Abstract
The Cosmos bipinnatus has been used in a traditional herbal remedy for various diseases such as jaundice, intermittent fever, and splenomegaly. The present study describes the preliminary evaluation of antioxidant activities and antigenotoxic effect of Cosmos bipinnatus flowers according to four different colors (white, pink, orange, and violet). The antioxidants properties were evaluated by determining TPC, DPPH RSA, ABTS RSA, and RP. The highest TPC of methanolic CFE (at concentration of 1 mg/ml) showed in violet colored CF (1,013 μM), and IC50 of DPPH RSA, ABTS RSA, and RP were also the lowest in violet colored CFE with values of 0.61, 1.48, and 0.82 mg/ml, respectively. The antigenotoxic effect of the CFE on DNA damage induced by H2O2 in human leukocytes was evaluated by Comet assay. Pretreatments with CFE produced significant reductions in oxidative DNA damage at the concentration of 500 μg/ml, except for violet colored CFE. The ED50 value of white colored CFE has shown the highest inhibition (0.40 mg/ml) on H2O2 induced DNA damage, followed by orange > pink > violet color. These results suggested that Cosmos bipinnatus has significant antioxidant activity and protective effect against oxidative DNA damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress generated by ROS, such as hydroxyl radicals and hydrogen peroxide, is thought to be implicated in pathological conditions such as inflammation and cancer, as well as neurodegenerative disorders [1]. Cells are equipped with different kinds of mechanisms to combat against ROS and to maintain the redox homeostasis of the cell. For example, superoxide dismutase, catalase, and glutathione play a critical role in neutralizing the free radicals produced during cellular functions [2].
There has been an increased interest in identifying antioxidant phytochemicals, because these molecules can inhibit the propagation of free radical reactions, and protect the human body from diseases [3]. An increasing number of investigations have been carried out to find antioxidative materials, which not only prolong the shelf life of food products but also participate as radical scavengers in living organisms. As with other synthetic food additives, commercial antioxidants have been criticized, mainly due to possible toxic effect. Therefore, there is an increasing interest in the antioxidative activity of natural compounds [4, 5].
Plants may contain phenolic compounds such as phenolic acids, coumarins, flavonoids, stilbenes, tannins, lignins, and lignans [6]. The antioxidant activity of phenolic compounds is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donators, and singlet oxygen quenchers [7]. Cosmos is a genus within the family Asteraceae. They are late-flowering annuals or tuberous perennials. Cosmos are the most widely studied species of the genus, and are generally considered to be short-day plants [8]. Most of cosmos, including Cosmos bipinnatus, are native to Mexico and the southwestern USA. C. bipinnatus offers flowers of white, pink, violet, or lavender color blooms. These plants can attain a height of 3 to 6 ft and have an open and sprawling habit. Finely cut and thread-like simple leaves are pinnately cut into deep lobes appearing compound [9]. The cosmos has been used in a traditional herbal remedy for various diseases such as jaundice, intermittent fever, and splenomegaly [10], and triterpene alcohols such as helianol of cosmos were reported to show anti-inflammatory activity [11]. Several other studies have been investigated in the cosmos [12, 13], however, there have been few reports regarding characteristics and physiological activities of cosmos flowers. The objective of this study was to evaluate the antioxidant and antigenotoxic activity in methanolic extracts of cosmos flowers according to various colors.
Materials and Methods
Material
Cosmos (Cosmos bipinnatus) flowers (CF) were collected in early fall 2007 at rural district of Masan City, Korea. DPPH, ABTS, and DMSO were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Folin–Ciocalteu reagents were from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Hydrogen peroxide, sodium chloride, sodium hydroxide, potassium chloride, and potassium phosphate were also purchased from Sigma Chemical Co. (St. Louis, MO). All the other organic solvents and chemicals used were of analytical grade.
Preparation of Extracts from CF
Each 5 g of fresh CF were extracted with 100 ml of methanol for overnight at room temperature and filtered through a Whatman no. 1 filter paper (Tokyo, Japan). Solvents were then removed by evaporation in vacuo, and the dried extracts were obtained. The solvent extracts from CF were named as CFE. The CFE were then dissolved in DMSO with concentration of 50 mg/ml for experiments, and diluted with DMSO when needed.
TPC
TPC of CFE were determined according to the method of Gutfinger [14]. Each CFE (1.0 ml) was mixed with 1.0 ml of 2% Na2CO3 and added 0.2 ml of 50% Folin–Ciocalteu reagent after standing for 5 min, and centrifuged at 13,400×g for 5 min after 30 min incubation at room temperature. The absorbance was measured with a spectrophotometer (Shimadzu UV-1601, Tokyo, Japan) at 750 nm. TPC were expressed as gallic acid equivalents.
DPPH RSA
The DPPH RSA of CFE was determined according to the method of Lee et al. [15]. After 0.1 ml of each concentration of CFE had been mixed with 0.9 ml of 0.041 mM DPPH in ethanol for 10 min, the absorbance of the sample was measured at 517 nm by a spectrophotometer (Shimadzu UV-1601, Tokyo, Japan). RSA was expressed as a percentage inhibition and it was calculated by using the following formula:
ABTS RSA
The ABTS RSA was evaluated with the method of Muller [16]. The CFE (0.1 ml), potassium phosphate buffer (0.1 ml, 0.1 M, pH 5.0) and hydrogen peroxide (20 μl, 10 mM) were mixed and incubated at 37 °C for 5 min. After preincubation, ABTS (30 μl, 1.25 mM, in 0.05 M phosphate-citrate buffer, pH 5.0) and peroxidase (30 μl, 1 IU/ml) were added to the mixture and then it was incubated at 37 °C for 10 min. The absorbance level was obtained with an enzyme-linked immunosorbent assay reader (Sunrise RC/TS/TS Color-TC/TW/BC/6Filter, Tecan Austria GmbH.) at 405 nm.
RP
RP of CFE was determined according to the method of Oyaizu [17]. CFE (1.0 ml), sodium phosphate buffer (1.0 ml, 0.2 M, pH 6.6) and potassium ferricyanide (1.0 ml, 10 mg/ml) were mixed and incubated at 50 °C for 20 min. Trichloroacetic acid (1.0 ml, 100 mg/ml) was added to the mixture and centrifuged at 13,400×g for 5 min. The supernatant (1.0 ml) was mixed with distilled water (1.0 ml) and ferric chloride (0.1 ml, 1.0 mg/ml), and then its absorbance was measured at 700 nm.
DNA Damage Determination by Alkaline Comet Assay
Leucocytes were isolated as a fraction of mononuclear cells (containing lymphocytes and monocytes) from anonymous buffy coat preserves by gradient centrifugation with Histopaque®-1077 (Sigma, Deisenhofen, Germany). Leukocytes were incubated with methanol extracts of cosmos dissolved in DMSO and diluted into concentrations 10, 50, 100, and 500 μg/ml for 30 min at 37 °C in a dark incubator. For oxidative stimulus they were then resuspended in PBS with 200 μM H2O2 for 5 min on ice. After each treatment, samples were centrifuged at 250×g for 5 min and washed with PBS. 1% DMSO without oxidative stimulus was treated for negative control. The leukocytes were then mixed with 75 μl of 0.7% low melting agarose, and added to the slides precoated with 0.5% agarose. The slide was then immersed in lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, and 1% sodium laurylasarcosine; 1% Triton X-100 and 10% DMSO) for 1 h at 4 °C. The slides were next placed into an electrophoresis tank containing 300 mM NaOH and 10 mM Na2EDTA (pH 13.0) for 40 min. For electrophoresis of the DNA, an electric current of 25 V/300 ± 3 mA was applied for 20 min at 4 °C. The slides were washed three times with a neutralizing buffer (0.4 M Tris, pH 7.5) for 5 min at 4 °C, and then treated with ethanol for another 5 min before staining with 50 μl of ethidium bromide (20 μg/ml). Measurements were made by image analysis (Kinetic Imaging, Komet 4.0, UK) and fluorescence microscope (Leica DMLB, Germany), determining the percentage of fluorescence in the tail (tail intensity, TI; 50 cells from each of two replicate slides).
Statistical Analysis
All measurements were done in triplicate, and antioxidant experimental values were expressed as means ± standard deviation. The data for Comet assay were the means of three determinations and was analyzed using the SPSS package for Windows (Ver. 12). The mean values of the DNA damage (tail intensity) from each treatment were compared using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test. P value of less than 0.05 was considered significant.
Results and Discussion
Antioxidant Activity of CFE
Polyphenolic compounds such as flavonoids and anthocyanins are of great interest for their radical scavenging activity. Intake of dietary antioxidants that act as radical-scavengers is expected to be effective in preventing many diseases [18, 19]. TPC, DPPH RSA, ABTS RSA, and RP of CFE are shown in Table 1. The TPC of each CFE at concentration of 1 mg/ml were listed in Table 1. Violet CFE (1012.93 ± 7.86 μM) had higher TPC values, while that of white CFE (361.94 ± 1.45 μM) showed the lowest value among the studied. Radical scavengers were evaluated by their reactivity toward a stable free radical, DPPH or ABTS. The DPPH and ABTS systems have both been commonly used to measure the total antioxidantive status of various biological specimens because of their good reproducibility and easy quality control [20, 21]. The highest DPPH radical scavenging effect was detected in violet CFE (IC50 = 0.61 ± 0.06 mg/ml, Table 1) followed by orange (IC50 = 0.84 ± 0.08 mg/ml), pink (IC50 = 1.45 ± 0.31 mg/ml), and white CFE (IC50 = 1.65 ± 0.29 mg/ml). However, IC50 values of ABTS RSA decreased in the following order: violet > white > pink > orange CFE. The power of certain antioxidants is associated with their reducing power (RP) [22]. Duh [23] reported that the reducing properties of antioxidants are generally related with the presence of reductants. RP was the highest in violet CFE, and this order of the RP showed similar trends to the DPPH RSA.
DPPH RSA and RP showed high positive relationship with TPC of CFE. That means order of the amount of TPC of CFE (violet > orange > pink > white) exactly coincided with the order of DPPH and RSA. However, ABTS RSA showed different pattern. Though violet CFE exhibited the highest ABTS RSA, the other colored CFE presented different order of ABTS RSA with that of TPC and DPPH RSA. The antioxidant activities of some putative antioxidants have been attributed to various mechanisms such as the prevention of chain initiation, the binding of transition metal ion catalysts, the decomposition of peroxides, the prevention of continued hydrogen abstraction and radical scavenging [24]. Hence, there may not always be linear correlation between antioxidant activities each other.
Oh et al. [25] reported antioxidant activities of two (purple and white) different Wisteria floribunda flowers, and there was no direct relationship between color and antioxidant activity of the flowers. Cyanidins are major pigment compounds responsible for the color of flowers with biological activities including antioxidant and antimutagenic activities [26]. However, it is difficult to deduce direct proportion relation of the amount of cyanidins and antioxidant activity because there are too many other compounds in the extracts of flowers.
Protective Effect of Cosmos on Oxidative DNA Damage in Human Leukocytes
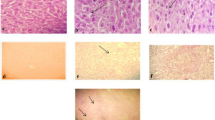
The genotoxic effects of H2O2 and the protective ability of various CFE were assessed in normal human leukocytes by the Comet assay (Fig. 1). The Comet assay, also known as single cell gel electrophoresis assay has been used successfully for evaluating DNA damage and has been suggested as an excellent technique for use with biological markers in the detection, monitoring, and prognosis of chronic degenerative disease including certain types of cancer, diabetes and atherosclerosis [27]. The protective effect of CFE increased as its concentration increases from 10 to 500 μg/ml. At concentration 500 μg/ml, all CFEs except violet CFE, exhibited the greatest protective effect on H2O2 induced DNA damage which was no statistical difference compared to DMSO treated negative control. The possible mechanism by which CFE inhibited oxidative DNA damage in human leukocytes can be ascribed to the chemical structure of the phenolic compound contained in CF. The phenolic compound in CF may act by providing hydrogen atoms from their phenolic hydroxyl groups to scavenge hydroxyl radical generated from hydrogen peroxide. The protective effect of polyphenolic compounds (tannic, ellagic, and gallic acid) on oxidative DNA damage measured by Comet assay has been demonstrated by Labieniec and Gabryelak [28].
The effect of supplementation in vitro with different concentration and colors of cosmos flowers extract on 200 μM H2O2-induced DNA damage in human leukocyte. Values are mean with standard deviation of triplicate experiments. NC, 1% DMSO (without oxidative stimulus) treated negative control; PC, 200 μM H2O2-treated positive control. Values not sharing the same letter are significantly different from one another (P < 0.05) by Duncan’s multiple range test
The concentrations of CFE that would produce 50% reduction in DNA damage in estimated from the regression equations were, in decreasing order of efficacy, white (40.2 μg/ml), orange (51.8 μg/ml), pink (55.1 μg/ml), and violet (107.6 μg/ml; Fig. 2). Although violet CFE might be expected to be more effective in inhibiting oxidative DNA damage than other colors CFE due to having high TPC, DPPH RSA, ABTS RSA, and RP, white CFE has shown the highest inhibition of cellular DNA damage induced by H2O2. These results are in concordance with the results of Rim et al. [29] who reported that leaves of Akebia quinata was higher in inhibiting of oxidative DNA damage, despite of having lower concentration of total phenol and weaker antioxidant activities than flowers of Akebia quinata. It is suggested that extract of white CFE might contain other possible antigenotoxic agents compared to that of violet CFE.
Abbreviations
- ABTS:
-
2,2′-azino-(bis 3-ethylbenzthiazoline-6-sulphonic acid)
- CF:
-
cosmos flowers
- CFE:
-
cosmos flowers extract
- DMSO:
-
dimethyl sulfoxide
- DPPH:
-
1,1-diphenyl-2-picrylhydrazyl
- ED50 :
-
the estimated dose for 50% reduction in oxidative DNA damage
- IC50 :
-
the concentration required for inhibiting 50% of activity
- PBS:
-
phosphate buffered saline
- ROS:
-
reactive oxygen species
- RP:
-
reducing power
- RSA:
-
radical scavenging activity
- TPC:
-
total phenolic contents
References
Gordon MH (1996) Dietary antioxidants in disease prevention. Nat Prod Red 13:265–273 doi:10.1039/np9961300265
Allen RG, Tresini M (2000) Oxidative stress and gene regulation. Free Radic Biol Med 28:463–499 doi:10.1016/S0891-5849(99)00242-7
Kinsella JE, Frankel E, German B, Kanner J (1993) Possible mechanisms for the protective role of antioxidants in wine and plant foods. Food Technol 47:85–89
Orhan I, Aydin A, Colkesen A, Isimer AI (2003) Free scavenging activities of some edible fruit seeds. Pharm Biol 41:163–165 doi:10.1080/13880200308951337
Lee BB, Cha MR, Kim SY, Park E, Park HR, Lee SC (2007) Antioxidative and anticancer activity of extracts of cherry (Prunus serrulata var. spontanea) blossoms. Plant Foods Hum Nutr 62:79–84 doi:10.1007/s11130-007-0045-9
Naczk M, Shahidi F (2006) Phenolics in cereals, fruits and vegetable: occurrence, extraction and analysis. J Pharm Biomed Anal 41:1523–1542 doi:10.1016/j.jpba.2006.04.002
Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB (1995) The relative antioxidant activities of plant derived polyphenolic flavonoids. Free Radic Res 22:375–383 doi:10.3109/10715769509145649
Molder M, Owens JN (1985) Cosmos. In: Halevy AH (ed) CRC handbook of flowering. CRC, Boca Raton, FL
Edward FG, Teresa H (1999) Cosmos bipinnatus. Fact sheet FPS-148, Institute of Food and Agricultural Sciences, University of Florida
Alexandros SB (2007) Plants used traditionally to treat malaria in Brazil. J Ethnobiol Ethnomed 3:18 doi:10.1186/1746-4269-3-18
Akihisa T, Yasukawa K, Oinuma H, Kasahara Y, Yamanouchi S, Takido M, Kumaki K, Tamura T (1996) Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. Phytochemistry 43:1255–1260 doi:10.1016/S0031-9422(96)00343-3
Kwon JH, Kang SW, Son KA, Park CS (1999) Anthracnose of cosmos caused by Colletotrichum acutatum in Korea. Plant Pathol J 15:172–174
Kanellos EAG, Pearson S (2000) Environmental regulation of flowering and growth of Cosmos atrosanguineus (Hook.) Voss. Sci Hortic (Amsterdam) 83:265–274 doi:10.1016/S0304-4238(99)00081-3
Gutfinger T (1981) Polyphenol in olive oils. J Am Oil Chem Soc 58:996–998 doi:10.1007/BF02659771
Lee SC, Kim JH, Jeong SM, Kim DR, Ha JU, Nam KC, Ahn Du (2003) Effect of far-infrared radiation on the antioxidant activity of rice hulls. J Agric Food Chem 51:4400–4403 doi:10.1021/jf0300285
Muller HE (1985) Detection of hydrogen peroxide produced by microorganisms on ABTS-peroxidase medium. Zentralbl Bakteriol Mikrobiol Hyg 259:151–158
Oyaizu M (1986) Antiangiogenic properties of natural polyphenols from red wine and green tea. Jpn J Nutr 44:307–315
Büyükbalci A, El SN (2008) Determination of in vitro antidiabetic effects, antioxidant activities and phenol contents of some herbal teas. Plant Foods Hum Nutr 63:27–33 doi:10.1007/s11130-007-0065-5
Mitsuru W (2007) An anthocyanin compound in buckwheat sprouts and its contribution to antioxidant capacity. Biosci Biotechnol Biochem 71:579–582 doi:10.1271/bbb.60471
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol 28:25–30 doi:10.1016/S0023-6438(95)80008-5
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evan C (1999) Antioxidant activity applying improved ABTS radical cation decolorization assay. Free Radic Res 26:1231–1237
Jayaprakasha GK, Singh RP, Sakariah KK (2001) Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem 73:285–290 doi:10.1016/S0308-8146(00)00298-3
Duh PD (1998) Antioxidant activity of budrock (Arctium lappa L.): its scavenging effect on free radical and active oxygen. J Am Oil Chem Soc 75:455–461 doi:10.1007/s11746-998-0248-8
Diplock AT (1997) Will the good fairies please prove to us that vitamin E lessens human degenerative disease? Free Radic Res 27:511–532 doi:10.3109/10715769709065791
Oh WG, Jang IC, Jeon GI, Park EJ, Park HR, Lee SC (2008) Antioxidative activity of extracts from Wisteria floribunda flowers. J Korean Soc Food Sci Nutr 37:677–683
Calvano F, Fauci LL, Lazzarino G, Fogliano V, Ritieni A, Ciappellano S, Battistini NC, Tavazzi B, Galvano G (2004) Cyanidins: metabolism and biological properties. J Nutr Biochem 15:2–11 doi:10.1016/j.jnutbio.2003.07.004
Collins AR, Gedik CM, Olmedilla B, Southon S, Bellizzi M (1998) Oxidative DNA damage measured in human lymphocytes: large differences between sexes and between countries, and correlation with heart disease mortality rates. FASEB J 12:1397–1400
Labieniec M, Gabryelak T (2005) Measurement of DNA damage and protein oxidation after the incubation of B14 Chinese hamster cells with chosen polyphenols. Toxicol Lett 155:15–25 doi:10.1016/j.toxlet.2004.06.008
Rim AR, Kim SJ, Joen KI, Park E, Park HR, Lee SC (2006) Antioxidant activity of extracts from Akebia quinata Decne. J Food Sci Nutr 11:84–27
Acknowledgement
This study was supported by Kyungnam University Research Fund, 2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jang, IC., Park, JH., Park, E. et al. Antioxidative and Antigenotoxic Activity of Extracts from Cosmos (Cosmos bipinnatus) Flowers. Plant Foods Hum Nutr 63, 205–210 (2008). https://doi.org/10.1007/s11130-008-0086-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-008-0086-8