Abstract
Ascophyllum nodosum is a brown seaweed that grows abundantly in the US Northeast coastal region. This study examined the seasonal variation of A. nodosum in phenolic contents and subsequent antioxidant, α-glucosidase and α-amylase inhibitory activities. A. nodosum was harvested monthly and extracted in hot water and the resulting extracts were spray-dried. The results indicate a clear seasonal variation in terms of phenolic content, with June and July being the highest (36.4 and 37 mg/g, respectively) and May the lowest (21.8 mg/g). The antioxidant activities, in terms of 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity, correlated with the phenolic contents observed (r = 0.81), with the month of July being the highest (58%) and April the lowest (26%). Similarly in terms of Trolox equivalent, July had the highest activity (15.53 μM) and April and May the lowest (8.40 and 8.27 μM, respectively). α-glucosidase inhibitory activity exhibited a pattern similar to the phenolic contents observed with July having the highest inhibitory activity (IC70 2.23 μg) and April the lowest (IC70 26.13 μg), resulting in an inverse correlation between IC70 values and total phenolic content (r = −0.89). Such seasonal variation is believed to be caused by temperature-related stress considering that A. nodosum is a cold water species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes is a metabolic disease usually linked to insulin resistance and cells that do not use insulin properly resulting in hyperglycemia. The major source of blood glucose are dietary carbohydrates that are hydrolyzed by pancreatic α-amylase, followed by α-glucosidase before being absorbed in the small intestine [1]. One of the therapeutic approaches for decreasing postprandial hyperglycemia is to prevent or delay absorption of glucose by the inhibition of carbohydrate hydrolyzing enzymes, α-amylase and α-glucosidase, in the digestive organs [2]. Although the mode of action is not clear, recent studies showed that phenolic phytochemicals from botanical sources are natural inhibitors of α-amylase and α-glucosidase [3–6] with a strong inhibitory effect on α-glucosidase, but a mild inhibitory effect on α-amylase and thus can be used as an effective measure to prevent postprandial hyperglycemia with minimal side effects [4–6]. Therefore, phenolic antioxidant-mediated inhibition of these enzymes can significantly decrease the postprandial hyperglycemia after ingestion of a mixed carbohydrate diet and could be an effective strategy in the control of type 2 diabetes [7].
Type 2 diabetes accounts for about 90% to 95% of all diagnosed cases of diabetes in adults [8] and at least 220 million people worldwide have diabetes and this figure is likely to double by 2030 [9]. In the United States, in 2007, 23.7 million people (10% of American adults) had diabetes and by 2050 this figure is expected to jump to 33%, or one-third of all American adults [8]. Diabetes cost Americans $174 billion to manage in 2007 - a figure that is expected to skyrocket based on the CDC’s latest estimates [8].
Ascophyllum nodosum is a dominant rocky intertidal brown seaweed species belonging in the class Phaeophyceae. It is commonly found on the northeastern coast of North America and the northwestern coast of Europe [10] and is widely used as food, cosmetic, fertilizer and recently as a functional food ingredient [11]. A. nodosum is currently considered an underutilized brown seaweed species found in abundance along the US Northeast coast that contains large molecular weight phenolic phytochemicals such as phlorotannins [12, 13]. Previous studies have determined that the solvent extracts of A. nodosum and Ecklonia stolonifera have high phenolic content and that this phenolic content correlates with reduction of blood glucose levels in rats [13, 14]. A recent study by Apostolidis & Lee [15] showed that water extracts of A. nodosum have inhibitory effect against carbohydrate hydrolyzing enzymes and this inhibitory activity correlated well with the observed phenolic content.
The seasonal variation of phenolic phytochemicals is well known in terrestrial plants [16–19]. Marine plants produce phenolic phytochemicals as a by-product of internal resource balances [20–23] in response to nutrient stress [21–25] or as a result of severe defoliation [26]. The seasonal variation in the phenolic content in A. nodosum and other brown seaweeds has been reported in the past [27–29] and should be monitored to help standardize the finished products. The metabolic production of polyphenolics depends on the harvesting location and season [28]. A. nodosum harvested in Norway had a maximum polyphenolic content in the winter season [28], while those harvested from the Scottish west coast showed a maximum phenolic content in July [29]. To date there is no published report regarding the seasonal variation of A. nodosum harvested from the US Northeast coast.
For the past three years, the landings of A. nodosum in the Gulf of Maine have increased from 5 million pounds in 2005 to 7 million pounds in 2006 and 2007, with steady price (around 4¢/ lb) for the last 10 years [30]. In addition to its abundant supply at a low cost, this resource has been well managed for its long-term sustainability [30]. Previous studies revealed that 80 °C water extract of A. nodosum has the highest level of phenolics and the strongest α-glucosidase inhibitory activity among seaweeds in the northeast region [15]. A. nodosum is a regionally available natural resource in abundance, thus allowing its utilization on a commercial scale for novel nutraceutical ingredient development. Currently A. nodosum is used in dietary supplements mainly for iodine supplementation. To determine the potential use of A. nodosum extract as a more sophisticated nutritional supplement or for medicinal purposes, the most favorable time for collection, from the US Northeast coast, of this algal material needs to be determined.
The objective of this study was to determine the seasonal effect on the phenolic phytochemical content. This is an essential step following characterization of phenolic profile and the antioxidant and carbohydrate digesting enzyme inhibitory activities for the determination of the type 2 diabetes management potential and mode of action of A. nodosum, as well as for the translational research to be followed, where standardized raw materials are required.
Materials and Methods
Fresh A. nodosum was harvested from Narragansett Bay (RI, USA) in the first week of each month for one year (April 2009 until March 2010). Fresh plants were manually harvested, during low-tide period, with a knife cut above the lateral shoot to ensure sustainable plant regeneration. α-amylase (porcine pancreatic, EC 3.2.1.1), α-glucosidase (yeast, EC 3.2.1.20) and acarbose (EC 260.030.7) were purchased from Sigma-Aldrich (St. Louis, MO). Acarbose is a known α-glucosidase and α-amylase inhibitor currently used for type 2 diabetes management. Unless otherwise specified, all chemicals were purchased from Sigma-Aldrich.
Sample Preparation
Fresh A. nodosum plants were extracted in hot water following the previously optimized conditions for maximal phenolic extraction [15]. Briefly, 1 kg of freshly harvested A. nodosum was manually cleaned for any impurities and then chopped for 2 min using a food cutter (Hobart, Troy, OH). The chopped product was further ground to finer particles for 2 min using a Stephan chopper (UM 5, capacity 2.5 kg, Stephan Machinery Corp., Columbus, OH). The resulting product was placed in a steam-jacketed kettle (Vulcan Equipment Co., Ooltewah, TN) which contained 8 L pre-heated water at 80 °C. Mixing was carried out using a Stir-Pak mixer (Cole-Parmer Instrument Vernon Hills, IL). Extraction took place under agitation at 80 °C for 30 min. The extract was filtered through a No. 25 sieve (710 μm) and the filtrate was spray-dried (APV Anhydro AS, Sobarg, Denmark). The resulting spray-dried powder was kept at refrigerated temperature (4 °C). Before analysis, 100 mg spray-dried powder was fully dissolved in 10 ml water at room temperature and this solution was used for further analysis.
Total Phenolics Assay
The total phenolics were determined following the procedure modified from Shetty et al. [31]. Briefly, 1 mL of 10x diluted stock extract was transferred into a test tube and mixed with 1 mL 95% ethanol and 5 mL distilled water. To each sample, 0.5 mL 50% (v/v) Folin-Ciocalteu reagent was added and vortex mixed. After 5 min, 1 mL 5% Na2CO3 was added to the reaction mixture and allowed to stand for 60 min. The absorbance was read at 725 nm using a Thermo Scientific Genesys 10uv spectrophotometer (Madison, WI). The absorbance values were converted to total phenolics and were expressed in mg gallic acid/g sample fresh weight (FW). Standard curve was established using various concentrations of gallic acid in ethanol.
Antioxidant Activity by 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Radical Inhibition Assay
The antioxidant activity by DPPH free radical scavenging inhibition was measured following the procedure modified from Shetty et al. [31]. To 0.8 ml 60 μM DPPH in ethanol, 3 doses (50, 100 and 200 μl) of each extract was added. The decrease in absorbance, due to radical scavenging, was monitored at 517 nm using a Thermo Scientific Genesys 10uv spectrophotometer (Madison, WI) until a constant reading was obtained. The readings were compared with the controls which contained 200 μl of water instead of the extract. The % inhibition was calculated as follows:
Oxygen Radical Absorbance Capacity (ORAC) Assay
Antioxidant activities of A. nodosum extracts were measured for their peroxyl and hydroxyl radical-scavenging capacities employing the ORAC assay system. The assay was carried out using a Tecan GENios multi-functional plate reader (GENios; Tecan Trading AG, Salzburg, Austria) with fluorescent filters (excitation wavelength: 485 nm, emission filter: 535 nm). In the final assay mixture, fluorescein (40 nM) was used as a target of free radical attack with either 2, 2′-azobis (2-amidinopropane) dihydrochloride (AAPH, 20 mM) as a peroxyl radical generator in peroxyl radical-scavenging capacity (ORACROO·) assay [32]. Trolox (1 μM) as a standard was prepared fresh on a daily basis. The analyzer was programmed to record the fluorescence of fluorescein every 2 min after AAPH or H2O2 - CuSO4 was added. All fluorescence measurements were expressed relative to the initial reading. Final results were calculated based on the difference in the area under the fluorescence decay curve between the blank and each sample. All data were expressed as μmoles of Trolox equivalents (TE). One ORAC unit is equivalent to the net protection area provided by 1 μM of Trolox.
α−amylase Inhibition Assay
A mixture of 50 μl extract or acarbose and 50 μL 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M sodium chloride) containing α−amylase solution (13U/ml) were incubated at 25 °C for 10 min. After pre-incubation, 50 μL 1% soluble starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) was added to each tube at timed intervals. The reaction mixtures were then incubated at 25 °C for 10 min followed by addition of 100 μl dinitrosalicylic acid color reagent. The test tubes were then placed in a boiling water bath for 5 min to stop the reaction and cooled to room temperature. The reaction mixture was then diluted with 1 mL distilled water and absorbance was read at 540 nm. α-amylase inhibitory activity was expressed as % inhibition and calculated as follows.
α−glucosidase Inhibition Assay
A mixture of 50 μL extract or acarbose solution and 100 μl of 0.1 M phosphate buffer (pH 6.9) containing α-glucosidase solution (1.0 U/ml) was incubated in 96 well plates at 25 °C for 10 min. After pre-incubation, 50 μl of 5 mM p-nitrophenyl-α-D-glucopyranoside solution in 0.1 M phosphate buffer (pH 6.9) was added to each well at timed intervals. The reaction mixtures were incubated at 25 °C for 5 min. Before and after incubation, absorbance was recorded at 405 nm by microplate reader (VMax, Molecular Device Co., Sunnyvale, CA) and compared to that of the control which had 50 μl buffer solution in place of the extract. The α-glucosidase inhibitory activity was expressed as % inhibition and calculated as follows:
Statistical Analysis
All experiments were performed twice and analysis for each experiment was carried out in triplicate. Means, standard deviations, the degree of significance (p < 0.05 - One way ANOVA and t-test) and correlation (r-Pearson Correlation Coefficient) were determined using Microsoft Excel XP. Inhibition concentration (IC) values were calculated using ED50plus vol.1 developed by Vargas (http://www.softlookup.com/display.asp?id=2972, accessed May 2009).
Results and Discussion
Total Phenolic Content
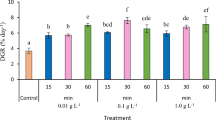
When our extracts were assayed for total phenolic contents, a strong seasonal variation was observed (Fig. 1a). Specifically, the total phenolic contents varied from a minimum content of 22 mg/g to a maximum of 37 mg/g, observed on May and July, respectively. These findings confirm that phenolic phytochemical levels in the brown seaweed A. nodosum harvested from Narragansett Bay (RI) greatly depend on the harvesting season.
a Seasonal variation in the ORAC value of spray-dried A. nodosum powder and correlation with total phenolic contents. (Trolox Eq. values with different letters are significantly different, p < 0.05), b Dose-dependent seasonal variation in the 1, 1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity of spray-dried A. nodosum powder. (Values with different letters within the same dose are significantly different, p < 0.05. Normal letter: 10 mg/ml, normal letter in parenthesis: 5 mg/ml, italic letter: 2.5 mg/ml)
Previously it was shown that A. nodosum harvested in Norway had a maximum polyphenolic content in the winter season [29], while those harvested from the Scottish west coast showed a maximum phenolic content in July [29]. This is the first report of the seasonal variation of phenolic contents in A. nodosum harvested from the Northeast US Atlantic coast and our results show that there are two stand-out periods for phenolic contents, one in summer (June and July) and one in fall (October) (Fig. 1a). A similar pattern was observed by Parys et al. [29] who observed that after the maximum phenolic content in July there was a significant drop in August followed by a steep increase in the month of September (values ranging from 1% to 0.3% fresh weight).
There are many factors that affect the production of phenolic metabolites in seaweeds including environmental stress [20–23] and nutrient stress [21–25], and severe defoliation [26]. Water temperature could be a stress factor, especially for the cold-water loving A. nodosum. This can explain the phenolic peak observed during the summer months, since it is possible that under stress more phenolic metabolites are produced. The phenolic peak observed at the month of October could be due to other environmental stress factors, which were previously mentioned and is similar to the observations of Steinberg [28].
Antioxidant Activity
When tested with both the DPPH free radical scavenging and ORAC assays, all samples had antioxidant activities (Figs. 1a and b) with the exception of acarbose which had no antioxidant activity.
With DPPH free radical scavenging assay, a dose-dependent inhibition was observed with July having the highest (68%) and April the lowest (25%) inhibitory activities (Fig. 1b). A strong correlation between phenolic contents and DPPH free radical scavenging activity was observed (r = 0.81), indicating that the observed antioxidant activity could possibly be phenolic dependent. Similarly with ORAC assay system, July had the highest antioxidant activity (16 μM TE) and May the lowest (8 μM TE) (Fig. 1a). A strong correlation between ORAC antioxidant activity and phenolic contents was observed (r = 0.844) (Fig. 1a), confirming the phenolic-dependent nature of the observed antioxidant activity.
These results suggest that higher phenolic content does confer higher antioxidant activity linked to free-radical scavenging.
α−amylase and α-glucosidase Inhibition Assay
None of the seaweed samples tested had inhibitory activity against α-amylase at the tested concentration (10 mg/ml), with the exception of acarbose. However, all the tested extracts had significant and dose-dependent α-glucosidase inhibitory activity (Fig. 2). The highest α-glucosidase inhibitory activity was observed in July (ranging from 100% at the highest dose to 62% at the lowest dose) (Fig. 2), while the minimum inhibitory activity was observed in April (ranging from 72% at the highest dose to 0% at the lowest dose) (Fig. 2). Due to the high inhibitory activity of the tested samples at the given concentrations, instead of IC50, the IC70 value (the concentration that results in 70% inhibition) was estimated. This allowed comparison of all samples on the same basis. A strong inverse correlation between phenolic contents and IC70 values was observed (r = −0.89, Fig. 3). When the IC70 values were estimated based on phenolic content, all extracts, with the exception of the month of April, had higher inhibitory activity than that of the type 2 diabetes drug compound, acarbose (Table 1).
Seasonal variation in the α-glucosidase inhibitory activity of spray-dried A. nodosum powder. (Values with different letters within the same dose are significantly different, p < 0.05. Normal letter: 500 μg/ml, normal letter in parenthesis: 200 μg/ml, italic letter: 100 μg/ml, italic letter in parenthesis: 50 μg/ml)
These results support our previous findings that 80 °C water extracts of A. nodosum have strong phenolic-dependent α-glucosidase inhibitory activity with lower IC50 value (phenolic base) when compared to that of acarbose [15]. Earlier reports have shown that phenolic phytochemicals from plant sources could be effective α-glucosidase inhibitors [4–6]. Furthermore, a purified fraction of 50% ethanol extract of A. nodosum had α-glucosidase inhibitory activity values (IC50 22 μg A. nodosum) which correlated with the phenolic content of the extract [13]. Our findings further confirm the strong inhibitory activity of A. nodosum water extracts against α-glucosidase and suggest that this inhibitory activity is due to the phenolic compounds present in A. nodosum although the specificity of the inhibitory effect was not evaluated in this study. It is important to point out that acarbose is a chemical drug specifically designed for α-glucosidase inhibition and has various side effects which include abdominal distention, flatulence, meteorism and possibly diarrhea [33]. It has been suggested that such side effects might be caused by the excessive inhibition of pancreatic α-amylase resulting in the abnormal bacterial fermentation of undigested carbohydrates in the colon [7, 33]. On the other hand, A. nodosum water extracts have significantly lower inhibitory activity against α-amylase than α-glucosidase [15]. In the present study, no extracts were shown to have α-amylase inhibitory activity possibly due to the low tested concentrations. Higher concentrations were not tried due to the inability of our extract to be fully solubilized in water.
Conclusions
This study is the first report on the phenolic content variation of A. nodosum harvested from the Northeast U.S. Atlantic coast. Our findings indicate that the total phenolic contents varied from a minimum content of 22 mg/g to a maximum of 37 mg/g, observed in May and July, respectively. All tested samples had strong α-glucosidase inhibitory activities that correlated well with the observed phenolic contents. Furthermore, A. nodosum water extracts could also provide antioxidant relief from high glucose-induced oxidative stress in contrast to acarbose, a commercial drug for type 2 diabetes management. Based on our findings, the optimum harvest time of A. nodosum for higher phenolic content, antioxidant activities and α-glucosidase inhibitory potential relevant to type 2 diabetes management, is the month of July. In summary, this research provides a strong biochemical rationale for further animal and clinical trials for A. nodosum mediated type 2 diabetes management, with suggested mechanism action of the inhibition of carbohydrate hydrolysis enzyme, α - glucosidase.
References
Elsenhans B, Caspary WF (1987) Absorption of carbohydrates. In: Caspary WF (ed), Structure and Function of the small intestine. Excerptia Medica, Amsterdam, pp 139–159
Krentz AJ, Bailey CJ (2005) Oral antidiabetic agents: Current role in type 2 diabetes mellitus. Drugs 65:385–411
Andlauer W, Furst P (2003) Special characteristics of non-nutrient food constituents of plants-phytochemicals. Introductory lecture. Int J Vitam Nut Res 73:55–62
Apostolidis E, Kwon Y-I, Shetty K (2006) Potential of cranberry - Based herbal synergies for diabetes and hypertension management. Asia Pac J Clin Nutr 15:433–41
Kwon Y-I, Apostolidis E, Kim Y-C, Shetty K (2007) Health benefits of traditional corn, beans and pumpkin: In vitro studies for hyperglycemia and hypertension management. J Med Food 10:266–275
Kwon Y-I, Vattem DA, Shetty K (2006) Clonal herbs of Lamiaceae species against diabetes and hypertension. Asia Pac J Clin Nutr 15:107–118
Horii S, Fukasse K, Matrua T, Kameda K, Asano N, Masui Y (1987) Synthesis and α-D- glucosidase inhibitory activity of N-substituted valiolamine derivatives as potent oral antidiabetic agents. J Med Chem 29:1036–1048
Centers For Disease Control And Prevention, National Center for Health Statistics (2010) Division of Health Interview Statistics, data from the National Health Interview Survey. U.S. Bureau of the Census, census of the population and population estimates. Available from: www.cdc.gov/diabetes/statistics/. Accessed October, 2010
World Health Organization (2009) Diabetes, Fact sheet N°312. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/index.html . Accessed December, 2010
Taylor WR (1962) Marine algae of the northeastern coast of North America. University of Michigan Press, Ann Arbor. ISBN 0-472-04904-6
Chan C-X, Ho C-L, Phang S-M (2006) Trends in seaweed research. Trends Plant Sci 11:165–166
Ragan MA, Glombitza KW (1986) Phlorotannins, brown algal polyphenols. In: Round FE, Chapman DJ (eds), Progress in Phycological research. Biopress Ltd., Bristol, pp 129–241
Zhang J, Tiller C, Shen J, Wang C, Girouard GS, Dennis D, Barrow CJ, Miao M, Ewart S (2007) Antidiabetic properties of polysaccharide and polyphenolic enriched fractions from the brown seaweed Ascophyllum nodosum. Can J Physiol Pharmacol 85:1116–1123
Iwai K (2008) Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-Ay Mice. Plant Foods Hum Nutr 63:163–169
Apostolidis E, Lee CM (2010) In vitro potential of Ascophyllum nodosum phenolic antioxidant mediated α-glucosidase and α-amylase inhibition. J Food Sci 75:97–102
McIntyre KL, Harris CS, Saleem A, Beaulieu LP, Ta CA, Haddad PS, Arnason JT (2009) Seasonal phytochemical variation of anti-glycation principles in lowbush blueberry (Vaccinium angustifolium). Planta Med 75:286–292
Jalal MAF, Readand DJ, Haslam E (1982) Phenolic composition and its seasonal variation in Calluna vulgaris. Phytochem 21:1397–1401
Erturk Y, Ercisli S, Sengul M, Eser Z, Haznedar A, Turan M (2010) Seasonal variation of total phenolic, antioxidant activity and minerals in fresh tea shoots (Camellia sinensis var. sinensis). Pak J Pharm Sci 23:69–74
Salminen J-P, Roslin T, Karonen M, Sinkkonen J, Pihlaja K, Pulkkinen P (2004) Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. J Chem Ecol 30:1693–1711
Mooney HA (1972) The carbon balance of plants. Annu Rev Ecol Systemat 33:15–346
Mattson WJ Jr (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Evol Systemat 11:119–161
Bryant JP, Chapin FS, Klein DR (1983) Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357–368
Ilvessalo H, Tuomi J (1989) Nutrient availability and accumulation of phenolic compounds in the brown alga Fucus vesiculosus. Mar Biol 101:115–119
Gershenzon J (1984) Changes in the levels of plant secondary metabolites under water and nutrient stress. In: Timmermann BN, Steelink C, Loewus FA (eds), Phytochemical Adaptations to Stress. Plenum, New York, pp 273–320
Fajer ED, Bowers MD, Bazzaz FA (1992) The effect of nutrients and enriched CO2 environments on production of carbon-based allelochemicals in Plantago: A test of the carbon/nutrient balance hypothesis. Am Nat 140:707–723
Bryant JP, Heitkonig I, Kuropat P, Owen-Smith N (1991) Effects of severe defoliation on the long-term resistance to insect attack and on leaf chemistry in six woody species of the southern African savanna. Am Nat 137:50–63
Yates JL, Peckol P (1993) Effects of nutrient availability and herbivory on polyphenolics in the seaweed Fucus versiculosus. Ecol 74:1757–1766
Steinberg PD (1989) Biogeographical variation in brown algal polyphenolics and other secondary metabolites: Comparison between temperate Australasia and North America. Oecologia 78:373–382
Parys S, Kehraus S, Pete R, Kupper F, Glombitza KW, Konig GM (2009) Seasonal variation of polyphenolics in Ascophyllum nodosum (Phaeophycae). Eur J Phycol 44:331–338
Maine Seaweed Council (2010) Rockweed Research Priority Symposium, March 2010
Shetty K, Curtis OF, Levin RE, Witkowsky R, Ang W (1995) Prevention of vitrification associated with in vitro shoot culture of oregano (Origanum vulgare) by Pseudomonas spp. J Plant Physiol 147:447–451
Kurihara H, Fukami H, Asami S, Totoda Y, Nakai M, Shibata H, Yao XS (2004) Effects of oolong tea on plasma antioxidative capacity in mice loaded with restraint stress assessed using the oxygen radical absorbance capacity (ORAC) assay. Biol Pharm Bull 27:1093–1098
Bischoff H, Puls W, Krause HP, Schutt H, Thomas G (1985) Pharmacological properties of the novel glucosidase inhibitors BAY m 1099 (miglitol) and BAY o 1248. Diabetes Res Clin Pract 1:53–62
Acknowledgement
We would like to thank Professor Charles Yarish (Department of Ecology and Evolutionary Biology, University of Connecticut) for his valuable advice on A. nodosum collection sites and harvesting technique.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Apostolidis, E., Karayannakidis, P.D., Kwon, YI. et al. Seasonal Variation of Phenolic Antioxidant-mediated α-glucosidase Inhibition of Ascophyllum nodosum . Plant Foods Hum Nutr 66, 313–319 (2011). https://doi.org/10.1007/s11130-011-0250-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-011-0250-4