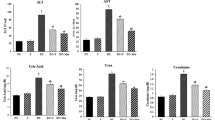Abstract
This report presents a complex analysis of changes proceeding in the gut, blood and internal organs of rats with induced oxidative stress, glucose intolerance and hyperlipidemia after dietary supplementation with an extract from black chokeberry (Aronia melanocarpa) fruit, that is a condensed source of polyphenols (714 mg/g), especially anthocyanin glycosides (56.6%). The disturbances mimicking those observed in metabolic syndrome were induced by a high-fructose diet and simultaneous single injection of streptozotocin (20 mg/kg). Dietary supplementation with the chokeberry fruit extract (0.2%) decreased activity of maltase and sucrase as well as increased activity of lactase in the mucosa of the small intestine. Its ingestion led also to the improvement of antioxidant status, especially, the concentration of a lipid peroxidation indicator (TBARS) in organ tissues (liver, kidney and lung) was normalized; some cholesterol-lowering and distinct hypoglycemic actions were also observed. The mechanism of glucose reduction is likely to be multifactorial, and we suggest the factors related with the decreased activity of mucosal disaccharidases important for further investigation. In conclusion, chokeberry fruit derivatives may act as a promising supplementary therapeutic option in the prevention and treatment of disorders occurring in metabolic syndrome, as well as their complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Black chokeberry (Aronia melanocarpa (Michx.) Elliott) belongs to the Rosaceae family, subfamily Malodieae, and is a deciduous shrub originating from the eastern part of North America [1]. In the 19th century, the chokeberry was introduced to Europe and has been cultivated in its central and eastern countries [1] mainly for production of homemade or commercial juices, jams, teas, wine and natural food colorants [2].
Recent studies have pointed at hepatoprotective [3], hypolipidemic [4] and hypoglycemic [5] actions of a chokeberry fruit juice, as well as antiaggregatory [6], antiinflammatory [7–8], vasoactive, vasoprotective [9] and hypotensive [7] activities of chokeberry extracts. In the foregoing studies scientists have frequently pointed out the high in vitro or in vivo antiradical activity of polyphenols, especially anthocyanins, to be the main reason of these beneficial actions, however, their wide range suggests another mechanisms whereby the chokeberry fruit constituents could affect an organism. These positive effects imply also that chokeberry fruit derivatives might be a useful nutritional factor in the prevention of type 2 diabetes and cardiovascular diseases as well as their complications. Well known risk factors of these diseases are impaired glucose tolerance (prediabetic state) and dyslipidemia, and together with obesity they constitute a metabolic syndrome [10].
The aim of this study was to scrutinize complex changes proceeding in the gut, blood and internal organs of rat prooxidative model with prediabetes and hyperlipidemia, after dietary supplementation with a polyphenol-rich extract from black chokeberry fruit. In order to induce disturbances in the metabolism of rats mimicking the metabolic syndrome, we used a combination of a high-fructose diet, described as hypertriglyceridemic and prooxidative [11] supplemented with saturated fat, as well as an injection of a low dose of streptozotocin (STZ), known as a causative agent of oxidative stress and partly necrosis of the β islet cells [12].
Materials and Methods
Plant Material
A commercial extract from black chokeberry fruits was purchased from Agropharm Co. (Łódź, Poland). The total concentration of polyphenols was estimated using Folin–Ciocalteu’s reagent with sinapic acid as a standard, and equaled 714 mg/g. Individual polyphenol components were characterized using the high performance liquid chromatography method described by Oszmianski and Wojdylo [13]. Chemical composition of polyphenols in the tested extract was described previously by Frejnagel [14] and is given in Table 1. Anthocyanins, namely cyanidin glycosides, were the dominant phenolic constituents (56.6%).
Animals, Diets and Treatments
The experiment was conducted on 24 male Wistar rats, weighed 161 ± 8 g, randomly divided into three groups of eight animals each, and fed over four weeks with semi-purified diets (Table 2). Rats from the control group (C) were fed a standard casein diet enriched with 0.5% of cholesterol, while in the experimental groups (E and EP) the diets were modified by 8% of lard and 65% of fructose added at the expense of soybean oil and maize starch, respectively; in the EP group the diet was additionally supplemented by the extract from chokeberry fruits (0.2%) added at the expense of corn starch. At the beginning of the experiment rats from both E and EP group received a single intraperitoneal injection of 20 mg/kg body weight of streptozotocin (STZ, Sigma Chemical Co., St. Louis, MO, USA) freshly dissolved in 0.05 mol/L sodium citrate buffer (pH 4.5), while in group C the rats received 20 mg/kg body weight of sodium citrate buffer. The animals have free access to water and diets and were maintained individually in cages of glass under a 12-h light/dark cycle, controlled temperature of 21 °C to 22 °C, relative humidity of 50% to 70%, and intensive ventilation of rooms (15 times per hour).
Sample Preparation, Analytical Procedures, and Statistical Analysis of Results
At the termination of the experiment, the rats were anaesthetized with sodium pentobarbital according to the recommendations for euthanasia of experimental animals. After laparotomy, blood samples were taken from caudal vein, then small intestine, cecum, liver, heart, kidneys, lungs and spleen were removed and weighed.
The small intestine was divided into four equal sections, and the second section from the stomach side was rinsed with cold physiological saline and cut open. Mucosal samples were collected by scraping with glass slides onto an iced glass plate. After the homogenization with four parts of cold physiological saline (v/w) and centrifugation for 10 min (10,000 rpm, 4 °C), the obtained supernatant was subsequently stored at −20 °C. Sucrase, maltase and lactase activities were assayed by the method of Dahlqvist [15] with some modifications: glucose oxidase reagent was from Alpha Diagnostic Ltd. (Warsaw, Poland), and 0.2 mol/L of sodium phosphate buffer (pH 6.0) was used instead of maleate one. Disaccharidase activities were expressed as μmol of particular disaccharide hydrolyzed per minute per g protein. Protein content of the supernatant was estimated using standard Lowry’s method with bovine serum albumin as a standard. The pH of ileal and cecal digesta were measured using a microelectrode and a pH/ION meter (model 301, Hanna Instruments). Fresh cecal digesta was used for analysis of ammonia concentration while determination of microbial glycolytic activity, as well as the concentration of short-chain fatty acids (SCFA) were performed after samples storage at −20 °C. Ammonia was extracted and trapped in a solution of boric acid, then determined by direct titration with sulfuric acid [16]. The microbial glycolytic activity (α- and β-glucosidase, α- and β-galactosidase, β-glucuronidase) were measured by the rate of p- or o-nitrophenol released from their nitrophenyl-glucosides, and expressed as μmol of product formed per h per g of cecal digesta [17]. The concentration of SCFA was measured using gas chromatography under conditions described previously [17].
Serum concentrations of glucose, triglycerides, urea, total cholesterol and its HDL fraction were estimated with reagents from Alpha Diagnostics Ltd. (Warsaw, Poland). The activities of glutathione peroxidase (GPx) in the heparinized blood and superoxide dismutase (SOD) in erythrocyte lysate were determined using reagents from Randox Laboratories Ltd. (Crumlin, UK). Serum ACH (antioxidant capacity of hydrophilic substances, kit Analytik Jena AG no. 400.801) and ACL (antioxidant capacity of lipophilic substances, kit Analytik Jena AG no. 400.803) were determined with photochemiluminescence detection method using a Photochem (Analytik Jena AG, Jena, Germany). In the photochemiluminescence assay, the generation of free radicals was partially eliminated by the reaction with antioxidants present in serum samples, and the remaining radicals were quantified by luminescence generation. Ascorbate and Trolox calibration curves were used in order to evaluate ACH and ACL, respectively, and the results were expressed as μmol of ascorbate or Trolox equivalent per mL serum. The concentration of thiobarbituric acid-reactive substances (TBARS) in the tissue of internal organs was estimated after storage at −70 °C according to Uchiyama and Mihara [18].
Results were elaborated statistically using a one-way analysis of variance and the Duncan’s multiple range post hoc test. Differences were considered significant at p ≤ 0.05. The calculations were made using the STATISTICA software package version 6.0 (StatSoft Corp, Krakow, Poland).
Results
Diet Intake, Body Weight, Mass of Internal Organs, and Indicators of Gut Functioning
Diet intake was statistically lower in group E, whereas after extract supplementation it increased to the level comparable with the control group (Table 3). The body weight gain was significantly reduced in group E and EP after 4 week of feeding, while food efficiency ratio, as well as relative mass of liver and kidneys was significantly increased, when compared to the control group. The lowest relative mass of the small intestine with digesta was in group C (Table 4). In turn, it was insignificantly higher in group E and significantly higher in the group E. The pH of ileal digesta was diversified statistically between all groups with the lowest value in the control group and the highest in group E (p ≤ 0.05). The extract was found to significantly decrease the ileal pH value, yet not to the level recorded in the rats from group C. Sucrase activity was comparable among C and EP groups and considerably increased in the group E, while an opposite situation occurred in the case of lactase activity, which decreased considerably in group E when compared with groups C and EP. Furthermore, essentially lower maltase activity in the group EP was also noted when compared that determined in group E. The relative mass of cecal tissue and digesta, as well as pH did not differ between treatments, while ammonia concentration was significantly lower in the cecal digesta of rats from group EP, when compared to the group E (Table 4). Moreover, the activity of microbial enzymes determined in the cecal digesta did not differ statistically between the control and experimental groups, except β-glucuronidase whose activity considerably increased in group E, when compared to the control rats. However, the diet supplementation with the chokeberry fruit extract decreased β-glucuronidase activity to the level not significantly different vs. control group. The lowest concentration of butyrate was recorded in group E, the extract increased it significantly, yet not to the level observed in the control group. Moreover, total concentration of SCFA in the cecal digesta decreased essentially in group E, whereas extract dietary supplementation elevated it when compared with group C.
Blood Parameters and Antioxidant Status
There were no significant differences in serum glucose level among C and EP groups, while in the group E the level was remarkably higher (Table 5, p ≤ 0.05). When compared to the control group, total cholesterol concentration was statistically higher in group E, and the extract decreased it to the level not significantly different against the control group; nevertheless, the concentration of HDL-cholesterol did not differ between all animals. Furthermore, experimental treatments caused more than twofold increase in serum triglyceride levels as well as a significant increase in serum urea concentration. The activity of SOD converted into whole blood was decreased upon experimental treatments vs. control rats, in turn the extract from chokeberry fruits was found to elevate it (Table 5). In comparison with rats from group C, the ACL serum level was remarkably increased in the rats from group E, while the extract decreased it. Moreover, enhanced concentration of TBARS in liver, kidney, and lung tissues observed in group E was completely reduced by the extract to the level recorded in the organs of control rats, while the concentration in heart and spleen tissues was not affected by all experimental treatments.
Discussion
Although a high-fructose diet is recognized as hypertriglyceridemic, prooxidative and hyperinsulinemic, the elevation of glycemia and cholesterolemia has not been presented [11]. Additionally, some authors have also questioned its prooxidative properties [19]. In our study, the dietary modifications applied to animals from group E (fructose and saturated fat inclusion), combined with an injection of a low dose of STZ, caused a significant increase of glucose, triglyceride and total cholesterol concentration in the serum, elevation of the indicator of lipid peroxidation (TBARS) in liver, kidney and lung tissue, as well as decrease of SOD activity, yet with confusing increase of ACL serum level. An explanation of the ACL rise is that vitamin E has main influence on lipid phase and, probably, its larger quantities were spent to protect against oxidation of unsaturated fatty acid from soybean oil, used as dietary component in the control group. Nevertheless, all the foregoing results should be considered as desired modeling changes mimicking disorders occurring in metabolic syndrome. They were connected with some side effects, such as decreased diet intake, body weight gain and increased mass of selected internal organs. These changes, although significant, have not been exposed so extremely as in fully manifested experimental diabetes, where the required STZ dose (50–65 mg/kg) led to far more advanced disturbances, such as severe weight loss and hyperphagia [20, 21].
The tested chokeberry extract was a condensed source of polyphenols, especially anthocyanin glycosides (56.6%). This is in agreement with other studies investigating the composition of chokeberry phytochemicals [2, 22]. In our study, the extract intake led to overall improvement of antioxidant status disturbed by experimental treatments, especially, it normalized concentrations of the lipid peroxidation indicator (TBARS) in organ tissues. Although it was not transferred to the alleviation of organs hypertrophy and reduction of urea level, as the marker of renal failure, an improved antiradical activity may, to some extent, inhibit pathological changes in the internal organs. A confirmation of this supposition can be partly the report of Valcheva–Kuzmanova et al. [3] in which chokeberry fruit juice intake reduced necrotic changes induced by carbon tetrachloride in rat liver, where it is transformed to free radical metabolites, and inhibited an increased activity of plasma transaminases
We observed a distinct hypoglycemic action of the extract tested. It is in accordance with the hypoglycemic effect of chokeberry fruit juice observed in experiment on STZ-induced diabetic rat model, where authors have pointed at chokeberry polyphenols and discussed possible mechanisms of action, such as cells stimulation of glucose uptake and glycogen synthesis, increase of insulin secretion, as well as protection of pancreatic β cells from STZ- and glucose-induced oxidative stress [5]. Nevertheless, a wide range of possible mechanisms, which are probably responsible of the hypoglycemic effect of chokeberry phytochemicals, encouraged us to future more extensive investigations. Therefore, we additionally decided to trace processes ongoing in the gut. Adachi et al. [23] stated that an increase activity of α-glucosidases in the brush border of small intestine might be one of the reason of postprandial hyperglycemia. In our study, the extract significantly reduced the activity of sucrase and maltase, and it seems to be one of the factors which explain the discussed hypoglycemic action of the chokeberry fruit derivatives. It is recognized that polyphenol glycosides, i.e. quercetin, genistein and daidzein glucosides, can be hydrolyzed by lactase [24]. On the one hand it points at the possibility that glycolytic activity of brush border enzymes may be directly affected by the phenolic compounds, while on the other, it is in agreement with increased lactase activity in rats fed the chokeberry extract (vs. group E) reported in our study. Nevertheless, depression of sucrase and maltase activity in group EP may also be the secondary effect of the decreased serum glucose level, since hyperglycemia is partly responsible for increased activity of maltase, sucrase, and lactase in diabetes [25]. Interestingly, in our model of prediabetic state the activity of lactase was strongly inhibited (group E), whereas completely reverse situation is to be found in rats with experimental diabetes [25].
It is known that fructose is not so efficiently absorbable in upper part of the small intestine as most of mono- and disaccharides [26], thus its high dietary content in our study (65%) might increased osmotic pressure in the ileum with simultaneous detrimental influence on local microflora; probably the elevated ileal pH in experimental groups was a consequence of these disorders, yet a dietary inclusion of the extract lowered ileal pH when compared with group E but not to the level observed in the control one. All the aforementioned fluctuations, as well as increased mass of the small intestine in group EP against the control one, were not transferred on pH and mass of the cecum in both experimental groups, but an influence of the chokeberry extract was observed on the other indicators of the cecal functioning disturbed by experimental treatments. Especially, worthy of notice is the decreased β-glucuronidase activity and ammonia concentration, that is the intestinal neoplasia promoters, as well as increased total SCFA and butyrate concentration, widely suggested as protective against many colonic diseases [17].
In our study, a cholesterol-lowering effect of the extract tested was exhibited as well with simultaneous lack of influence on its HDL fraction and triglyceride levels. Available data have pointed out a thorough antihyperlipidemic effect (total cholesterol and triglycerides) of the chokeberry fruit juice in rats fed with a high-cholesterol diet [4]. Nonetheless, all these potential effects in connection with antiaggregatory [6], antiinflammatory [7–8], vasoactive and vasoprotective [9] actions point at chokeberry constituents as promising factors in the prevention and treatment of cardiovascular diseases. Partly, it has already been confirmed by the latest clinical trial where chokeberry fruit extract enhanced reduction in cardiovascular risk markers of patients after myocardial infraction [7].
In conclusion, the rat model of prediabetes and hyperlipidemia obtained in our study may serve as a function in scientific approach to induce disturbances mimicking those observed in metabolic syndrome. The chokeberry fruit extract was attributed with distinct antioxidative and hypoglycemic properties, as well as some cholesterol-lowering action. The mechanism of glucose reduction is likely to be multifactorial, and this study suggests that factors related to increased mucosal disaccharidase activities (maltase and sucrase), as well as oxidative stress are important for further investigation. Moreover, the findings indicate that chokeberry fruit derivatives may provide a viable supplementary therapeutic option in the prevention and treatment of disorders occurring in metabolic syndrome, as well as their complications.
Abbreviations
- ACL:
-
antioxidant capacity of lipophilic substances
- ACH:
-
antioxidant capacity of hydrophilic substances
- STZ:
-
streptozotocin
- GPx:
-
glutathione peroxidase
- HDL:
-
high-density lipoprotein
- SCFA:
-
short-chain fatty acids
- SOD:
-
superoxide dismutase
- TBARS:
-
thiobarbituric acid-reactive substances
References
Persson Hovmalm HA, Jeppsson N, Bartish IV, Nybom H (2004) RAPD analysis of diploid and tetraploid populations of Aronia points to different reproductive strategies within the genus. Hereditas 141:301–312
Sueiro L, Yousef GG, Seigler D, de Mejia EG, Grace MH, Lila MA (2006) Chemopreventive potential of flavonoid extracts from plantation-bred and wild Aronia melanocarpa (Black chokeberry) fruits. J Food Sci 71:480–488
Valcheva-Kuzmanova S, Borisova P, Galunska B, Krasnaliev I, Belcheva A (2004) Hepatoprotective effect of the natural fruit juice from Aronia melanocarpa on carbon tetrachloride-induced acute liver damage in rats. Exp Toxicol Pathol 56:195–201
Valcheva-Kuzmanova S, Kuzmanov K, Mihova V, Krasnaliev I, Borisova P, Belcheva A (2007a) Antihyperlipidemic effect of Aronia melanocarpa fruit juice in rats fed a high cholesterol diet. Plant Food Hum Nutr 62:19–24
Valcheva-Kuzmanova S, Kuzmanov K, Tancheva S, Belcheva A (2007b) Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Method Find Exp Clin Pharmacol 29:1–5
Ryszawa N, Kawczyńska-Dróżdż A, Pryjma J, Czesnikiewicz-guzik M, Adamek-guzik T, Naruszewicz M, Korbut R, Guzik TJ (2006) Effects of novel plant antioxidants on platelet superoxide production and aggregation in atherosclerosis. J Physiol Pharmacol 57:611–626
Naruszewicz M, Łaniewska I, Millo B, Dłużniewski M (2007) Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis 194:79–184
Ohgami K, Ilieva I, Shiratori K, Koyama Y, Jin X-H, Yoshida K, Kase S, Kitaichi N, Suzuki Y, Tanaka T, Ohno S (2005) Effects of novel plant antioxidants on platelet superoxide production and aggregation in atherosclerosis. Invest Ophth Vis Sci 46:275–281
Bell DR, Gochenaur K (2006) Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J Appl Physiol 100:1164–1170
Després J-P, Lemieux I (2006) Abdominal obesity and metabolic syndrome. Nature 444:881–887
Busserolles J, Gueux E, Rock E, Demigne C, Mazur A, Rayssiguier Y (2003) Oligofructose protects against the hypertriglyceridemic and pro-oxidative effects of a high fructose diet in rats. J Nutr 133:1903–1908
Garg MC, Ojha S, Bansal DD (1996) Antioxidant status of streptozotocin diabetic rats. Indian J Exp Biol 34:264–266
Oszmianski J, Wojdylo A (2005) Aronia melanocarpa phenolics and their antioxidant activity. Eur Food Res Technol 221:809–813
Frejnagel S (2007) Comparison of polyphenolic composition of extracts from honeysuckle, chokeberries and green tea—a short report. Pol J Food Nutr Sci 57:83–86
Dahlqvist A (1964) Method for assay of intestinal disaccharidases. Anal Biochem 7:18–25
Hofirek B, Haas D (2001) Comparative studies of ruminal fluid collected by oral tube or by puncture of the caudorental ruminal sac. Acta Vet Brno 70:27–33
Juśkiewicz J, Zduńczyk Z (2004) Effects of cellulose, carboxymethylcellulose and inulin fed to rats as single supplements or in combinations on their caecal parameters. Comp Biochem Phys A 139:513–519
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Girard A, Madani S, El Boustani ES, Belleville J, Prost J (2005) Changes in lipid metabolism and antioxidant defense status in spontaneously hypertensive rats and Wistar rats fed a diet enriched with fructose and saturated fatty acids. Nutrition 21:240–248
Chavez M, Seeley RJ, Havel PJ, Friedman MI, Matson CA, Woods SC, Schwartz MW (1998) Effect of a high-fat diet on food intake and hypothalamic neuropeptide gene expression in streptozotocin diabetes. J Clin Invest 102:340–346
Okada M, Shibuya M, Yamamoto E, Murakami Y (1999) Effect of diabetes on vitamin B6 requirement in experimental animals. Diabetes Obes Metab 1:221–225
Benvenuti S, Pellati F, Melegari M, Bertelli D (2004) Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of rubus, ribes, and aronia. J Food Sci 69:164–169
Adachi T, Mori C, Sakurai K, Shihara N, Tsuda K, Yasuda K (2003) Morphological changes and increased sucrase and isomaltase activity in small intestines of insulin deficient and type 2 diabetic rats. Endocr J 50:271–279
Day AJ, Gee JM, DuPont MS, Johnson IT, Williamson G (2003) Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: the role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem Pharmacol 65:1199–1206
Murakami I, Ikeda T (1998) Effects of diabetes and hyperglycemia on disaccharidase activities in the rat. Scand J Gastroentero 33:1069–1073
Skoog SM, Bharucha AE (2004) Dietary fructose and gastrointestinal symptoms: a review. Am J Gastroenterol 99:2046–2050
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jurgoński, A., Juśkiewicz, J. & Zduńczyk, Z. Ingestion of Black Chokeberry Fruit Extract Leads to Intestinal and Systemic Changes in a Rat Model of Prediabetes and Hyperlipidemia. Plant Foods Hum Nutr 63, 176–182 (2008). https://doi.org/10.1007/s11130-008-0087-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-008-0087-7