cell lines tested. In addition, trans-R affected both cell cycle distribution and cellular apoptosis in a dose-dependent manner since low trans-R concentrations induced an increase of cells in the S phase, an accumulation that was higher in the leukemia cell lines than those derived from breast cancer cells. However, a high trans-R concentration increased the number of cells in the G0/G1 phase in all three cell lines. These results suggest that treatment with low concentrations of trans-R decreased cell proliferation by blocking the cell cycle, rather than by inducing cell death. In contrast, a high concentration of trans-R decreased cell proliferation as a result of apoptosis which is induced in all the cell lines. In leukemia cell lines, trans-R up-regulated the expression of caspase-3 but Bcl-2 expression was not significantly up-regulated. In contrast, trans-R-induced apoptosis in breast cancer MCF-7 cells could occur through a caspase-3-independent mechanism mediated by the down-regulation of Bcl-2.
Abbreviations
- CDs:
-
Cyclodextrins
- IC50 :
-
Median inhibitory concentration
- trans-R:
-
trans-Resveratrol
References
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorm AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, Das DK, Delmas D, Gottfried C, Lin HY, Ma QY, Mukhopadhyay P, Nalini N, Pezzuto JM, Richard T, Shukla Y, Surh YJ, Szekeres T, Szkudelski T, Walle T, Wu JM (2011) What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One 6:e19881
Mikstacka R, Rimando AM, Ignatowicz E (2010) Antioxidant effect of trans-resveratrol, pterostilbene quercetin and their combinations in human erythrocytes in vitro. Plant Foods Hum Nutr 65:57–63
Giovinazzo G, Ingrosso I, Paradiso A, De Gara L, Santino A (2012) Resveratrol biosynthesis: Plant metabolic engineering for nutritional improvement of food. Plant Foods Hum Nutr 67:191–199
Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A (2007) Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol 73:550–560
Pezzuto JM (2008) Resveratrol as an inhibitor of carcinogenesis 1, 2. Pharmaceut Biol 46:443–573
Singh N, Nigam M, Ranjan V, Sharma R, Balapure AK, Rath SK (2009) Caspase mediated enhanced apoptotic action of cyclophosphamide-and resveratrol-treated MCF-7 cells. J Pharmacol Sci 109:473–485
Komina O, Wesierska-Gadek J (2008) Action of resveratrol alone or in combination with roscovitine, a CDK inhibitor, on cell cycle progression in human HL-60 leukemia cells. Biochem Pharmacol 76:1554–1562
Jazirehi AR, Bonavida B (2004) Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin’s lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther 3:71–84
Belchí-Navarro S, Almagro L, Lijavetzky D, Bru R, Pedreño MA (2012) Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep 31:81–89
Calderón AA, Zapata JM, Muñoz R, Pedreño MA, Ros-Barceló A (1993) Resveratrol production as a part of the hypersensitive-like response of grapevine cells to an elicitor from Trichoderma viride. New Phytol 124:455–463
Lijavetzky D, Almagro L, Belchí-Navarro S, Martínez-Zapater JM, Bru-Martinez R, Pedreño MA (2008) Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res Notes 1:132–136
Fernández-Pérez F, Almagro L, Pedreño MA, Gómez Ros LV (2012) Synergistic and cytotoxic action of indole alkaloids produced from elicited cell cultures of Catharanthus roseus. Pharmaceut Biol. doi:10.3109/13880209.2012.722646
Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB (2002) Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res 8:893–903
Lee SK, Zhang W, Sanderson BJS (2008) Selective growth inhibition of human leukemia and human lymphoblastoid cells by resveratrol via cell cycle arrest and apoptosis induction. J Agric Food Chem 56:7572–7577
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Jiménez-Atiénzar M, Pedreño MA, Caballero N, Cabanes J, García-Carmona F (2007) Characterization of polyphenol oxidase and peroxidase from peach mesocarp (Prunus persica L. cv. Babygold). J Sci Food Agric 87:1682–1690
Wesierska-Gadek J, Kramer MP, Maurer M (2008) Resveratrol modulates roscovitine-mediated cell cycle arrest of human MCF-7 breast cancer cells. Food Chem Toxicol 46:1327–1333
Vitrac X, Desmouliere A, Brouillaud B, Krisav S, Deffieux G, Barthe N, Rosenbaum J, Mérillon JM (2003) Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci 72:2219–2233
Wenzel E, Somoza V (2005) Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res 49:472–481
Cakir Z, Saydam G, Sahin F, Baran Y (2011) The roles of bioactive sphingolipids in resveratrol-induced apoptosis in HL60 acute myeloid leukemia cells. J Canc Res Clin Oncol 137:279–286
Nagakawa H, Kiyozuka Y, Uemura Y, Senzaki H, Shikata N, Hioki K, Tsubura A (2001) Resveratrol inhibits human breast cancer cell growth and may mitigate the effect of linoleic acid, a potent breast cancer cell stimulator. J Cancer Res Clin Oncol 127:258–264
Pozo-Guisado E, Alvarez-Barrientos A, Mulero-Navarro S, Santiago-Josefat B, Fernandez-Salguero PM (2002) The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: Cell-specific alteration of the cell cycle. Biochem Pharmacol 64:1375–1386
Basly JP, Marre-Fournier F, Le Bail JC, Habrioux G, Chulia AJ (2000) Estrogenic/antiestrogenic and scavenging properties of (E)-and (Z)-resveratrol. Life Sci 66:769–777
Pozo-Guisado E, Lorenzo-Benayas MJ, Fernandez-Salguero PM (2004) Resveratrol modulates the phosphoinositide 3-kinase pathway through an estrogen receptor α-dependent mechanism: Relevance in cell proliferation. Int J Cancer 109:167–173
Locatelli GA, Savio M, Forti L, Shevelev I, Ramadan K, Stivala LA, Vannini V, Hübscher U, Spadari S, Maga G (2005) Inhibition of mammalian DNA polymerases by resveratrol: Mechanism and structural determinants. Biochem J 389:259–268
Holmes-McNary M, Baldwin AS Jr (2000) Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of IκB kinase. Cancer Res 60:3477–3483
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X (1997) Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 275:1129–1132
Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y (2004) Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA 101:2476–2481
Janicke RU (2009) MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res Treat 117:219–221
Pozo-Guisado E, Merino JM, Mulero-Navarro S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A, Fernández-Salguero PM (2005) Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-κB. Int J Cancer 115:74–84
Acknowledgments
F. Fernández-Pérez and L. Almagro have grants from the MICINN. This study was supported by the Fundación Séneca, Agencia de Ciencia y Tecnología de la Región de Murcia en el marco de II PCTRM 2007-10 (08799/PI/08) and by MICINN-FEDER (BIO2008-2941 and BIO2011-29856-C02-02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Francisco Fernández-Pérez, Sarai Belchí-Navarro and Lorena Almagro contributed equally to this work.
This work is dedicated in memoriam of Prof. Alfonso Ros-Barceló our mentor and friend.
Rights and permissions
About this article
Cite this article
Fernández-Pérez, F., Belchí-Navarro, S., Almagro, L. et al. Cytotoxic Effect of Natural trans-Resveratrol Obtained from Elicited Vitis vinifera Cell Cultures on Three Cancer Cell Lines. Plant Foods Hum Nutr 67, 422–429 (2012). https://doi.org/10.1007/s11130-012-0327-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-012-0327-8