Abstract
Pomegranate juice may improve cardiovascular risk because of its content of antioxidant polyphenols. We conducted a randomized placebo-controlled parallel study to examine the effect of pomegranate juice on pulse wave velocity (PWV), blood pressure (BP) and plasma antioxidant status (ferric reducing power; FRAP) in 51 healthy adults (30–50 years). Participants consumed 330 ml/day of pomegranate juice or control drink for four weeks. Measurements were made at baseline and at four weeks. There was no effect of the intervention on PWV (P = 0.694) and plasma FRAP (P = 0.700). However, there was a significant fall in systolic blood pressure (−3.14 mmHg, P < 0.001), diastolic blood pressure (−2.33 mmHg P < 0.001) and mean arterial pressure (−2.60 mmHg, P < 0.001). Change in weight was similar in the two groups over the intervention period (P = 0.379). The fall in BP was not paralleled by changes in concentration of serum angiotensin converting enzyme. We conclude that pomegranate juice supplementation has benefits for BP in the short term, but has no effect on PWV. The mechanism for the effect is uncertain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pomegranates are a rich source of bioactive compounds that may have beneficial health effects [1]. Pomegranate juice is claimed to have anti-atherosclerotic effects; rodent studies show that supplementation increases antioxidant status, reduces oxidative stress, limits atherogenic modification of low density lipoprotein-cholesterol and diminishes the size of atherosclerotic lesions [2, 3]. Supplementation studies in humans have also shown decreased plasma lipid susceptibility to oxidation [2], and reduced carotid intima-media thickness [4]. However, statistical comparison with a control group was lacking in these studies, and a more recent supplementation study, which had a randomized placebo-controlled design, showed no benefit for carotid intima-media thickness [5].
Pulse wave velocity (PWV) is a non-invasive marker of arterial stiffness that has been shown to be a predictor of future cardiovascular events [6, 7]. Arterial stiffness is determined by the structural properties of the arterial wall, blood pressure (BP), and mediators released by the endothelium such as nitric oxide (NO), which can alter smooth muscle tone [8]. Several studies have found that short-term supplementation with dietary antioxidant micronutrients can lower PWV [9, 10], but others have not supported this [11, 12].
Pomegranate juice is a particularly rich source of polyphenolic antioxidant compounds, particularly hydrolyzable tannins and anthocyanins [13, 14]. Pomegranate juice and extracts of pomegranate can influence a number of processes which would be expected to have beneficial effects on endothelial function and by extrapolation arterial stiffness. In a rodent study, pomegranate juice increased plasma nitrate and nitrite levels and vascular expression of endothelium nitric oxide synthase [3]. Pomegranate juice consumption has also been shown to improve brachial artery flow-mediated dilation in adolescent subjects with metabolic syndrome (as did grape juice), but the study did not have an appropriate concurrent control group [15]. Furthermore, in an uncontrolled study of hypertensive patients, consumption of pomegranate juice for two weeks inhibited serum angiotensin converting enzyme (ACE) activity and moderately reduced systolic blood pressure (SBP) [16]. Pharmacological inhibitors of ACE reduce arterial stiffness passively via a reduction in blood pressure as well as by promoting structural changes of the arterial wall [17, 18].
This study aimed to determine whether a daily supplement of pomegranate juice for four weeks compared to a placebo drink influenced PWV and blood pressure in healthy, young and middle-aged adults. The study also examined the effects of supplementation on serum ACE and antioxidant status.
Materials and Methods
Pomegranate Juice and Placebo Beverage
Pomegranate juice (Pomepure Punica granatum L., cv Hicaz) was supplied by The Pure Juice Company Ltd. (Twickenham, UK). The juice was manufactured by Göknur Foods Import Export, Trading and Production Company, Kayseri Nidğe, Turkey. Whole pomegranates (cv Hicaz) were washed, cut into quarters and juice was extracted by an industrial centrifugal juice extractor. The juice was then filtered and stored in large temperature controlled tanks, before being flash pasteurized and bottled. The total phenol content of the pomegranate juice was measured using the Folin-Ciocalteu method and is expressed as gallic acid equivalents [19]. The total antioxidant capacity of the juice was determined by the ferric reducing power (FRAP) method using a COBAS bioanalyzer [20]. The potassium content of the juice was measured on a Beckman-Coulter DcX analyzer (USA) using an ion selective electrode.
Placebo beverage was a commercial lemonade drink (7UP). Lemonade was selected as the control drink because it was devoid of bioactive plant compounds, antioxidants or vitamins, and contained only a trace amount of sodium. Energy and carbohydrate content of the pomegranate juice and lemonade were similar (167 kJ/100 ml and 10 g/100 ml, respectively for the pomegranate juice and 192 kJ/100 ml and 11.4 g/100 ml, respectively for the 7UP). Sucrose is the carbohydrate present in 7UP manufactured in the UK.
Participants and Study Design
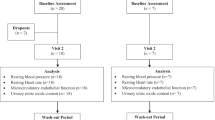
Participants were healthy, non-smoking volunteers aged 30 to 50 years, recruited through University email distribution lists and posters and through personal contacts. A total of 51 participants (35 female, 16 male) consented to take part in the study. Exclusion criteria included taking medication for heart disease, hypertension or diabetes, regular consumption of pomegranate juice or pomegranates and use of antioxidant supplements. The University of Sheffield Ethics Committee approved the study, and all volunteers gave written informed consent. The study was conducted between May 2007 and January 2008.
The study was a four-week parallel open-label intervention of a daily supplement of pomegranate juice (330 ml/day) versus a control drink of lemonade (330 ml/day; 7UP). Both drinks were delivered weekly to participants; those consuming the pomegranate juice were instructed to store the juice in their refrigerators before consumption. Participants consumed the drinks during the day, according to their preference.
Participants were stratified by gender and block randomized to treatment by an investigator who was not involved in recruitment. The investigator responsible for recruitment was unaware of which study arm the subject had been allocated to until recruitment was complete. All participants were asked to maintain their usual diet and exercise regimen throughout the intervention. A daily tick-sheet of drink consumption was completed by each subject in order to assess compliance.
Our primary outcome variable was change in PWV; our secondary outcome variable was change in SBP and diastolic blood pressure (DBP). Power calculations were based on a 10 % change in PWV. A sample size of 50 (25 per arm) was calculated to detect a 10 % change in PWV with 80 % power and an α value of 0.05, using variance estimates of PWV (SD = 106.0, n = 57) from a then on-going study of PWV (unpublished data).
Measurements
Participants attended the University of Sheffield on two occasions for vascular measurements and blood sampling. All measurements were taken in the early morning after an overnight fast. On both occasions, PWV, SBP, DBP and weight was measured and a blood sample was taken. Height was also measured at the baseline visit. Measurements were taken after a 15 min rest in the supine position to allow time for BP, cardiac function and vasomotor tone to reach resting levels.
PWV was measured in the supine position using a Nicolet Vasoguard Microlight system (VIASYS Healthcare, USA) [21]. Occlusion cuffs were placed around the upper arm and thigh (just above the knee) and pulse volume waveforms were recorded simultaneously until 10 complete waveforms were captured. Transit time was determined by a computerized algorithm that calculated the time delay between the foot of each simultaneously recorded waveform at each site. Surface distance between the two cuffs was measured and PWV was calculated using the equation PWV = distance/time (m/s). PWV was calculated from five measurements.
BP and heart rate were measured in a supine position using a semi-automated Accutorr Plus™ sphygmomanometer (Datascope®, USA). Mean SBP, DBP and heart rate were calculated from three measurements taken at 2.5 min intervals. Mean arterial pressure (MAP) was calculated as (2 DBP + SBP)/3. MAP has the advantage of being a summary measure of SBP and DBP.
Height was measured using a wall-mounted stadiometer (Seca, Germany) to the nearest cm. Weight was measured on an electronic scale to the nearest 100 g (Seca, Germany).
A fasting venous blood sample was drawn into an 8 ml lithium heparin tube and centrifuged at 2,000 g for 10 min at 4 °C to extract plasma. Antioxidant status was measured immediately as FRAP using a COBAS bioanalyzer [18]. The intra- and inter-assay CV for FRAP were 0.8 % and 3.6 %, respectively. Whole blood was collected into a serum separator tube and allowed to clot at room temperature for 30 min. Serum was separated by centrifugation at 2,000 g for 10 min at 4 °C and frozen at −80 °C until analysis. Serum ACE was measured using a commercially available ELISA kit from R&D Systems (Abingdon, UK). The intra-assay CV was 3.8 % and the inter-assay CV was 5.9 %.
Statistical Analysis
The effect of group on post-intervention measures used two-way ANOVA with sex as a random factor with adjustment for baseline value [22]. All analyses were conducted using SPSS version 16.0 (SPSS UK Ltd., Woking, UK).
Results
Analysis of the Juice
The mean total phenol content of the juice was 18.6 (SD 1.26) mmol/l (n = 5). The mean FRAP value of the juice was 45.51 (SD 0.18) mmol/l (n = 3) and the mean potassium content was 1,711 (SD 42.6) mg/l (n = 3).
Baseline Characteristics of Participants, Retention and Compliance
At baseline there were no significant differences between the groups for age, height, weight, BMI, MAP, SBP, DBP, FRAP and serum ACE (all P > 0.05) (Table 1).
Three participants failed to complete the study (one from the control group and two from the pomegranate juice group), giving a total of 24 per group (16 female and 8 male). The participant in the control group withdrew because of a change in personal circumstances, whilst those in the pomegranate group left because they had problems consuming the juice. Compliance was good in both groups (pomegranate and control groups reported drinking 96 % and 93 % of their drinks, respectively).
Effect of the Intervention
After the four-week intervention period there was no significant effect of pomegranate juice consumption on PWV, heart rate or FRAP. However, compared to the control group SBP and DBP fell significantly (both P < 0.001), as did MAP (P < 0.001) (Table 1). Change in weight was similar in the two groups over the intervention period (P = 0.379).
Discussion
The aim of this study was to investigate the chronic effects of pomegranate juice consumption on markers of vascular health. We were interested in the cumulative influence of pomegranate juice, thus, measurements were carried out at least 8 h after the last beverage consumption. Consumption of pomegranate juice for a four-week period did not influence PWV. This was contrary to our hypothesis that ingestion of a juice rich in polyphenolic antioxidant compounds would reduce arterial stiffness. Oral supplementation with 500 mg vitamin C for a four-week period has been shown to reduce arterial stiffness in diabetic patients [9], and an eight-week intervention using a combined oral dose of vitamin C and E resulted in a fall in PWV in hypertensive patients [10]. In an acute setting, vitamin C supplementation has been shown to ameliorate smoking-induced increases in arterial stiffness [23]. Also, a four-week intervention with cranberry juice, which is rich in polyphenolic compounds, especially anthocyanins, reduced arterial stiffness in patients with coronary artery disease [24]. In contrast, interventions in healthy volunteers with dietary antioxidants and beverages rich in polyphenolic compounds have been less positive; arterial stiffness did not respond to a seven-year intervention with a low-dose cocktail of antioxidant micronutrients [25], whilst in an uncontrolled trial, vitamin C supplementation had no effect on PWV [12]. Similarly, studies of green tea have documented no effect on arterial stiffness in healthy subjects [26, 27], although clover isoflavone supplementation was associated with a reduction in arterial stiffness in healthy normotensive volunteers [28]. Our null results are in agreement with practically all of the studies of healthy volunteers.
In the current study pomegranate juice did not elicit an increase in antioxidant status (plasma FRAP). The lack of effect on a plasma marker of total antioxidant activity indicates that the high in vitro antioxidant potential of pomegranate juice was not translated into biological activity in vivo. However, the sensitivity of FRAP may not have been sufficient to detect a small increment in antioxidant activity and our fasting measurement may have missed an acute change in antioxidant status. After consumption of pomegranate juice, punicalagin (the main ellagitannin present) undergoes hydrolysis in the gut producing the potent antioxidant compound, ellagic acid [29]. The metabolism of ellagic acid is rapid, with plasma concentrations peaking 1 h post consumption and clearance occurring within 5 h [29]. Such transient changes in antioxidant status may be insufficient to elicit a fall in PWV.
Some studies of pomegranate supplementation have reported an increase in plasma total antioxidant status [2, 4, 30, 31]. These effects are difficult to align with the time course of ellagic acid metabolism, since blood sampling in these studies occurred when only colonic metabolites of ellagic acid, which have low antioxidant activity, would be present in plasma [13, 29].
Whilst pomegranate juice consumption failed to reduce PWV, a modest reduction (about 3 %) in MAP, SBP and DBP was recorded. Other studies of pomegranate juice consumption on BP are inconclusive. Summer et al. [32] did not observe a hypotensive effect of a three-month period of pomegranate juice supplementation in patients with heart disease compared with a group supplemented with a placebo beverage; however, the two groups were not equally matched for concurrent treatment with anti-hypertensive medication. In contrast, Aviram et al. [4] reported that systolic BP fell by 12 % after a one-year pomegranate juice intervention in patients with carotid artery stenosis, many of whom were already taking anti-hypertensive medication [4], although, there was no control comparison in the statistical analysis, Aviram & Dornfeld [16] had previously reported a 5 % fall in systolic BP in an uncontrolled study of hypertensive patients supplemented with pomegranate juice. The hypotensive effect was ascribed to an inhibition of serum ACE activity. In contrast, in the present study pomegranate juice had no effect on serum ACE concentration.
Each bottle of pomegranate juice provided approximately 15 mmol of potassium and this may have contributed to its modest hypotensive effect (2.6 mmHg for MAP, 3.1 mmHg for SBP and 2.3 mmHg for DBP). In a meta-analysis of randomized controlled trials of potassium supplementation in normotensive subjects [33], the effect for systolic blood pressure was 1.8 mmHg and for diastolic 2.0 mmHg with a potassium dose greater than 60 mmol/day. However, Naismith & Braschi [34] reported a greater reduction in BP (6–7 mmHg for MAP) than this, with a lower dose of potassium (24 mmol/day) in a six-week supplementation study of normotensive volunteers. The supplement had greatest effect in the last three weeks of the six-week supplementation period. Studies of at least six weeks duration have been recommended [34] to test the influence of potassium supplementation on blood pressure. In our study, a more prolonged consumption of pomegranate juice may have evoked a larger fall in BP.
The observed fall in BP, although modest, is greater than the observed in studies of dietary sodium restriction in normotensive individuals [35]. This systematic review [35] reported that a reduction in dietary salt of 4.4 g/d (about half of usual intake) fashioned a mean fall in SBP of 2.03 mmHg with a corresponding decrease in DBP of 0.99 mmHg.
This study has several limitations. First, it would have been useful to confirm compliance with the pomegranate juice intervention using a biomarker of intake. However, the bioavailability of the main polyphenol present in pomegranate juice, punicalagin, is low and its metabolism is highly variable [13, 29]. Second, we used FRAP to assess antioxidant status. The main determinants of FRAP activity are uric acid and vitamin C, and the contribution of any punicalagin metabolites to overall FRAP activity is likely to be minor [20]. Third, we did not assess dietary intake during the intervention, therefore, it was not possible to rule the observed decrease in blood pressure was caused by dietary change in the group receiving pomegranate juice. However, participants were instructed to maintain their habitual diet throughout the study period. Fourth, the intervention period of four weeks was short, thus, we cannot rule out the possibility that a longer period of supplementation may have exerted beneficial effects on arterial stiffness. Finally, we did not profile the pomegranate juice to check for verify adulteration, however, the total antioxidant capacity of the juice and its content of phenolic compounds and potassium were consistent with reported values for pomegranate juice [36–38].
The current study has provided evidence that pomegranate juice consumption lowers BP but does not influence PWV in normotensive adults. The underlying mechanism for the effect on BP is unclear. We found no effect on serum ACE, but there is the possibility that the juice’s content of polyphenols and/or potassium was responsible for the hypotensive effect. In a meta-analysis of randomized controlled trials [39], chocolate, which is a rich source of polyphenols, was shown to elicit a fall in BP of similar magnitude to our study. The hypotensive effects of potassium supplementation are also well recognized [33]. Even when the supplementation period was short, the effects observed on BP were highly significant and similar in size to those achieved by dietary interventions of sodium and potassium. Further studies are warranted to confirm that consumption of pomegranate juice can reduce blood pressure.
Abbreviations
- PWV:
-
Pulse wave velocity
- BP:
-
Blood pressure
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- MAP:
-
Mean arterial pressure
- NO:
-
Nitric oxide
- ACE:
-
Angiotensin converting enzyme
- FRAP:
-
Ferric reducing power
- BMI:
-
Body mass index
- SD:
-
Standard deviation
References
Caligiani A, Bonzanini F, Palla G, Cirlin M, Bruni R (2010) Characterization of a potential nutraceutical ingredient: Pomegranate (Punica granatum L.) seed oil unsaponifiable fraction. Plant Foods Hum Nutr 6:277–283
Aviram M, Dornfeld L, Rosenblat M, Volkova N, Kaplan M, Coleman R, Hayek T, Presser D, Fuhrman B (2000) Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr 71:1062–1076
De Nigris FD, Williams-Ignarro S, Lerman LO et al (2005) Beneficial effects of pomegranate juice on oxidation-sensitive genes and endothelial nitric oxide synthase activity at sites of perturbed shear stress. Proc Natl Acad Sci USA 102:4896–4901
Aviram M, Rosenblat M, Gaitini D et al (2004) Pomegranate juice consumption for three years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr 23:423–433
Davidson MH, Maki KC, Dicklin MR, Feinstein SB, Witchger M, Bell M, McGuire DK, Provost JC, Liker H, Aviram M (2009) Effects of pomegranate juice consumption on carotid intima-media thickness in men and women at moderate risk from cardiovascular disease. Am J Cardiol 104:936–942
Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S (2002) Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension 39:10–15
Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hirayama Y, Yamamoto Y, Hori S (2003) Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res 26:615–622
Wilkinson IB, Franklin SS, Cockcroft JR (2004) Nitric oxide and the regulation of large artery stiffness: From physiology to pharmacology. Hypertension 44:112–116
Mullan BA, Young IS, Fee H, McCance DR (2002) Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension 40:804–809
Platinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S, Salvetti A (2007) Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens 20:392–397
Rasool AH, Rehman A, Wan Yusuf WN, Rahman AR (2003) Vitamin E and its effect on arterial stiffness in postmenopausal women - A randomized controlled trial. Int J Clin Pharmacol Ther 41:587–592
Eskurza I, Monahan KD, Robinson JA, Seals DR (2004) Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol 286:H1528–H1534
Cerda B, Espin JC, Parra S, Martinez P, Tomas-Barberan FA (2004) The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolized into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr 43:205–220
Mastrodi Salgado J, FerreiraTR B, de Oliveira BF, Dos Santos Dias CT (2012) Increased antioxidant content in juice enriched with dried extract of pomegranate (Punica granatum) peel. Plant Foods Human Nutr 67:39–43
Hashemi M, Kelishadi R, Hashemipour M, Zakerameli A, Khavarian N, Ghatrehsamani S, Poursafa P (2010) Acute and long-term effects of grape and pomegranate juice consumption on vascular reactivity in pediatric metabolic syndrome. Cardiol Young 20:73–77
Aviram M, Dornfeld L (2001) Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis 158:195–198
Ahimastos AA, Natoli AK, Lawler A, Blombery PA, Kingwell BA (2005) Ramipril reduces large-artery stiffness in peripheral arterial disease and promotes elastogenic remodelling in cell culture. Hypertension 45:1194–1199
Van Bortel LM, Kool MJ, Boudier HA, Struijker Boudier HA (1995) Effects of antihypertensive agents on local arterial distensibility and compliance. Hypertension 26:531–534
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma The (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 239:70–76
Khandanpour N, Armon MP, Jennings B, Finglas PM, Willis G, Clark A, Meyer FT (2009) Randomised controlled trial of folate supplementation in patients with peripheral arterial disease. Br J Surg 96:990–998
Vickers AJ, Altman DJ (2001) Analysing controlled trials with baseline and follow up measurements. BMJ 323:1123–1124
Katayama Y, Shige H, Yamamoto S, Hirata F, Yasuda H (2004) Oral vitamin C ameliorates smoking-induced arterial wall stiffness in healthy volunteers. J Atheroscler Thromb 11:354–357
Dohadwala MM, Holbrook M, Hamburg NM et al (2011) Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr 93:934–940
Zureik M, Galan P, Bertrais S, Mennen L, Czernichow S, Blacher J, Ducimetière P, Hercberg S (2004) Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arterioscler Thromb Vasc Biol 24:1485–1491
Ryu OH, Lee J, Lee KW, Kim HY, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM (2006) Effects of green tea consumption on inflammation, insulin resistance and pulse wave velocity in type 2 diabetes patients. Diabetes Res Clin Pract 71:356–358
Vlachopoulos C, Alexopoulos N, Dima I, Aznaouridis K, Andreadou I, Stefanadis C (2006) Acute effect of black and green tea on aortic stiffness and wave reflections. J Am Coll Nutr 25:216–223
Teede HJ, McGrath BP, DeSilva L, Cehun M, Fassoulakis A, Nestel PJ (2003) Isoflavones reduce arterial stiffness: A placebo-controlled study in men and postmenopausal women. Arterioscler Thromb Vasc Biol 23:1066–1071
Seeram NP, Henning SM, Zhang Y, Suchard M, Li A, Heber D (2006) Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr 136:2481–2485
Rosenblat M, Hayek T, Aviram M (2006) Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis 187:363–371
Guo C, Wei J, Yang J, Xu J, Pang W, Jiang Y (2008) Pomegranate juice is potentially better than apple juice in improving antioxidant function in elderly subjects. Nutr Res 28:72–77
Sumner MD, Elliott-Eller M, Weidner G, Daubenmier JJ, Chew MH, Marlin R, Raisin CJ, Ornish D (2005) Effects on pomegranate juice on myocardial perfusion in patients with coronary heart disease. Am J Cardiol 96:810–814
Whelton PK, He J, Jiang MD, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ (1997) Effect of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 277:1624–1632
Naismith DJ, Braschi A (2003) The effect of low-dose potassium supplementation on blood pressure in apparently healthy volunteers. Br J Nutr 90:53–60
He FJ, MacGregor GA (2004) Effect of longer-term modest salt reduction on blood pressure. Cochrane Database of Systematic Reviews, Issue 3. Art No.: CD004937
Borges G, Mullen W, Crozier A (2010) Comparison of the polyphenolic composition and antioxidant activity of European commercial fruit juices. Food Funct 1:73–83
Kruegar DA (2012) Composition of pomegranate juice. J AOAC Int 95:163–168
Zhang Y, Kruegar D, Durst R, Lee R, Wang D, Seeram N, Heber D (2009) International multidimensional authenticity specification (IMAS) algorithm for detection of commercial pomegranate juice adulteration. J Agric Food Chem 57:2550–2557
Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A (2008) Flavonoids, flavonoid-rich foods, and cardiovascular risk; A meta-analysis of randomized controlled trials. Am J Clin Nutr 88:38–50
Acknowledgements
AL and MEB designed the study and wrote the manuscript. JR carried out the statistical analysis. HH and WL were responsible for data collection. All authors read and approved the final manuscript. We thank The Pure Juice Company Ltd. for supplying the pomegranate juice and Dr. Kritika Mahadevan from Sheffield Hallam University for measuring the phenolic content of the pomegranate juice. We are grateful to all our volunteers for their time and commitment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lynn, A., Hamadeh, H., Leung, W.C. et al. Effects of Pomegranate Juice Supplementation on Pulse Wave Velocity and Blood Pressure in Healthy Young and Middle-aged Men and Women. Plant Foods Hum Nutr 67, 309–314 (2012). https://doi.org/10.1007/s11130-012-0295-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-012-0295-z