Abstract
The scavenging activity against DPPH (1,1-diphenil-2-picrylhydrazyl) radical and the antifungal effect against chloroform, ethyl acetate and 50% methanolic extracts of Verbena officinalis leaves were investigated. The activity of different fractions of 50% methanolic extract and some isolated compounds were also investigated. The results suggest that 50% methanolic extract and caffeoyl derivatives could potentially be considered as excellent and readily available sources of natural antifungal and antioxidant compounds.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Deterioration of food quality occurs during processing and storage and it is related to oxidative processes and microorganisms. Oxidative degradation affects lipids mainly, but also carbohydrates, proteins and nucleic acids. Many additives have long been used by food industry to preserve their products from oxidation, usually synthetic antioxidants such as butylhydroxianisole (BHA) and butylhydroxitoluene (BHT), with no clear effects on human health after chronic consumption [1, 2].
Many plants are sources of compounds with antioxidant activity that might be used as natural preservatives [3–5]. In addition, antioxidants play a special role in human healthcare and in last years prevention of cardiovascular diseases and cancer has been associated with the intake of fruits and vegetables, rich in natural antioxidants [6, 7]. Reactive oxygen species (ROS) are a class of highly reactive molecules derived from the oxygen and generated by metabolic processes in human beings and some external factors such as pollution, radiation or some dietary habits. ROS can cause DNA mutation, protein oxidation and lipid peroxidation, contributing to the development of atherosclerosis [8–9], inflammation [10], neurodegenerative diseases [11, 12], cataracts [13, 14], cancer [15] and aging.
On the other hand, fruits and vegetables suffer from infections during harvesting and packing. Rhizopus stolonifer, Alternaria alternata, Penicillium expansum and Botrytis cinerea are reported to cause decay in stoned fruits, particularly peaches, but also in strawberries, raspberries and grapes [16]. The disease normally develops after harvest, during transportation and as the fruit ripen prior to consumption. The post-harvest losses are fastly increased by the rapid spread of the fungus to adjacent fruits during ripening because the pathogen is not efficiently controlled by registered fungicides and treatments [17].
In the attempt to reduce the use of chemicals due to the concern about human health and environmental pollution, new alternative control approaches are being developed as the use of medicinal and aromatic plant extracts. Plants contain secondary metabolites, some of them with antimicrobial properties [18, 19].
Verbena officinalis, commonly called verveine, grow widespread in all temperature regions of the globe and finds use in folk medicine as diuretic, expectorant and anti-rheumatic. It is listed in the Chinese Pharmacopoeia and the British Herbal Pharmacopoeia. In Navarra (Spain), it is used extensively in traditional medicine mainly because of its anti-inflammatory properties [20]. Preliminary pharmacological studies indicate anti-inflammatory activities of the chloroform and methanolic extract of the plant in the carrageenan paw oedema model [21]. In a previous study we reported that 50% methanolic extract from the leaves of V. officinalis L. showed significant topical anti-inflammatory and analgesic properties in experimental models [22, 23]. The main components of V. officinalis L. are triterpenic acids and sterols [21], iridoids [24, 25], caffeoyl derivatives [26], and flavonoids [27].
The aim of this work was to analyze the antioxidant and antifungal activity of V. officinalis by in vitro approaches. The antioxidant capacity was measured by DPPH method and the antifungal activity by inhibition of in vitro cell cultures of A. alternata, B. cinerea, P. expansum and R. stolonifer.
Materials and Methods
Plant Material
V. officinalis L. (Verbenacea), leaves were collected in June 2001, at Ilundain (Navarra, Spain). A voucher is deposited in the Botany Department’s Herbarium, at the Faculty of Pharmacy, University of Navarra, Pamplona, Spain, and was authenticated by Dra. M.L. López.
Tested Material
Crude chloroform, ethyl acetate and 50% methanolic extracts of V. officinalis L. were obtained by 24-h maceration at 4 °C. Fifty percent of methanolic extract was fractionated by Sephadex® LH-20 to afford five fractions. The analysis of them by HPLC [27] showed the presence of iridoids in fraction I, flavonoids in fraction II and caffeoyl derivatives in fraction IV. Further purifications of fractions II and IV with Sephadex® G-25 afforded three flavonoids (luteolin-7-glucoside (1), luteolin 7-diglucuronide (2), apigenin 7-glucuronide (3)), and two phenolic acids (chlorogenic acid (4) and verbascoside (5); Fig. 1). All crude extracts, fractions I, II, IV and pure compounds (1–5) were tested for antioxidant and antifungal activity.
Antioxidant Activity
Among the radical scavenging assays, one based on the utilization of DPPH was chosen due to its simplicity and worldwide acceptance for comparative purpose. DPPH method is independent of the substrate polarity. This method is based on the reduction of alcoholic DPPH solutions at 517 nm in the presence of an antioxidant compound that donates hydrogen or electron. Non-radical form DPPH-H is formed. The procedure of Brand-Williams et al. [28] has been adapted for evaluation of the free radical scavenging capacity of the tested material. One milliliter of several concentrations of each crude extract and pure compounds was added to 4 ml of 0.004% methanolic solution of DPPH. After 30 min incubation period at room temperature, the absorbance was read against a blank at 517 nm. Inhibition of free radical by DPPH in (%) was calculated:
Where A blank is the absorbance of the control reaction (containing all reagents except the test compound), and A sample is the absorbance of the test compound. IC50 was calculated using nonlinear regression module of Statistica 5.0 as a concentration exposing 50% of maximum activity. The antioxidant activity was compared with the activity of natural rutin, l-ascorbic acid and butylated hydroxyanisole (BHA). The results are given as a mean ± standard deviation (SD) of experiments done in triplicate.
Antifungal Activity
The fungal microorganisms were A. alternata, B. cinerea, P. expansum and R. stolonifer. These fungi cause different infections in many plant crops and stored products. The fungi were obtained from “Colección Española de Cultivos Tipo” edited by Department of Microbiology (University of Valencia).
In order to obtain microorganism growth, potato dextrose agar (PDA) was used (200 g potato juice, 20 g d(+)-glucose, 15 g agar, and 1,000 ml distilled water). Czapex Dox Agar was used for the determination of antifungal effect (30 g sucrose, 3 g sodium nitrate, 0.5 g magnesium sulfate, 1 g potassium hydrogen phosphate, 13 g agar, and 1,000 ml distilled water). One hundred fifty milliliter culture medium (Czapex Dox Agar) was prepared for each sample. Prepared agars were sterilized at 121 °C for 20 min in autoclave. Two hundred fifty parts per million of each tested material was added into sterilized agar medium. These mediums were poured into Petri dishes after being shaken. After keeping the agars in room conditions for one night fungi colonies with a 0.5 cm diameters corkpore were taken from the disks. Petri dishes were incubated at 24–25 °C. Assays were carried out in triplicates and with control samples. Benzoic and gallic acids were used as positive controls at the same concentration.
In order to evaluate the inhibition of mycelia growth of fungi by samples, the fungi colonies diameters were measured since the day of incubation with Leica Q Win Program (v.2.2.) and converted into percentage of inhibition (I%) according to the following formula: [(Control − Treatment / Control)] × 100.
Results and Discussion
Antioxidant Activity
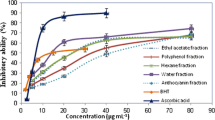
The DPPH radical scavenging activity decreased in order 50% methanol > ethyl acetate > chloroform (Fig. 2). Phytochemical investigations of 50% methanolic extract showed the presence of iridoids, caffeoyl derivatives as well as flavonoid compounds [22].
After fractionation of 50% methanolic extract, DPPH assay clearly indicated that the highest shown by the fraction which contained flavonoids and caffeoyl derivatives (Table 1), and the results (2.20 and 7.23 μg/ml, respectively) were comparable to those of rutin (4.8 μg/ml). Therefore, flavonoids and caffeoyl derivatives might have been the active principles responsible for the antioxidant activity of V. officinalis.
The isolated compounds from V. officinalis showed a similar DPPH antiradical activity when compared with rutin, but much higher activity than ascorbic acid and BHA. In that it concerns to the flavonoids, luteolin 7-diglucuronide is the compound with more activity of the three-isolated (5.86 ± 1.8). The antioxidant activity decreased when the substitution is a glucose molecule (13.12 ± 2.1). Apigenin 7-glucuronide presents an intermediate antioxidant activity (10.95 ± 2.3). In a first approach with these results it is possible to establish that the substitution with one or several glucuronic acid molecules is related to the antioxidant activity of the flavonoids. Both caffeoyl derivatives (chlorogenic acid and verbascoside) presented high antioxidant activity in DPPH assay.
Antifungal Activity
The effect of three different extracts of V. officinalis on the mycelial growth of four plant pathogen fungi is presented in Fig. 3. None of the extracts showed significant inhibition against A. alternata and B. cinerea (<10%). The 50% methanolic crude extract was the most active against P. expansum and R. stolonifer (32.55% and 28.98%, respectively). The inhibition was more pronounced in the caffeoyl derivative fraction against P. expansum (87.45%) and R. stolonifer (79.11%), being even higher than pure compounds (Table 2). These results suggest a possible synergism between the caffeoyl derivatives present in the extract.
Although most of the natural products in agricultural area are used against insects, there are several reports on the effects of essential oils against food and cereal storage fungi, leaf pathogens, and soil-borne fungi [29, 30]. However, there are few studies on the effects of plant extract and pure natural products, so the antioxidant and antifungal abilities of caffeoyl derivatives are of particular interest for applications within the food, storage products and cosmetic industries.
References
Imaida K, Fukushima S, Shivai T, Ohtani M, Nakanish K, Ito N (1983) Promoting activities of butylated hydroxyanisole and butylated hydroxitoluene on 2-stage urinary bladder carcinogenesis and inhibition of g-glutamyl transpeptidase-positive foci development in the liver of rats. Carcinogenesis 4:895–899
Yanishlieva N (2001) Inhibiting oxidation. In: Pokorny J, Yanishlieva N, Gordon M (eds) Antioxidants in food: practical applications. CRC, Boca Raton, Florida, p 380
Zhang JD, Xu Z, Cao YB, Chen HS, Yan L, An MM, Gao PH, Wang Y, Jia XM, Jiang YY (2006) Antifungal activities and action mechanisms of compounds from Tribulus terrestris L. J Ethnopharmacol 103:76–84
Milovanović V, Radulović N, Todorović Z, Stanković M, Gordana Stojanović G (2007) Antioxidant, antimicrobial and genotoxicity screening of hydro-alcoholic extracts of five Serbian Equisetum species. Plant Food Hum Nutr 62:113–119
López V, Akerreta S, Casanova E, García-Mina JM, Cavero RY, Calvo MI (2007) In vitro antioxidant and anti-rhizopus activities of lamiaceae herbal extracts. Plant Food Hum Nutr 62:151–155
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Sun Y (1990) Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med 8:583–599
Fernando RL, Varghese Z, Moorhead JF (1998) Differential ability of cells to promote oxidation of low density lipoproteins in vitro. Clin Chim Acta 269:159–173
Navab M, Fogelman AM, Berliner JA, Territo MC, Demer LL, Frank JS, Watson AD, Edwards PA, Lusis AJ (1995) Pathogenesis of atherosclerosis. Am J Cardiol 76:18C–23C
Maron J (2004) Flavonoids for reduction of atherosclerotic risk. Curr Atheroscler Rep 6:73–78
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Frank B, Gupta H (2005) A review of antioxidants and Alzheimer's disease. Ann Clin Psychiatry 17:269–286
Li S, Zhang Z, Cain A, Wang B, Long M, Taylor J (2005) Antifungal activity of camptothecin, trifolin, and hyperoside isolated from Camptotheca acuminata. J Agric Food Chem 53:32–37
Martensson J, Steinherz R, Jain A, Meister A (1989) Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci USA 86:8727–8731
Ingram D, Sanders K, Kolybaba M, Lopez D (1997) Case–control study of phyto-oestrogens and breast cancer. Lancet 350:990–994
Northover J, Zhou T (2002) Control of rhizopus rot of peaches with postharvest treatments of tebuconazole, fludioxonil, and Pseudomonas syringae. Can J Plant Pathol 24:144–153
Karabulut OA, Arslan U, Kuruoglu G (2004) Control of postharvest diseases of organically grown strawberry with preharvest applications of some food additives and postharvest hot water dips. J Phytopathol 152:224–228
De Lucca AJ, Boue S, Palmgren MS, Maskos K, Cleveland TE (2006) Fungicidal properties of two saponins from Capsicum frutescens and the relationship of structure and fungicidal activity. Can J Microbiol 52:336–342
Oh KB, Mar W, Kim S, Kim JY, Lee TH, Kim JG, Shin D, Sim CJ, Shin J (2006) Antimicrobial activity and cytotoxicity of bis(indole) alkaloids from the sponge Spongosorites sp. Biol Pharm Bull 29:570–573
Fernández M (1981) Las plantas en la medicina popular navarra 1. Navarra húmeda del N.O. Pamplona. Sociedad de Estudios Vascos-Eusko Ikaskuntza
Deepak M, Handa SS (2000) Anti-inflammatory activity and chemical composition of extracts of Verbena officinalis. Phytother Res 14:463–465
Calvo MI, Vilalta N, San Julian A, Fernández M (1998) Anti-inflammatory activity of leaf extract of Verbena officinalis L. Phytomedicine 5:465–467
Calvo MI (2006) Anti-inflammatory and analgesic activity of the topical preparation of Verbena officinalis L. J Ethnopharmacol 107:380–382
Rimpler H, Schaefer BZ (1979) Hastatoside, a new iridoid from Verbena hastata L. and Verbena officinalis L. Naturforscher 3:311–318
Makboul AM (1986) Chemical constituents of Verbena officinalis. Fitoterapia 57:50–51
Hänsel R, Kallmann S (1986) Identitätsprüfung von Verbenae herba: Verbascosid als Leitstoff. Arch Pharm 319:227–230
Calvo MI, San Julián A, Fernández M (1997) Identification of the major compounds in extracts of Verbena officinalis L. (Verbenaceae) by HPLC with post-column derivatization. Chromatographia 46:241–244
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm-Wiss Technol 28:25–30
Muller-Riebau F, Berger B, Yegen O (1995) Chemical composition and fungitoxic properties to phytopathogenic fungi of essential oils of selected aromatic plants growing wild in Turkey. J Agric Food Chem 43:2262–2266
Bowers JH, Locke JC (2000) Effect of botanical extracts on the population density of Fusarium oxysporum in soil and control of Fusarium wilt in the greenhouse. Plant Dis 84:300–305
Acknowledgements
The authors are grateful to the Botany Department for the identification of the plant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casanova, E., García-Mina, J.M. & Calvo, M.I. Antioxidant and Antifungal Activity of Verbena officinalis L. Leaves. Plant Foods Hum Nutr 63, 93–97 (2008). https://doi.org/10.1007/s11130-008-0073-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-008-0073-0